Recent Advances in Characterization and Valorization of Lignin and Its Value-Added Products: Challenges and Future Perspectives
Abstract
:1. Introduction
| Feature | Kraft Lignin | Lignosulfonates | Organosolv Lignin |
|---|---|---|---|
| Production Process | Kraft pulping (NaOH & Na2S) | Sulfite pulping (SO2 & salts) | Organosolv pulping (Organic solvents) |
| Sulfur Content | High | High | Low/Sulfur-free |
| Molecular Weight | High | Low | Low/Medium |
| Solubility | Low (water) | High (water) | Variable (solvent dependent) |
| Applications | Adhesives, dispersants, chemicals/materials precursor | Concrete additives, animal feed binders, dispersants | High-purity lignin derivatives, specialty chemicals, carbon fibers, resins, composites |
| Advantages | Most widely produced | Water-soluble, versatile | Relatively pure, sulfur-free |
| Disadvantages | High sulfur content, complex processing | High sulfur content, environmental challenges | Variable solubility |
| References | [17,18] | [19,20] | [21,22] |
Research Gap and Objective of the Study
2. Methodology
2.1. Understanding Lignin: Characterization as the Foundation for Sustainable Valorization
2.2. Chemical Analysis (Compositional Methods)
2.3. Spectroscopic Techniques for Characterization of Lignin
2.4. Microscopic Techniques for Characterization of Lignin
3. Pretreatment: Loosening Bonds with Other Biomass Components
3.1. Thermal Depolymerization of Lignin
3.2. Catalytic Depolymerization
3.3. Ionic Liquid Pretreatment for Lignin Depolymerization
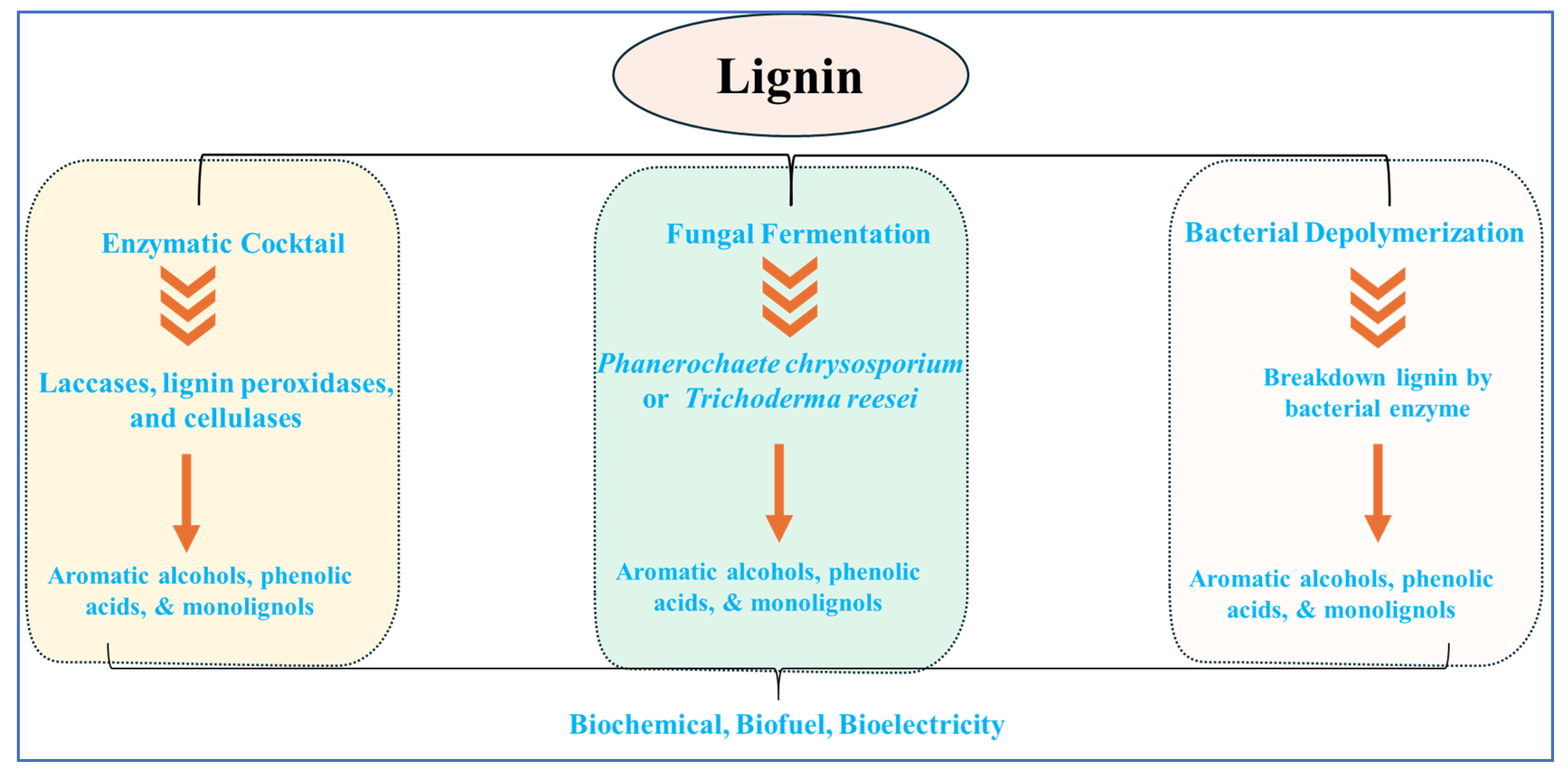
3.4. Biological Depolymerization of Lignin
3.5. Emerging Techniques for Valorization of Lignin
4. Challenges and Readiness of Lignin Depolymerization
5. Advancements in the Valorization of Lignin
5.1. Lignin as a Source of Biofuels
5.2. Lignin-Derived Chemicals and Materials
5.3. Lignin in Polymer Blends and Composites
5.4. Lignin as a UV Protector and Antioxidant
5.5. Functionalization and Modification of Lignin
5.6. Economic and Environmental Benefits of Lignin Valorization
6. Exploring the Expanding Applications of Upgraded Lignin
6.1. Lignin as Precursors for Biofuels and Bio-Based Chemicals
6.2. Role of Lignin as a Functional Additive in Biocomposites
6.3. Source of Aromatic Building Blocks for Novel Biomaterials
6.4. Reduced Reliance on Fossil Resources
6.5. Efficient Use of Natural Resources
6.6. Lignin Valorization for Polyurethane
6.7. Lignin for Bioplastics
7. Conclusions
Future Prospects and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dar, M.A.; Xie, R.; Zabed, H.M.; Ali, S.; Zhu, D.; Sun, J. Termite Microbial Symbiosis as a Model for Innovative Design of Lignocellulosic Future Biorefinery: Current Paradigms and Future Perspectives. Biomass 2024, 4, 180–201. [Google Scholar] [CrossRef]
- Ali, S.; Shah, T.A.; Afzal, A.; Tabassum, R. Exploring Lignocellulosic Biomass for Bio-Methane Potential by Anaerobic Digestion and Its Economic Feasibility. Energy Environ. 2018, 29, 742–751. [Google Scholar] [CrossRef]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A Guide to Lignin Valorization in Biorefineries: Traditional, Recent, and Forthcoming Approaches to Convert Raw Lignocellulose into Valuable Materials and Chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Ali, S.; Shah, T.A.; Afzal, A.; Tabassum, R. Analysis of Agricultural Substrates for Nutritive Values and Biomethane Potential. Curr. Sci. 2018, 115, 292–299. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.; Zhang, C.-C.; Nan, J.; Ren, N.; Lee, D.-J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2021, 343, 126123. [Google Scholar] [CrossRef]
- Rosini, E.; Molinari, F.; Miani, D.; Pollegioni, L. Lignin Valorization: Production of High Value-Added Compounds by Engineered Microorganisms. Catalysts 2023, 13, 555. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Elder, T.; Kim, H.; Ralph, J. Lignin Monomers from beyond the Canonical Monolignol Biosynthetic Pathway: Another Brick in the Wall. ACS Sustain. Chem. Eng. 2020, 8, 4997–5012. [Google Scholar] [CrossRef]
- Dixon, R.; Barros-Rios, J. Lignin Biosynthesis: Old Roads Revisited and New Roads Explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Ferra, J.; Paiva, N.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Lignosulphonates as an Alternative to Non-Renewable Binders in Wood-Based Materials. Polymers 2021, 13, 4196. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2020, 22, 63. [Google Scholar] [CrossRef]
- Giardino, G.J.; Wang, H.; Niu, J.; Wang, D. From Technical Lignin to Native Lignin: Depolymerization, Functionalization, and Applications. Chem. Phys. Rev. 2024, 5, 021302. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.; Sethupathy, S.; Liaqat, F.; Shunkai, W.; Shah, T.A.; Zhu, D. Advanced Technologies for Transforming Biomass to Biofuels. In Integrated Solutions for Smart and Sustainable Environmental Conservation; Mabrouki, J., Azrour, M., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 47–64. [Google Scholar]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Ali, S.; Dar, M.A.; Liaqat, F.; Sethupathy, S.; Rani, A.; Khan, M.I.; Rehan, M.; Zhu, D. Optimization of Biomethane Production from Lignocellulosic Biomass by a Developed Microbial Consortium. Process Saf. Environ. Prot. 2024, 184, 1106–1118. [Google Scholar] [CrossRef]
- Sapouna, I.; van Erven, G.; Heidling, E.; Lawoko, M.; McKee, L.S. Impact of Extraction Method on the Structure of Lignin from Ball-Milled Hardwood. ACS Sustain. Chem. Eng. 2023, 11, 15533–15543. [Google Scholar] [CrossRef] [PubMed]
- Beaucamp, A.; Muddasar, M.; Amiinu, I.S.; Moraes Leite, M.; Culebras, M.; Latha, K.; Gutiérrez, M.C.; Rodriguez-Padron, D.; del Monte, F.; Kennedy, T.; et al. Lignin for Energy Applications—State of the Art, Life Cycle, Technoeconomic Analysis and Future Trends. Green Chem. 2022, 24, 8193–8226. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Smolarski, N. High-Value Opportunities for Lignin: Unlocking Its Potential Market Insights. Frost Sullivan 2012, 1, 1–15. [Google Scholar]
