Catalytic Evaluation of an Optimized Heterogeneous Composite Catalyst Derived from Fusion of Tri-Biogenic Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Collection and Preparation
Catalyst Preparation
2.2. Catalyst Characterization
2.2.1. XRF Analysis
2.2.2. Scanning Electron Microscopy (SEM) Analysis
2.2.3. Fourier Transform Infrared (FTIR) Analysis
2.2.4. X-Ray Diffraction Analysis
2.2.5. Analysis Method of Loss on Ignition (LOI)
2.3. Development of the Composite Catalyst
2.4. Catalytic Testing of the Developed Heterogeneous Composite Catalysts
3. Results and Discussion
3.1. Elemental Composition of the Raw Agricultural Residues
3.2. The Basic Oxide Composition of Selected Raw Agricultural Residues
3.3. Characterization of the Selected Calcined Agricultural Residue
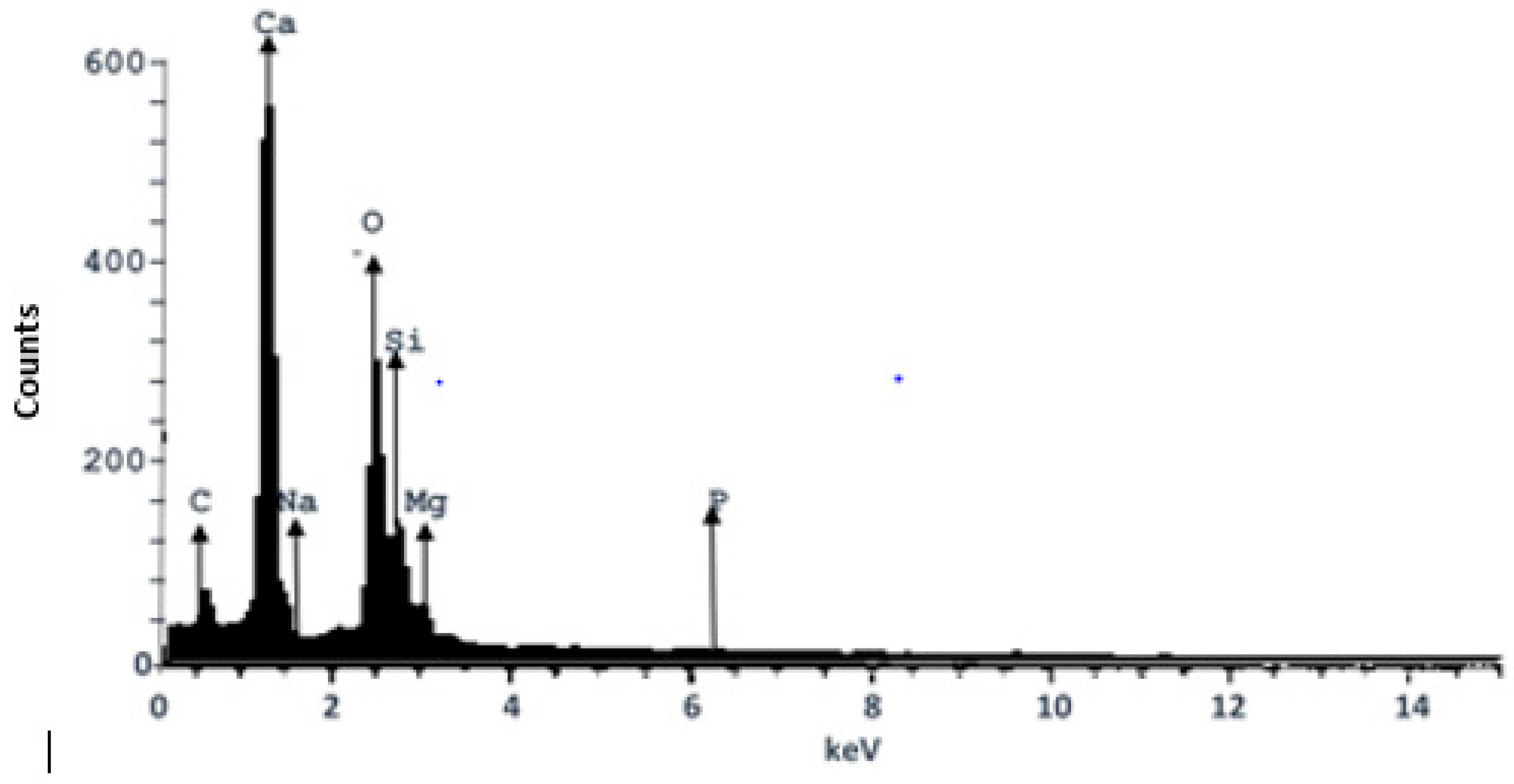
3.4. EDX Analysis of the Composite Heterogeneous Catalyst (CHC)
3.5. Scanning Electron Microscopy (SEM) for CHC
3.6. Functional Group Composition of the Raw and Calcined Composite Residue
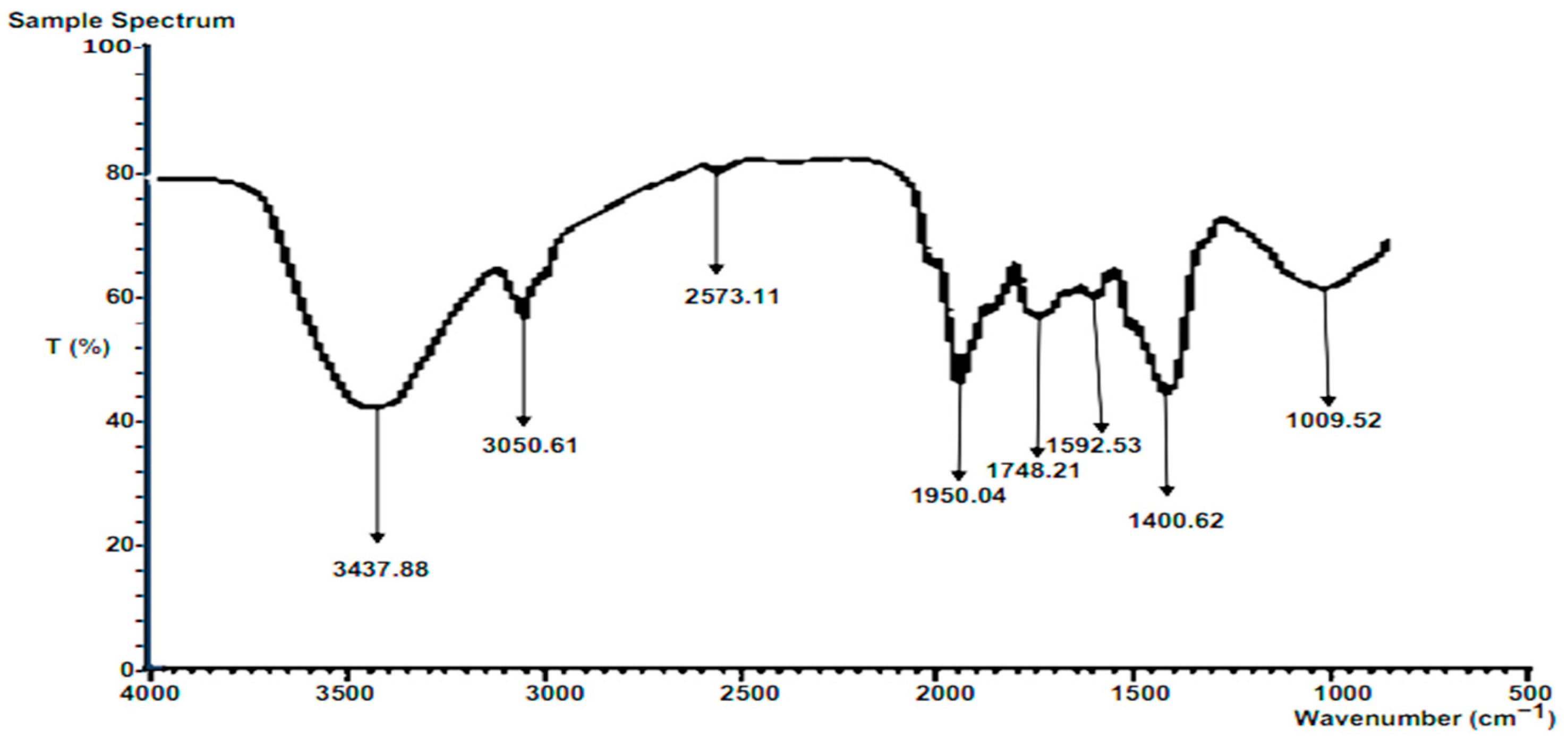
3.6.1. FTIR of Raw Composite Residue
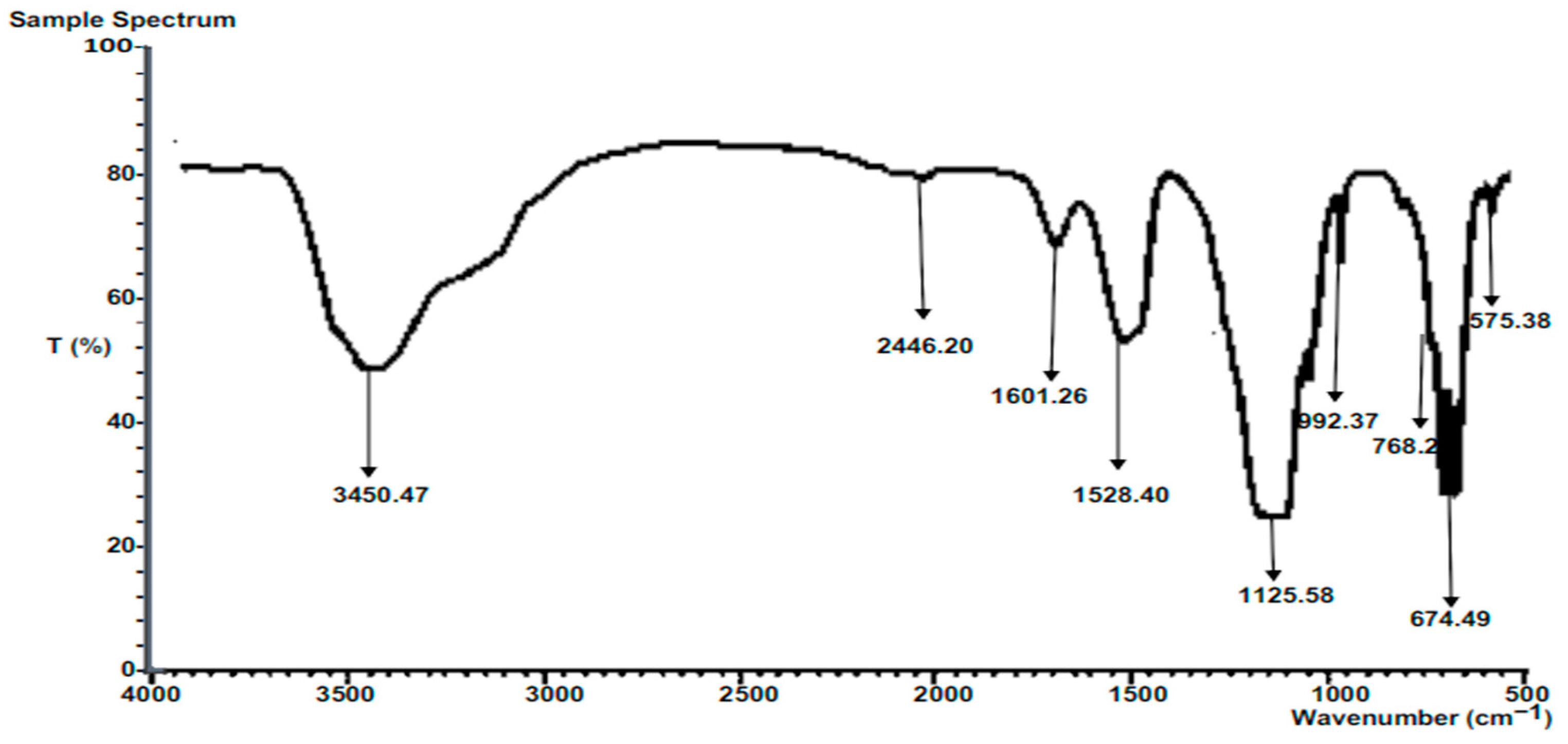
3.6.2. FTIR of Composite Calcined Heterogeneous Catalysts
3.7. XRD Analysis of Composite Heterogeneous Catalyst (CHC)
3.8. Responses from Experimental Data
3.8.1. Model Summary Statistics for the Responses
3.8.2. ANOVA for the Developed Catalyst Composite
3.8.3. Regression Statistics for the Development of Catalyst Composite
3.8.4. Model Equations of Responses for the Development of Catalyst Composite
3.9. Physicochemical Properties of WFO for Testing the Composite Catalyst Developed
3.10. Physicochemical Properties of the Waste Frying Oil Biodiesel (WFOB) Produced
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. BioEnergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Yang, L.; Takase, M.; Zhang, M.; Zhao, T.; Wu, X. Potential non-edible oil feedstock for biodiesel production in Africa: A survey. Renew. Sustain. Energy Rev. 2014, 38, 461–477. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, B.; Kalita, P. Application of agro-waste derived materials as heterogeneous base catalysts for biodiesel synthesis. J. Renew. Sustain. Energy 2018, 10, 10. Available online: https://pubs.aip.org/aip/jrse/article/10/4/043105/383974 (accessed on 3 October 2024). [CrossRef]
- Gupta, A.; Kumar, H. Multi-dimensional perspectives on electric vehicles design: A mind map approach. Clean. Eng. Technol. 2022, 8, 100483. [Google Scholar] [CrossRef]
- Jekayinfa, S.O.; Scholz, V. Assessment of Availability and Cost of Energetically Usable Crop Residues in Nigeria. Nat. Gas 2007, 24, 25. [Google Scholar]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) using Calcium Oxide (CaO) Nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef]
- Kamat, S.; Bandyopadhyay, S. Optimization of regeneration temperature for energy integrated water allocation networks. Clean. Eng. Technol. 2022, 8, 100490. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M.; Ahmed, N.A.; Jekayinfa, S.O.; Ogunkunle, O. Modelling the effects of particle size pretreatment method on biogas yield of groundnut shells. Waste Manag. Res. J. Sustain. Circ. Econ. 2022, 40, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Okpalaeke, K.E.; Ibrahim, T.H.; Latinwo, L.M.; Betiku, E. Mathematical modeling and optimization studies by Artificial neural network, genetic algorithm and response surface methodology: A case of ferric sulfate–catalyzed esterification of Neem (Azadirachta indica) seed oil. Front. Energy Res. 2020, 8, 614621. [Google Scholar] [CrossRef]
- Etim, A.O.; Musonge, P.; Eloka-Eboka, A.C. An effective green and renewable from the fusion of bi-component transesterification of linseed oil methyl ester. Biofuels Bioprod. Biorefining 2021, 15, 1461–1472. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in Enzymatic Biodiesel Production and Commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Onukwuli, D.O.; Emembolu, L.N.; Ude, C.N.; Aliozo, S.O.; Menkiti, M.C. Optimization of biodiesel production from refined cotton seed oil and its characterization. Egypt. J. Pet. 2017, 26, 103–110. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Su, C.H.; Ong, H.C.; Juan, H.Y.; Wu, S.J. Bio-derived catalysts: A current trend of catalysts used in biodiesel production. Catalysts 2021, 11, 812. [Google Scholar] [CrossRef]
- Iribarren, D.; Peters, J.F.; Dufour, J. Life cycle assessment of waste-to-energy in the European Union: Assessing environmental impacts of biofuels production. Renew. Energy 2020, 157, 66–73. [Google Scholar]