- Norgren, M.; Edlund, H. Lignin: Recent Advances and Emerging Applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Brodin, I.; Ernstsson, M.; Gellerstedt, G.; Sjöholm, E. Oxidative Stabilisation of Kraft Lignin for Carbon Fibre Production. Holzforschung 2011, 66, 141–147. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Hao, N.; Wang, Y.-Y.; Dou, C.; Lin, F.; Shen, R.; Bura, R.; Hodge, D.B.; Dale, B.E.; Ragauskas, A.J.; et al. Transforming Biorefinery Designs with ‘Plug-In Processes of Lignin’ to Enable Economic Waste Valorization. Nat. Commun. 2021, 12, 3912. [Google Scholar] [CrossRef] [PubMed]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-Lignin: Supply, Extraction Processes and Use as Bio-Based Material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Anthony, W.E.; Carr, R.R.; DeLorenzo, D.M.; Campbell, T.P.; Shang, Z.; Foston, M.; Moon, T.S.; Dantas, G. Development of Rhodococcus opacus as a Chassis for Lignin Valorization and Bioproduction of High-Value Compounds. Biotechnol. Biofuels 2019, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Shah, T.A.; Afzal, A.; Tabbassum, R. Evaluating the co-digestion effects on chicken manure and rotten potatoes in batch experiments. Int. J. Biosci. 2017, 10, 150–159. [Google Scholar]
- Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent Advances in Lignin-Based Biofuel Production. Energies 2023, 16, 3382. [Google Scholar] [CrossRef]
- Shah, T.A.; Ali, S.; Afzal, A.; Tabassum, R. Simultaneous Pretreatment and Biohydrogen Production from Wheat Straw by Newly Isolated Ligninolytic Bacillus sp. Strains with Two-Stage Batch Fermentation System. BioEnergy Res. 2018, 11, 835–849. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Gao, Y.; Walker, M.J.; Barrett, J.A.; Hosseinaei, O.; Harper, D.P.; Ford, P.C.; Williams, B.J.; Foston, M.B. Analysis of Gas Chromatography/Mass Spectrometry Data for Catalytic Lignin Depolymerization Using Positive Matrix Factorization. Green Chem. 2018, 20, 4366–4377. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Guo, T.; Zhao, H.; Jin, Y. Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy. Polymers 2018, 10, 736. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X. An Integral Method for Determining the Molecular Composition of Lignin and Its Application. Sci. Rep. 2022, 12, 19136. [Google Scholar] [CrossRef] [PubMed]
- Richel, A.; Vanderghem, C.; Simon, M.; Wathelet, B.; Paquot, M. Evaluation of Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for Second-Generation Lignin Analysis. Anal. Chem. Insights 2012, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, M.; Jiao, L.; Dai, H. Molecular Weight Distribution and Dissolution Behavior of Lignin in Alkaline Solutions. Polymers 2021, 13, 4166. [Google Scholar] [CrossRef]
- Levdansky, A.V.; Kondrasenko, A.A.; Malyar, Y.N.; Levdansky, V.A.; Kuznetsov, B.N. Study of Organosolv Lignins by Methods of FTIR and NMR Spectroscopy. J. Sib. Fed. Univ. Chem. 2019, 12, 201–220. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, G.; Deng, J.; Dong, M.; Murugadoss, V.; Liu, C.; Shao, Q.; Wu, S.; Guo, Z. Structural Characterization of Lignin from D. sinicus by FTIR and NMR Techniques. Green Chem. Lett. Rev. 2019, 12, 235–243. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of Lignin Structures and Lignin–Carbohydrate Complex (LCC) Linkages by Quantitative 13C and 2D HSQC NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole Plant Cell Wall Characterization Using Solution-State 2D NMR. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef]
- Blindheim, F.H.; Ruwoldt, J. The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy. Polymers 2023, 15, 2901. [Google Scholar] [CrossRef]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Molecules Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef]
- Meraj, A.; Jawaid, M.; Singh, S.P.; Nasef, M.M.; Ariffin, H.; Fouad, H.; Abu-Jdayil, B. Isolation and Characterisation of Lignin Using Natural Deep Eutectic Solvents Pretreated Kenaf Fibre Biomass. Sci. Rep. 2024, 14, 8672. [Google Scholar] [CrossRef]
- Fattahi, S.H.; Kazemi, A.; Khojastehnazhand, M.; Roostaei, M.; Mahmoudi, A. The classification of Iranian wheat flour varieties using FT-MIR spectroscopy and chemometrics methods. Expert Syst. Appl. 2024, 239, 122175. [Google Scholar] [CrossRef]
- Dütsch, L.; Sander, K.; Brendler, E.; Bremer, M.; Fischer, S.; Vogt, C.; Zuber, J. Chemometric Combination of Ultrahigh Resolving Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy for a Structural Characterization of Lignin Compounds. ACS Omega 2024, 9, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Biswas, B.; Kaur, R.; Rani, R.; Krishna, B.B.; Bhaskar, T. Structure Elucidation of Prot, Alkali and Dealkaline Lignin(s) by NMR, FT-IR and Py-GC/MS: Effect of Solid Acid and Base Catalysts. Sustain. Energy Fuels 2023, 7, 1942–1954. [Google Scholar] [CrossRef]
- Lundquist, K. Proton (1H) NMR Spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 242–249. ISBN 978-3-642-74065-7. [Google Scholar]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Furlanetto, R.H.; Nanni, M.R.; Antunes, W.C. Simple, Fast and Efficient Methods for Analysing the Structural, Ultrastructural and Cellular Components of the Cell Wall. Plants 2022, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Fromm, J.; Rockel, B.; Lautner, S.; Windeisen, E.; Wanner, G. Lignin Distribution in Wood Cell Walls Determined by TEM and Backscattered SEM Techniques. J. Struct. Biol. 2003, 143, 77–84. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Xu, F. Chemical Characteristics of Wood Cell Wall with an Emphasis on Ultrastructure: A Mini-Review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]
- Magalhaes, D.; Gürel, K.; Matsakas, L.; Christakopoulos, P.; Pisano, I.; Leahy, J.; Kazanç, F.; Trubetskaya, A. Prediction of Yields and Composition of Char from Fast Pyrolysis of Commercial Lignocellulosic Materials, Organosolv Fractionated and Torrefied Olive Stones. Fuel 2021, 289, 119862. [Google Scholar] [CrossRef]
- Reza, M.; Kontturi, E.; Jääskeläinen, A.-S.; Vuorinen, T.; Ruokolainen, J. Transmission Electron Microscopy for Wood and Fiber Analysis—A Review. Bioresources 2015, 10, 6230–6261. [Google Scholar] [CrossRef]
- Ton-That, M.T.; Ngo, T.; Lebarbe, T.; Ahvazi, B.; Bélanger, C.; Hu, W.; Hawari, J.; Monteil-Rivera, F.; Pilon, A.; Langlois, A.; et al. Development of Ligno-Polyol for the Production of Polyurethanes. In Proceedings of the Polyurethanes 2010 Technical Conference, Houston, TX, USA, 11–13 October 2010. [Google Scholar]
- Gracia-Vitoria, J.; Rubens, M.; Feghali, E.; Adriaensens, P.; Vanbroekhoven, K.; Vendamme, R. Low-Field Benchtop versus High-Field NMR for Routine 31P Analysis of Lignin, a Comparative Study. Ind. Crops Prod. 2022, 176, 114405. [Google Scholar] [CrossRef]
- Melone, F.; Saladino, R.; Crestini, C. Tannin Structural Elucidation and Quantitative 31P NMR Analysis. 1. Model Compounds. J. Agric. Food Chem. 2013, 61, 9307–9315. [Google Scholar] [CrossRef]
- LibreTexts, C. 4.7 Identifying Characteristic Functional Groups. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Introduction_to_Organic_Spectroscopy/04:_Infrared_Spectroscopy/4.07_Identifying_Characteristic_Functional_Groups (accessed on 6 June 2024).
- Materials, A. FTIR Basic Organic Functional Group Reference Chart. Available online: https://www.thermofisher.cn/blog/materials/a-gift-for-you-an-ftir-basic-organic-functional-group-reference-chart/ (accessed on 8 June 2024).
- Enders, A.A.; North, N.M.; Fensore, C.M.; Velez-Alvarez, J.; Allen, H.C. Functional Group Identification for FTIR Spectra Using Image-Based Machine Learning Models. Anal. Chem. 2021, 93, 9711–9718. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in Understanding the Humins: Formation, Prevention and Application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Li, M.; Bian, J.; Sun, R. Revealing the Structure and Distribution Changes of Eucalyptus Lignin during the Hydrothermal and Alkaline Pretreatments. Sci. Rep. 2017, 7, 593. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Alwani, M.S.; Omar, A.K.M. Chemical Composition, Anatomy, Lignin Distribution, and Cell Wall Structure of Malaysian Plant Waste Fibers. Bioresources 2006, 1, 220–232. [Google Scholar] [CrossRef]
- Morita, K.; Takenaka, M.; Tomita, K.; Ishii, J.; Kawaguchi, H.; Murakami, D.; Amo, H.; Fujii, M.; Maruyama, T.; Matsumoto, T.; et al. Nanoscopic Lignin Mapping on Cellulose Nanofibers via Scanning Transmission Electron Microscopy and Atomic Force Microscopy. Cellulose 2023, 30, 11357–11367. [Google Scholar] [CrossRef]
- Letourneau, D.; Volmer, D. Mass Spectrometry-based Methods for the Advanced Characterization and Structural Analysis of Lignin: A Review. Mass Spectrom. Rev. 2021, 42, 144–188. [Google Scholar] [CrossRef]
- Kusch, P. Pyrolysis-Gas Chromatography/Mass Spectrometry of Polymeric Materials. In Advanced Gas Chromatography-Progress in Agricultural, Biomedical and Industrial Applications; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0298-4. [Google Scholar]
- Reyes-Rivera, J.; Terrazas, T. Lignin Analysis by HPLC and FTIR. In Xylem; Springer: New York, NY, USA, 2017; Volume 1544, pp. 193–211. ISBN 978-1-4939-6720-9. [Google Scholar]
- AZoOptics. The Future of Lignin Research Using Mass Spectrometry. Available online: https://www.azooptics.com/Article.aspx?ArticleID=2438 (accessed on 8 June 2024).
- Prothmann, J.; Spégel, P.; Sandahl, M.; Turner, C. Identification of Lignin Oligomers in Kraft Lignin Using Ultra-High-Performance Liquid Chromatography/High-Resolution Multiple-Stage Tandem Mass Spectrometry (UHPLC/HRMS(n)). Anal. Bioanal. Chem. 2018, 410, 7803–7814. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, Y.; Ma, C.; Ge, J.; Hu, Q.; Yue, F.J.; Li, S.L.; Volmer, D.A. Characterization of Lignin Compounds at the Molecular Level: Mass Spectrometry Analysis and Raw Data Processing. Molecules 2021, 26, 178. [Google Scholar] [CrossRef]
- Zhu, L.; Cui, C.; Liu, H.; Zhou, Z.; Qi, F. Thermochemical Depolymerization of Lignin: Process Analysis with State-of-the-Art Soft Ionization Mass Spectrometry. Front. Chem. Eng. 2022, 4, 982126. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Zhai, R.; Wen, Z.; Jin, M. Recent Advances in Lignin Valorization with Bacterial Cultures: Microorganisms, Metabolic Pathways, and Bio-Products. Biotechnol. Biofuels 2019, 12, 32. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A Review on Trends in Lignin Extraction and Valorization of Lignocellulosic Biomass for Energy Applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S.; Rupasinghe, H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Syed, M.W.; Kazmi, W.W.; Hussain, A.; Shah, S.F.A.; Kariim, I.; Mehdi, A.M.; Omer, A.; Bhatti, A.H.; Eze, F.; Bhatti, U.H. Lignin Liquefaction: Unraveling the Effect of Process Conditions and Sustainable Pathways for Biofuel Production—A Comprehensive Review. Energy Convers. Manag. 2024, 313, 118615. [Google Scholar] [CrossRef]
- Han, Y.; Simmons, B.A.; Singh, S. Perspective on Oligomeric Products from Lignin Depolymerization: Their Generation, Identification, and Further Valorization. Ind. Chem. Mater. 2023, 1, 207–223. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, W.; Xu, B.; Teng, Z.; Tao, D.; Lou, Y.; Gao, Y. Screening and Identification of Lignin-Degrading Bacteria in Termite Gut and the Construction of LiP-Expressing Recombinant Lactococcus lactis. Microb. Pathog. 2017, 112, 63–69. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the Shape Selectivity of Zeolite Catalysts for Biomass Conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Cui, C.; Zhu, Y.; Zhou, Z.; Qi, F. In Situ Atmospheric Pressure Photoionization Mass Spectrometric Monitoring of Initial Pyrolysis Products of Biomass in Real Time. Anal. Chem. 2020, 92, 603–606. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Krishna, B.B.; Baltrusaitis, J.; Adhikari, S.; Bhaskar, T. Catalytic Depolymerization of Lignin for the Selective Production of Phenolic Monomers over Cobalt-Supported Calcium Catalysts. Energy Fuels 2023, 37, 3813–3824. [Google Scholar] [CrossRef]
- Katahira, R.; Mittal, A.; McKinney, K.; Chen, X.; Tucker, M.P.; Johnson, D.K.; Beckham, G.T. Base-Catalyzed Depolymerization of Biorefinery Lignins. ACS Sustain. Chem. Eng. 2016, 4, 1474–1486. [Google Scholar] [CrossRef]
- Gao, D.; Ouyang, D.; Zhao, X. Electro-Oxidative Depolymerization of Lignin for Production of Value-Added Chemicals. Green Chem. 2022, 24, 8585–8605. [Google Scholar] [CrossRef]
- Rana, M.; Ghosh, S.; Nshizirungu, T.; Park, J.H. Catalytic Depolymerization of Kraft Lignin to High Yield Alkylated-Phenols over CoMo/SBA-15 Catalyst in Supercritical Ethanol. RSC Adv. 2023, 13, 30022–30039. [Google Scholar] [CrossRef]
- Deepa, A.K.; Dhepe, P.L. Solid Acid Catalyzed Depolymerization of Lignin into Value Added Aromatic Monomers. RSC Adv. 2014, 4, 12625–12629. [Google Scholar] [CrossRef]
- Baxter, K.; Driver, S.; Williamson, E. Stockley’s Herbal Medicines Interactions; Pharmaceutical Press: London, UK, 2013. [Google Scholar]
- Han, Y.; Ye, L.; Gu, X.; Zhu, P.; Lu, X. Lignin-Based Solid Acid Catalyst for the Conversion of Cellulose to Levulinic Acid Using γ-Valerolactone as Solvent. Ind. Crops Prod. 2019, 127, 88–93. [Google Scholar] [CrossRef]
- Bi, Y.; Lei, X.; Xu, G.; Chen, H.; Hu, J. Catalytic Fast Pyrolysis of Kraft Lignin over Hierarchical HZSM-5 and Hβ Zeolites. Catalysts 2018, 8, 82. [Google Scholar] [CrossRef]
- Mateo, W.; Lei, H.; Villota, E.; Qian, M.; Zhao, Y.; Huo, E.; Zhang, Q.; Lin, X.; Wang, C. One-Step Synthesis of Biomass-Based Sulfonated Carbon Catalyst by Direct Carbonization-Sulfonation for Organosolv Delignification. Bioresour. Technol. 2021, 319, 124194. [Google Scholar] [CrossRef]