- Demirbas, A. Biofuels securing the planet’s future energy needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- Traven, L. Sustainable energy generation from municipal solid waste: A brief overview of existing technologies. Case Stud. Chem. Environ. Eng. 2023, 8, 100491. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Maniatis, K.; Janulis, P. Sustainable energy production: The role of biofuels in decarbonizing transport. Renew. Sustain. Energy Rev. 2019, 102, 156–166. [Google Scholar]
- Oyedele, O.A.; Jekayinfa, S.O.; Alade, A.O. Evaluation of impact of calcination temperatures on kola nut pod residue as catalyst for biodiesel synthesis. J. Energy Res. 2024, 16, 48–55. [Google Scholar] [CrossRef]
- Betiku, E.; Akintunde, A.M.; Ojumu, T.V. Banana peels as a biobased catalyst for fatty acid methyl esters production using Napoleon’s plume (Bauhinia monandra) seed oil: A process parameters optimization study. Energy 2016, 103, 797–806. [Google Scholar] [CrossRef]
- Oladipo, B.; Ojumu, T.V.; Betiku, E. Potential of pawpaw peels as a base heterogeneous catalyst for biodiesel production: Modeling and optimization studies. In Proceedings of the Nigerian Society of Chemical Engineers 48th Annual Conference, Abuja, Nigeria, 26–30 November 2018; pp. 1–11. Available online: https://www.researchgate.net/profile/Eriola-Betiku/publication/329070825_Potential_of_pawpaw_peels_as_a_base_heterogeneous_catalyst_for_biodiesel_production_Modeling_and_optimization_studies/links/5bf41b0b92851c6b27cc42f8/Potential-of-pawpaw-peels-as-a-base-heterogeneous-catalyst-for-biodiesel-production-Modeling-and-optimization-studies.pdf (accessed on 3 October 2024).