- Scholz, D.; Xie, J.; Kröcher, O.; Vogel, F. Mechanochemistry-Assisted Hydrolysis of Softwood over Stable Sulfonated Carbon Catalysts in a Semi-Batch Process. RSC Adv. 2019, 9, 33525–33538. [Google Scholar] [CrossRef]
- Ovejero-Pérez, A.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Acidic Depolymerization vs Ionic Liquid Solubilization in Lignin Extraction from Eucalyptus Wood Using the Protic Ionic Liquid 1-Methylimidazolium Chloride. Int. J. Biol. Macromol. 2020, 157, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Achinivu, E.C. Protic Ionic Liquids for Lignin Extraction-A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Rahman, M.S.; Amit, T.A.; Jadhav, B. Recent Advances in Lignin Depolymerization Techniques: A Comparative Overview of Traditional and Greener Approaches. Biomass 2022, 2, 130–154. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin Depolymerization and Biotransformation to Industrially Important Chemicals/Biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- da Cruz, M.G.A.; Gueret, R.; Chen, J.; Piątek, J.; Beele, B.; Sipponen, M.H.; Frauscher, M.; Budnyk, S.; Rodrigues, B.V.M.; Slabon, A. Electrochemical Depolymerization of Lignin in a Biomass-Based Solvent. ChemSusChem 2022, 15, e202200718. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef]
- Gu, J.; Qiu, Q.; Yu, Y.; Sun, X.; Tian, K.; Chang, M.; Wang, Y.; Zhang, F.; Huo, H. Bacterial Transformation of Lignin: Key Enzymes and High-Value Products. Biotechnol. Biofuels Bioprod. 2024, 17, 2. [Google Scholar] [CrossRef]
- Weng, C.; Peng, X.; Han, Y. Depolymerization and Conversion of Lignin to Value-Added Bioproducts by Microbial and Enzymatic Catalysis. Biotechnol. Biofuels 2021, 14, 84. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Chai, L.Y.; Chen, Y.H.; Tang, C.J.; Yang, Z.H.; Zheng, Y.; Shi, Y. Depolymerization and Decolorization of Kraft Lignin by Bacterium Comamonas sp. B-9. Appl. Microbiol. Biotechnol. 2014, 98, 1907–1912. [Google Scholar] [CrossRef]
- Chen, Y.; Chai, L.; Tang, C.; Yang, Z.; Zheng, Y.; Shi, Y.; Zhang, H. Kraft Lignin Biodegradation by Novosphingobium sp. B-7 and Analysis of the Degradation Process. Bioresour. Technol. 2012, 123, 682–685. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chai, L.Y.; Zhu, Y.H.; Yang, Z.H.; Zheng, Y.; Zhang, H. Biodegradation of Kraft Lignin by a Bacterial Strain Comamonas sp. B-9 Isolated from Eroded Bamboo Slips. J. Appl. Microbiol. 2012, 112, 900–906. [Google Scholar] [CrossRef]
- Dar, M.A.; Xie, R.; Jing, L.; Qing, X.; Ali, S.; Pandit, R.S.; Shaha, C.M.; Sun, J. Elucidating the Structure, and Composition of Bacterial Symbionts in the Gut Regions of Wood-Feeding Termite, Coptotermes formosanus and Their Functional Profile towards Lignocellulolytic Systems. Front. Microbiol. 2024, 15, 1395568. [Google Scholar] [CrossRef]
- Dar, M.A.; Xie, R.; Pandit, R.S.; Danso, B.; Dong, C.; Sun, J. Exploring the Region-Wise Diversity and Functions of Symbiotic Bacteria in the Gut System of Wood-Feeding Termite, Coptotermes formosanus, toward the Degradation of Cellulose, Hemicellulose, and Organic Dyes. Insect Sci. 2022, 29, 1414–1432. [Google Scholar] [CrossRef]
- Suman, S.K.; Dhawaria, M.; Tripathi, D.; Raturi, V.; Adhikari, D.K.; Kanaujia, P.K. Investigation of Lignin Biodegradation by Trabulsiella sp. Isolated from Termite Gut. Int. Biodeterior. Biodegrad. 2016, 112, 12–17. [Google Scholar] [CrossRef]
- Xie, R.; Dong, C.; Wang, S.; Danso, B.; Dar, M.A.; Pandit, R.S.; Pawar, K.D.; Geng, A.; Zhu, D.; Li, X.; et al. Host-Specific Diversity of Culturable Bacteria in the Gut Systems of Fungus-Growing Termites and Their Potential Functions towards Lignocellulose Bioconversion. Insects 2023, 14, 403. [Google Scholar] [CrossRef]
- Li, H.; Yelle, D.J.; Li, C.; Yang, M.; Ke, J.; Zhang, R.; Liu, Y.; Zhu, N.; Liang, S.; Mo, X.; et al. Lignocellulose Pretreatment in a Fungus-Cultivating Termite. Proc. Natl. Acad. Sci. USA 2017, 114, 4709–4714. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—A review. Energy Reports 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Liao, H.D.; Ma, J.S.; Liu, X.M.; Zhang, L.Y.; Shi, X.W.; Yang, X.L.; Lu, X.N.; Zhu, Y.H. Isolation of a Rice Endophytic Bacterium, Pantoea sp. Sd-1, with Ligninolytic Activity and Characterization of Its Rice Straw Degradation Ability. Lett. Appl. Microbiol. 2014, 58, 123–129. [Google Scholar] [CrossRef]
- Yu, T.; Wu, W.; Liang, W.; Lever, M.A.; Hinrichs, K.U.; Wang, F. Growth of Sedimentary Bathyarchaeota on Lignin as an Energy Source. Proc. Natl. Acad. Sci. USA 2018, 115, 6022–6027. [Google Scholar] [CrossRef]
- Mallinson, S.J.B.; Machovina, M.M.; Silveira, R.L.; Garcia-Borràs, M.; Gallup, N.; Johnson, C.W.; Allen, M.D.; Skaf, M.S.; Crowley, M.F.; Neidle, E.L.; et al. A Promiscuous Cytochrome P450 Aromatic O-Demethylase for Lignin Bioconversion. Nat. Commun. 2018, 9, 2487. [Google Scholar] [CrossRef]
- Moraes, E.C.; Alvarez, T.M.; Persinoti, G.F.; Tomazetto, G.; Brenelli, L.B.; Paixão, D.A.A.; Ematsu, G.C.; Aricetti, J.A.; Caldana, C.; Dixon, N.; et al. Lignolytic-Consortium Omics Analyses Reveal Novel Genomes and Pathways Involved in Lignin Modification and Valorization. Biotechnol. Biofuels 2018, 11, 75. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, P.; Xie, C.; Zhang, W.; Sun, J.; Qian, W.J.; Yang, B. Biodegradation of Alkaline Lignin by Bacillus ligniniphilus L1. Biotechnol. Biofuels 2017, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Bharagava, R.N.; Mani, S.; Mulla, S.I.; Saratale, G.D. Degradation and Decolourization Potential of an Ligninolytic Enzyme Producing Aeromonas hydrophila for Crystal Violet Dye and Its Phytotoxicity Evaluation. Ecotoxicol. Environ. Saf. 2018, 156, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Priyadarshinee, R.; Kumar, A.; Mandal, T.; Dasgupta Mandal, D. Exploring Planococcus sp. TRC1, a Bacterial Isolate, for Carotenoid Pigment Production and Detoxification of Paper Mill Effluent in Immobilized Fluidized Bed Reactor. J. Clean. Prod. 2019, 211, 1389–1402. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Navas, L.E.; Fetherolf, M.M.; Liu, L.-Y.; Dalhuisen, T.; Renneckar, S.; Eltis, L.D.; Mohn, W.W. Discovery of Lignin-Transforming Bacteria and Enzymes in Thermophilic Environments Using Stable Isotope Probing. ISME J. 2022, 16, 1944–1956. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A. Lata Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Junghans, U.; Bernhardt, J.J.; Wollnik, R.; Triebert, D.; Unkelbach, G.; Pufky-Heinrich, D. Valorization of Lignin via Oxidative Depolymerization with Hydrogen Peroxide: Towards Carboxyl-Rich Oligomeric Lignin Fragments. Molecules 2020, 25, 2717. [Google Scholar] [CrossRef]
- Ayub, R.; Raheel, A. High-Value Chemicals from Electrocatalytic Depolymerization of Lignin: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 3767. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Chen, Y.; Li, G.; Li, H.; Tang, Y.; Wan, P. Electrochemical Depolymerization of Lignin into Renewable Aromatic Compounds in a Non-Diaphragm Electrolytic Cell. RSC Adv. 2014, 4, 29917–29924. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Zhong, X.; Jiang, W.; Liang, Y.; Niu, P.; Li, M.; Zhuang, G.; Li, X.; Wang, J. Electrocatalytic Upgrading of Lignin-Derived Bio-Oil Based on Surface-Engineered PtNiB Nanostructure. Adv. Funct. Mater. 2019, 29, 1807651. [Google Scholar] [CrossRef]
- Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical Depolymerisation of Lignin in a Deep Eutectic Solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Fang, Z.; Li, F.; Wang, M.; Li, F.; Wu, X.; Fan, K.; Tang, Q.; Sun, L.; Zhang, P. Selective Electrocatalytic Upgrading of Lignin to Aryl Aldehydes and Carboxylic Acids over Dodecyl Sulfate-Intercalated CoS Nanocones. Appl. Catal. B 2023, 323, 122149. [Google Scholar] [CrossRef]
- Umar, Y.; Velasco, O.; Abdelaziz, O.Y.; Aboelazayem, O.; Gadalla, M.A.; Hulteberg, C.P.; Saha, B. A Renewable Lignin-Derived Bio-Oil for Boosting the Oxidation Stability of Biodiesel. Renew. Energy 2022, 182, 867–878. [Google Scholar] [CrossRef]
- Akash, B. Thermal Depolymerization of Lignin. Int. J. Therm. Environ. Eng. 2016, 13, 17–22. [Google Scholar] [CrossRef]
- Peng, M.; Muraishi, T.; Hou, X.; Zhao, M.; Kamiya, K.; Qian, E.W. Oxidative Depolymerization of Lignin to Vanillin and Lactic Acid in an Aqueous Solution. Fuel 2023, 348, 128486. [Google Scholar] [CrossRef]
- Huang, Y.B.; Zhang, J.L.; Zhang, X.; Luan, X.; Chen, H.Z.; Hu, B.; Zhao, L.; Wu, Y.L.; Lu, Q. Catalytic Depolymerization of Lignin via Transfer Hydrogenation Strategy over Skeletal CuZnAl Catalyst. Fuel Process. Technol. 2022, 237, 107448. [Google Scholar] [CrossRef]
- Shorey, R.; Salaghi, A.; Fatehi, P.; Mekonnen, T.H. Valorization of Lignin for Advanced Material Applications: A Review. RSC Sustain. 2024, 2, 804–831. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. A Field of Dreams: Lignin Valorization into Chemicals, Materials, Fuels, and Health-Care Products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef]
- Ewuzie, R.N.; Genza, J.R.; Abdullah, A.Z. Catalytic Hydrogenolysis of Lignin in Isopropanol as Hydrogen Donor over Nickel-Cobalt Supported on Zeolite to Produce Aromatic and Phenolic Monomers. Appl. Catal. A Gen. 2023, 667, 119443. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Bjelić, S.; Hulteberg, C.P.; Riisager, A. Oxidative Depolymerization of Kraft Lignin to Aromatics Over Bimetallic V–Cu/ZrO_2 Catalysts. Topics in Catalysis 2023, 66, 1369–1380. [Google Scholar] [CrossRef]
- Huang, M.; Xu, J.; Ma, Z.; Yang, Y.; Zhou, B.; Wu, C.; Ye, J.; Zhao, C.; Liu, X.; Chen, D.; et al. Bio-BTX Production from the Shape Selective Catalytic Fast Pyrolysis of Lignin Using Different Zeolite Catalysts: Relevance between the Chemical Structure and the Yield of Bio-BTX. Fuel Process. Technol. 2021, 216, 106792. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef]
- Yuan, J.; Du, G.; Yang, H.; Liu, S.; Park, S.; Liu, T.; Ran, X.; Park, B.D.; Gao, W.; Yang, L. Fully Bio-Based Adhesive Designed through Lignin-Cellulose Combination and Interfacial Bonding Reinforcement. Ind. Crops Prod. 2023, 204, 117279. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, P.; Huang, D.; Wang, H.; Wang, Z.; Qin, H.; He, Y.; Li, R.; Yin, L.; Chen, S.; et al. The Driving Force of Biomass Value-Addition: Selective Catalytic Depolymerization of Lignin to High-Value Chemicals. J. Environ. Chem. Eng. 2023, 11, 109719. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-Derivatives Based Polymers, Blends and Composites: A Review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- Ma, C.; Kim, T.H.; Liu, K.; Ma, M.G.; Choi, S.E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef]
- Ridho, M.R.; Agustiany, E.A.; Rahmi Dn, M.; Madyaratri, E.W.; Ghozali, M.; Restu, W.K.; Falah, F.; Rahandi Lubis, M.A.; Syamani, F.A.; Nurhamiyah, Y.; et al. Lignin as Green Filler in Polymer Composites: Development Methods, Characteristics, and Potential Applications. Adv. Mater. Sci. Eng. 2022, 2022, 1363481. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S. Lignin-Based Polymer Blends: Analysis of Intermolecular Interactions in Lignin–Synthetic Polymer Blends. Compos. Part A Appl. Sci. Manuf. 2004, 35, 395–400. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gu, Y.; Feng, S.; Yang, W.; Dai, H.; Xiao, H.; Han, J. Lignin-Containing Biodegradable UV-Blocking Films: A Review. Green Chem. 2023, 25, 9020–9044. [Google Scholar] [CrossRef]
- Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2020, 26, 126. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Solubility of Lignin and Acetylated Lignin in Organic Solvents. Bioresources 2017, 12, 1548–1565. [Google Scholar] [CrossRef]
- Johansson, M.; Skrifvars, M.; Kadi, N.; Dhakal, H.N. Effect of Lignin Acetylation on the Mechanical Properties of Lignin-Poly-Lactic Acid Biocomposites for Advanced Applications. Ind. Crops Prod. 2023, 202, 117049. [Google Scholar] [CrossRef]
- Inwood, J.P.W. Sulfonation of Kraft Lignin to Water Soluble Value Added Products. Ph.D. Thesis, Lakehead University, Thunder Bay, ON, Canada, 2014. [Google Scholar]
- Wang, Y.Y.; Meng, X.; Pu, Y.; Ragauskas, A.J. Recent Advances in the Application of Functionalized Lignin in Value-Added Polymeric Materials. Polymers 2020, 12, 2277. [Google Scholar] [CrossRef]
- Jacobs, B.; Yao, Y.; Van Nieuwenhove, I.; Sharma, D.; Graulus, G.J.; Bernaerts, K.; Verberckmoes, A. Sustainable Lignin Modifications and Processing Methods: Green Chemistry as the Way Forward. Green Chem. 2023, 25, 2042–2086. [Google Scholar] [CrossRef]
- Libretti, C.; Santos Correa, L.; Meier, M.A.R. From Waste to Resource: Advancements in Sustainable Lignin Modification. Green Chem. 2024, 26, 4358–4386. [Google Scholar] [CrossRef]
- Wang, T.; Li, H.; Diao, X.; Lu, X.; Ma, D.; Ji, N. Lignin to Dispersants, Adsorbents, Flocculants and Adhesives: A Critical Review on Industrial Applications of Lignin. Ind. Crops Prod. 2023, 199, 116715. [Google Scholar] [CrossRef]
- Sethupathy, S.; Murillo Morales, G.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin Valorization: Status, Challenges and Opportunities. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef]
- Obydenkova, S.V.; Kouris, P.D.; Smeulders, D.M.J.; Boot, M.D.; van der Meer, Y. Evaluation of Environmental and Economic Hotspots and Value Creation in Multi-Product Lignocellulosic Biorefinery. Biomass Bioenergy 2022, 159, 106394. [Google Scholar] [CrossRef]
- Mandal, D.D.; Singh, G.; Majumdar, S.; Chanda, P. Challenges in Developing Strategies for the Valorization of Lignin—A Major Pollutant of the Paper Mill Industry. Environ. Sci. Pollut. Res. 2022, 30, 11119–11140. [Google Scholar] [CrossRef]
- Baral, N.R.; Banerjee, D.; Mukhopadhyay, A.; Simmons, B.A.; Singer, S.W.; Scown, C.D. Economic and Environmental Trade-Offs of Simultaneous Sugar and Lignin Utilization for Biobased Fuels and Chemicals. ACS Sustain. Chem. Eng. 2024, 12, 2563–2576. [Google Scholar] [CrossRef]
- Bilal, M.; Qamar, S.A.; Qamar, M.; Yadav, V.; Taherzadeh, M.J.; Lam, S.S.; Iqbal, H.M.N. Bioprospecting Lignin Biomass into Environmentally Friendly Polymers—Applied Perspective to Reconcile Sustainable Circular Bioeconomy. Biomass Convers. Biorefin. 2024, 14, 4457–4483. [Google Scholar] [CrossRef]
- Boarino, A.; Klok, H.A. Opportunities Challenges for Lignin Valorization in Food Packaging, Antimicrobial, and Agricultural Applications. Biomacromolecules 2023, 24, 1065. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; Rios-Del Toro, E.E.; Amezquita-Garcia, H.J.; Escamilla-Alvarado, C. 5-Advances in Biofuels and by-Products from Lignin. In Sustainable Biofuels; Ray, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 101–130. ISBN 978-0-12-820297-5. [Google Scholar]
- Silau, H.; Melas, A.; Dam-Johansen, K.; Wu, H.; Daugaard, A.E.; Høj, M. Solvent Fractionation and Depolymerization Provide Liquid Lignin Fractions Exploited as Bio-Based Aromatic Building Blocks in Epoxies. ACS Sustain. Chem. Eng. 2023, 11, 1591–1597. [Google Scholar] [CrossRef]
- Miranda-Valdez, I.Y.; Coffeng, S.; Zhou, Y.; Viitanen, L.; Hu, X.; Jannuzzi, L.; Puisto, A.; Kostiainen, M.A.; Mäkinen, T.; Koivisto, J.; et al. Foam-Formed Biocomposites Based on Cellulose Products and Lignin. Cellulose 2023, 30, 2253–2266. [Google Scholar] [CrossRef]
- Baniasadi, H.; Lipponen, S.; Asplund, M.; Seppälä, J. High-Concentration Lignin Biocomposites with Low-Melting Point Biopolyamide. Chem. Eng. J. 2023, 451, 138564. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, G.; Vaidya, U.; Wang, H. Past, Present and Future Prospective of Global Carbon Fibre Composite Developments and Applications. Compos. B Eng. 2023, 250, 110463. [Google Scholar] [CrossRef]
- Global and China Carbon Fiber and CFRP Industry Report, 2019–2025. Available online: https://www.researchandmarkets.com/reports/4770782/global-and-china-carbon-fiber-and-cfrp-industry (accessed on 22 June 2024).
- Qu, W.; Yang, J.; Sun, X.; Bai, X.; Jin, H.; Zhang, M. Towards Producing High-Quality Lignin-Based Carbon Fibers: A Review of Crucial Factors Affecting Lignin Properties and Conversion Techniques. Int. J. Biol. Macromol. 2021, 189, 768–784. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How Far Is Lignin from Being a Biomedical Material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzi, E.; Cardone, F.; Lu, X.; Canestrari, F. The Use of Lignin for Sustainable Asphalt Pavements: A Literature Review. Constr. Build. Mater. 2023, 362, 129773. [Google Scholar] [CrossRef]
- Vinod, A.; Pulikkalparambil, H.; Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Recent Advancements in Lignocellulose Biomass-Based Carbon Fiber: Synthesis, Properties, and Applications. Heliyon 2023, 9, e13614. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Ahmed Yagoub, A.E.; Ji, Q.; Zhou, C. Lignin Fractionation from Lignocellulosic Biomass Using Deep Eutectic Solvents and Its Valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From Lignin to Valuable Products–Strategies, Challenges, and Prospects. Bioresour. Technol. 2018, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, C.; Radu, A.; de Waard, D.F.; Wang, Y.; Behaghel de Bueren, J.T.; Kouris, P.D.; Boot, M.D.; Xiao, J.; Zhang, H.; et al. Carbon–Carbon Bond Cleavage for a Lignin Refinery. Nat. Chem. Eng. 2024, 1, 61–72. [Google Scholar] [CrossRef]
- Quinsaat, J.E.Q.; Falireas, P.G.; Feghali, E.; Torr, K.M.; Vanbroekhoven, K.; Eevers, W.; Vendamme, R. Depolymerised Lignin Oil: A Promising Building Block towards Thermoplasticity in Polyurethanes. Ind. Crops Prod. 2023, 194, 116305. [Google Scholar] [CrossRef]
- Vieira, F.R.; Magina, S.; Evtuguin, D.V.; Barros-Timmons, A. Lignin as a Renewable Building Block for Sustainable Polyurethanes. Materials 2022, 15, 6182. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-Based Polyurethane: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2021, 42, 2000492. [Google Scholar] [CrossRef]
- Lignin Industries Starts Production of Lignin-Based Material Renol®, to Replace Fossil-Based Plastics, with a Capacity of More than 1000 Tonnes/Year—Lignin Industries AB. Available online: https://www.lignin.se/news/2021/2/22/rencom-starts-the-production-of-its-lignin-based-material-renol-to-replace-fossil-based-plastics-with-a-capacity-of-more-than-1000-tonnesyear (accessed on 22 June 2024).
- RenCom Announces Company Name Change to Lignin Industries AB—Lignin Industries AB. Available online: https://www.lignin.se/news/-name-change-to-lignin-industries (accessed on 22 June 2024).
- UPM-Kymmene Corp Patent: Lignin-Based Bioplastic for Agriculture Items. Available online: https://www.packaging-gateway.com/data-insights/upm-kymmene-files-patent-for-lignin-based-bioplastic-material-for-agriculture-items/ (accessed on 22 June 2024).
- US Patent. Application for Lignin-Based Bioplastic Material, Processes for Preparing the Same and Uses Thereof Patent Application)—Justia Patents Search. US Patent Application #20240026157, 25 January 2024. Available online: https://patents.justia.com/patent/20240026157 (accessed on 22 June 2024).

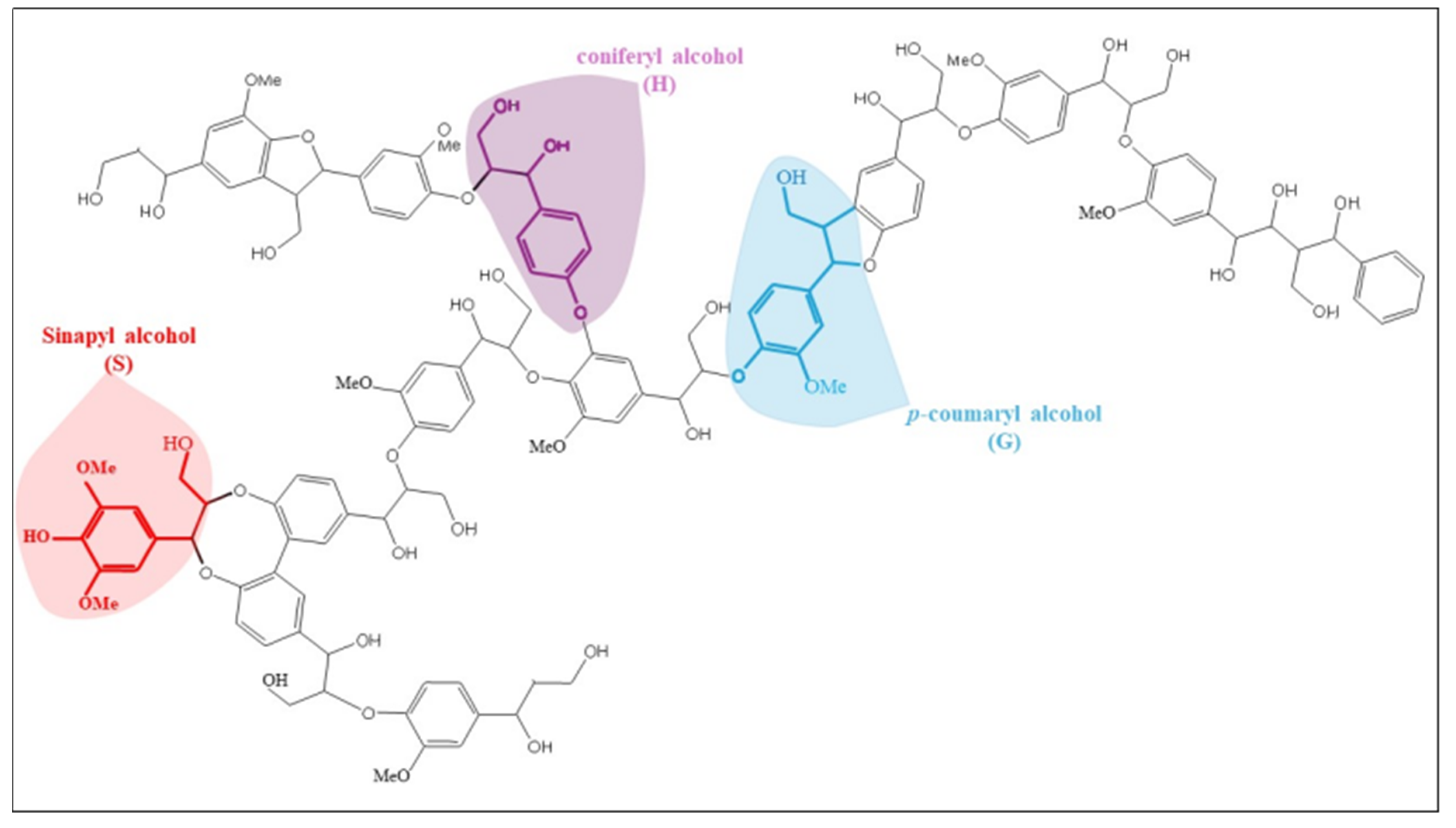
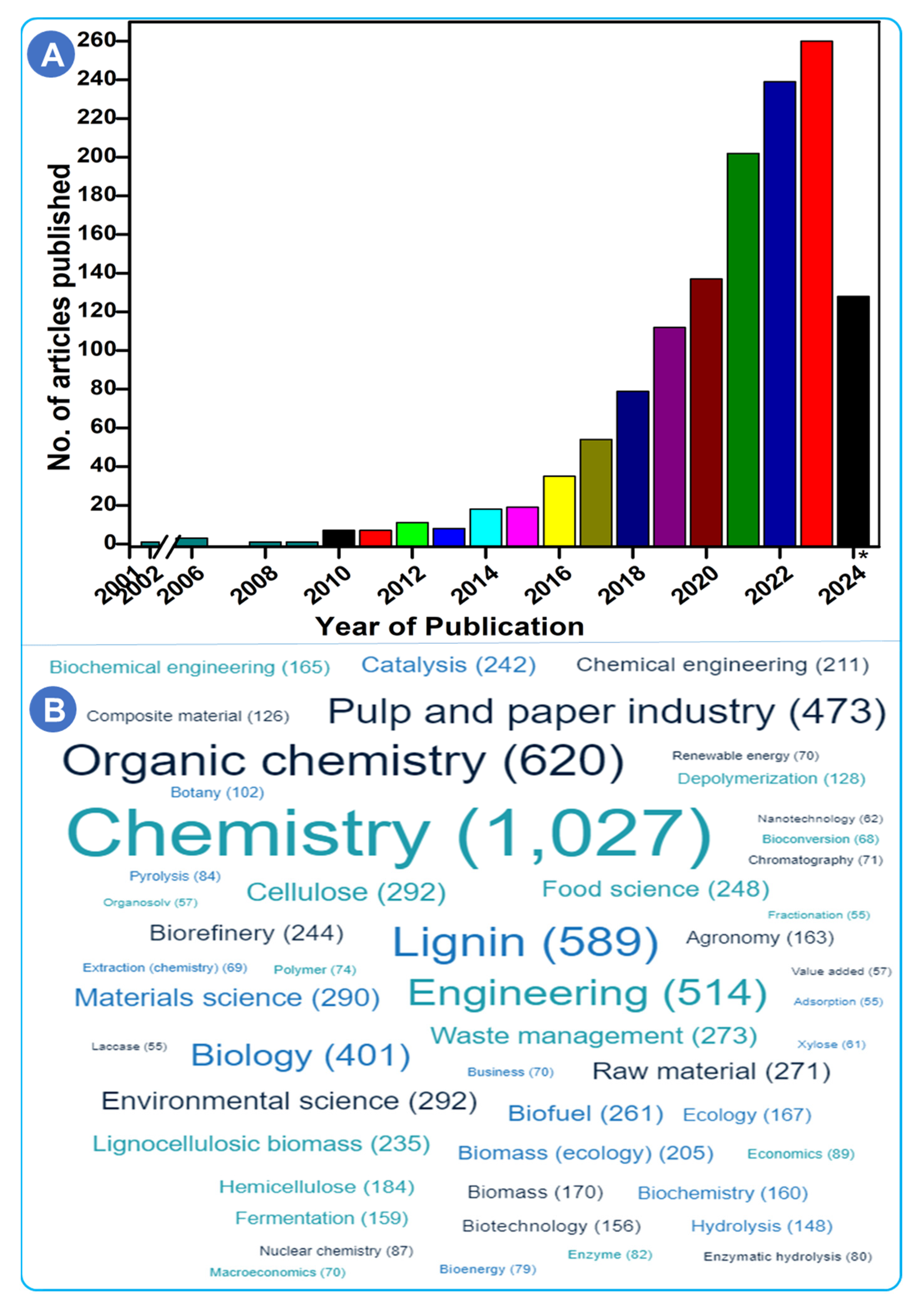
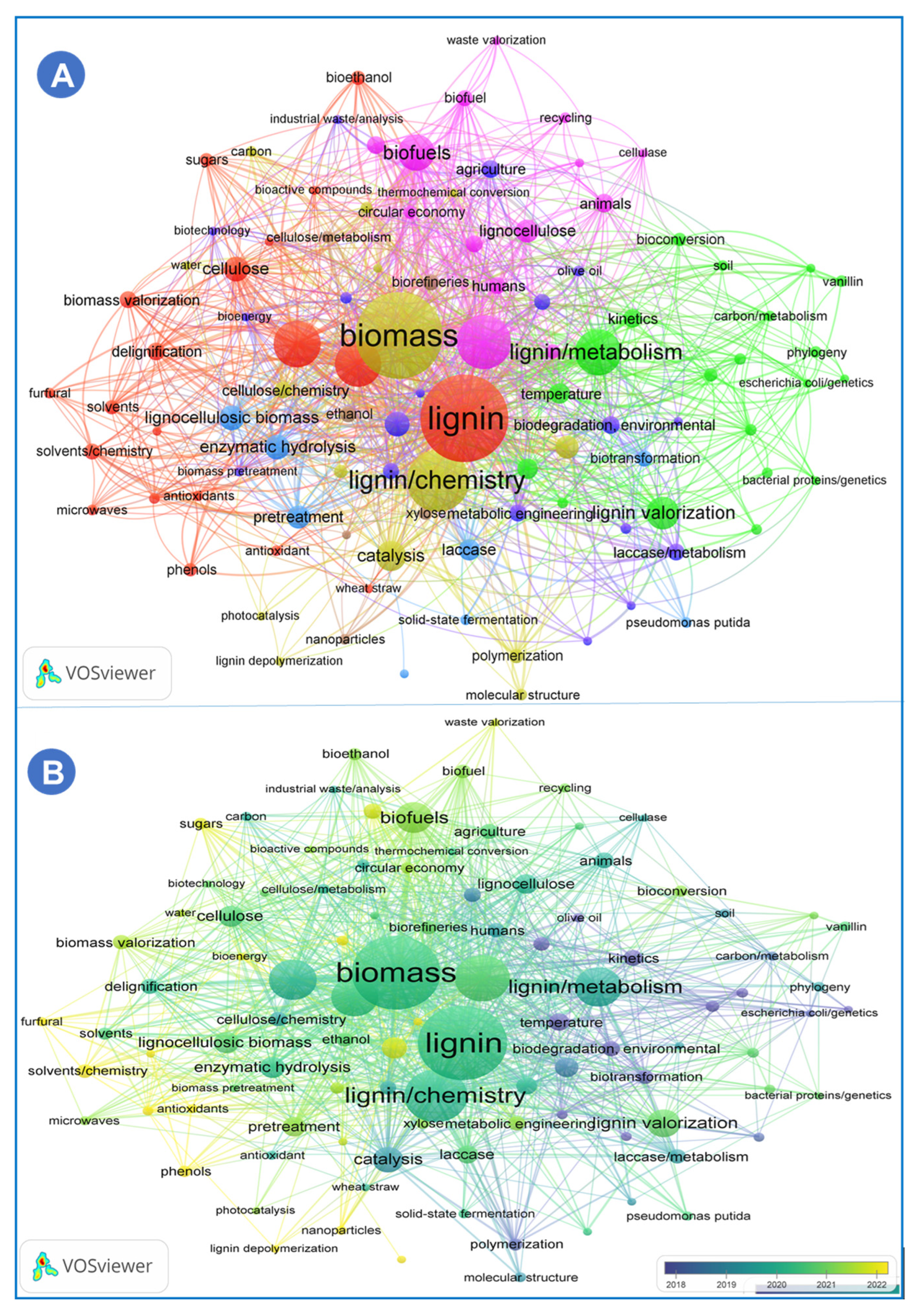
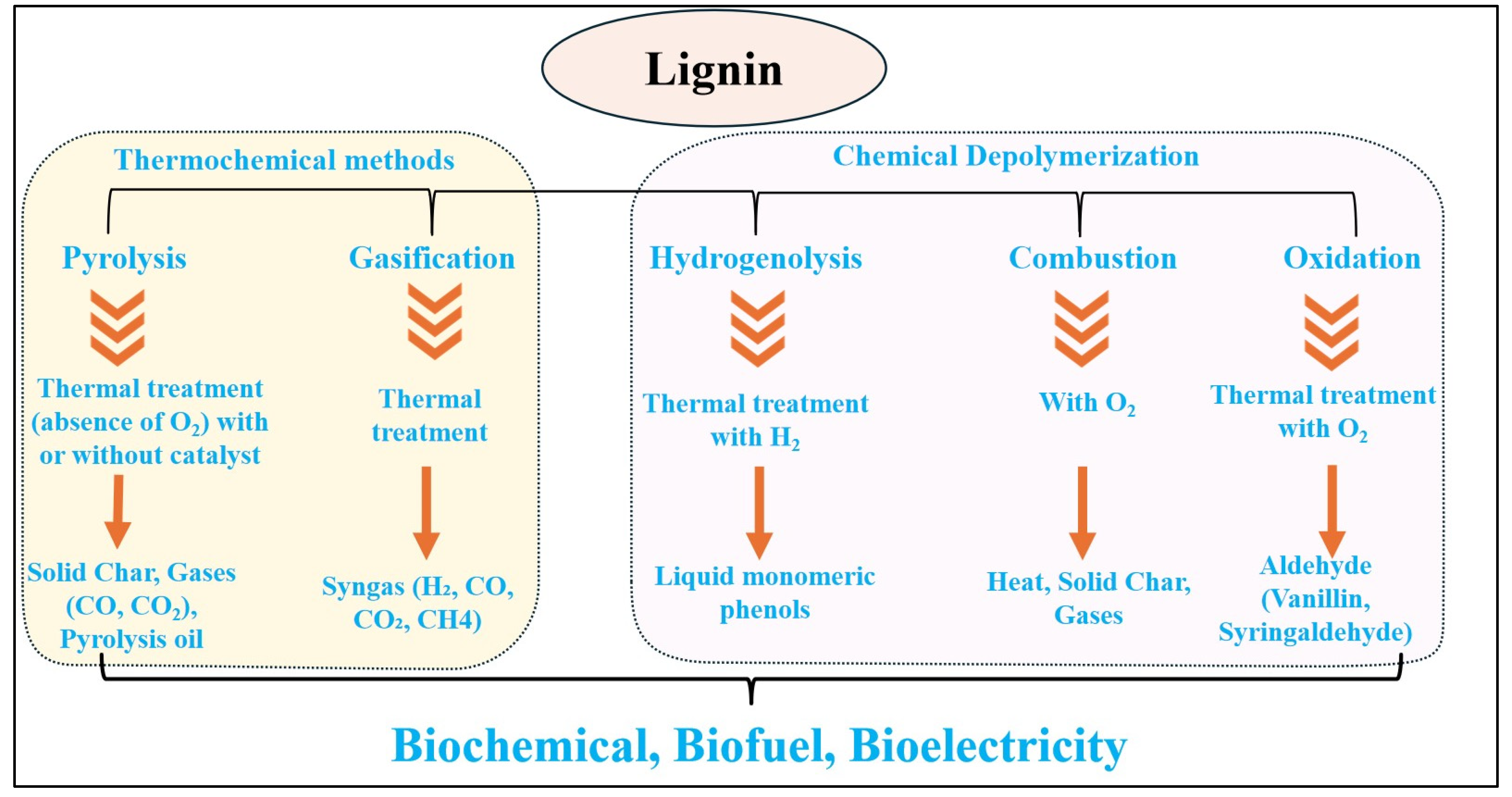

| Technique | Uses/Key Features | References |
|---|---|---|
| Functional group characterization | ||
| 31P NMR | Quantitative determination of different types of hydroxyl groups present in lignin, including aliphatic (α-OH, β-OH), phenolic (OH ph), and carboxylic acid groups | [53,54,55] |
| FTIR |
| [56,57,58] |
| Morphological analysis | ||
| SEM | Visualization of lignin morphology on cell wall surfaces, interaction with other components, surface topography, and modifications after pretreatment
| [59,60] |
Determination of lignin distribution across cell wall layers, interaction with cellulose microfibrils.
| [61] | |
| AFM | Nanoscopic mapping of lignin location and distribution on cellulose nanofibers:
| [62] |
| Structural elements characterization | ||
| Py-GC-MS | Identification of Monomers:
| [63,64] |
| NMR (1H, 13C, 2D) | Elucidation of structural elements and inter-unit linkages. | [65] |
| Molar mass distribution analysis | ||
| SEC | Determination of weight-average (Mw), number-average (Mn), and peak molar mass (Mp) | [66] |
| Other Techniques | ||
| XRD | Evaluation of crystallinity and amorphous regions | [66] |
| Thermal Analysis (TGA, DSC) | Thermal stability and phase transitions | [66] |
| Elemental Analysis | Determination of elemental composition (C, H, O, S, etc.) | [66] |
| LC-MS | Analysis of lignin degradation products, identification of monomers, dimers, and oligomers, structural elucidation | [66,67,68] |
| GC-MS | Analysis of volatile lignin degradation products, identification of monomers and dimers | [59,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Rani, A.; Dar, M.A.; Qaisrani, M.M.; Noman, M.; Yoganathan, K.; Asad, M.; Berhanu, A.; Barwant, M.; Zhu, D. Recent Advances in Characterization and Valorization of Lignin and Its Value-Added Products: Challenges and Future Perspectives. Biomass 2024, 4, 947-977. https://doi.org/10.3390/biomass4030053
Ali S, Rani A, Dar MA, Qaisrani MM, Noman M, Yoganathan K, Asad M, Berhanu A, Barwant M, Zhu D. Recent Advances in Characterization and Valorization of Lignin and Its Value-Added Products: Challenges and Future Perspectives. Biomass. 2024; 4(3):947-977. https://doi.org/10.3390/biomass4030053
Chicago/Turabian StyleAli, Shehbaz, Abida Rani, Mudasir A. Dar, Muther Mansoor Qaisrani, Muhammad Noman, Kamaraj Yoganathan, Muhammad Asad, Ashenafi Berhanu, Mukul Barwant, and Daochen Zhu. 2024. "Recent Advances in Characterization and Valorization of Lignin and Its Value-Added Products: Challenges and Future Perspectives" Biomass 4, no. 3: 947-977. https://doi.org/10.3390/biomass4030053
APA StyleAli, S., Rani, A., Dar, M. A., Qaisrani, M. M., Noman, M., Yoganathan, K., Asad, M., Berhanu, A., Barwant, M., & Zhu, D. (2024). Recent Advances in Characterization and Valorization of Lignin and Its Value-Added Products: Challenges and Future Perspectives. Biomass, 4(3), 947-977. https://doi.org/10.3390/biomass4030053