- Oladipo, B.; Ojumu, T.V.; Latinwo, L.M.; Betiku, E. Pawpaw (Carica papaya) peel waste as a novel green heterogeneous catalyst for moringa oil methyl esters synthesis: Process optimization and kinetic study. Energies 2020, 13, 5834. [Google Scholar] [CrossRef]
- Tunji Oloyede, C.; Olatayo Jekayinfa, S.; Olanrewaju Alade, A.; Ogunkunle, O.; Timothy Laseinde, O.; Oyejide Adebayo, A.; Veza, I.; Rizwanul Fattah, I.M.d. Potential Heterogeneous Catalysts from Three Biogenic Residues toward Sustainable Biodiesel Production: Synthesis and Characterization. ChemistrySelect 2022, 7, e202203816. [Google Scholar] [CrossRef]
- Betiku, E.; Etim, A.O.; Pereao, O.; Ojumu, T.V. Two-Step Conversion of Neem (Azadirachta indica) Seed Oil into Fatty Methyl Esters Using a Heterogeneous Biomass-Based Catalyst: An Example of Cocoa Pod Husk. Energy Fuels 2017, 31, 6182–6193. [Google Scholar] [CrossRef]
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Linggawati, A. Preparation and Characterization of Calcium Oxide Heterogeneous Catalyst Derived from Anadara Granosa Shell for Biodiesel Synthesis. KnE Eng. 2016, 1, 1–8. Available online: http://knepublishing.com/index.php/KnE-Engineering/article/view/494 (accessed on 8 October 2024). [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.; Carvalho, J.A., Jr. Biodiesel from waste oils: Production and Environmental Benefits. Renew. Sustain. Energy Rev. 2020, 27, 475–493. [Google Scholar] [CrossRef]
- Yogeeswara, T.; Devendra, U.; Kalaisselvane, A. Physical and chemical characterization of waste frying palm oil biodiesel and its blends with diesel. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2020; Available online: https://pubs.aip.org/aip/acp/article-abstract/2225/1/030003/721622 (accessed on 3 October 2024).
- Ismail, S.; Ahmed, A.S.; Anr, R.; Hamdan, S. Biodiesel Production from Castor Oil by Using Calcium Oxide Derived from Mud Clam Shell. J. Renew. Energy 2016, 2016, 5274917. [Google Scholar] [CrossRef]
- Falowo, O.A.; Oladipo, B.; Taiwo, A.E.; Olaiya, A.T.; Oyekola, O.O.; Betiku, E. Green heterogeneous base catalyst from ripe and unripe plantain peels mixture for the transesterification of waste cooking oil. Chem. Eng. J. Adv. 2022, 10, 100293. [Google Scholar] [CrossRef]
- Odude, V.O.; Adesina, A.J.; Oyetunde, O.O.; Adeyemi, O.O.; Ishola, N.B.; Etim, A.O.; Betiku, E. Application of Agricultural Waste-Based Catalysts to Transesterification of Esterified Palm Kernel Oil into Biodiesel: A Case of Banana Fruit Peel Versus Cocoa Pod Husk. Waste Biomass Valorization 2019, 10, 877–888. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Buasri1, A.; Chaiyut, N.; Loryuenyong, V.; Wongweang, C.; Khamsrisu, S. Application of eggshell wastes as a heterogeneous catalyst for biodiesel production. Sustain. Energy 2013, 1, 7–13. [Google Scholar]
- Almadani, E.A.; Abdelghani, K.A.; Omar, F.A. Calcium oxide as an efficient heterogeneous catalyst for production of biodiesel. Sci. J. Univ. Benghazi 2023, 36, 139–143. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P.; Yang, C.; Zhang, Y. XRD and FTIR Analysis of Calcium-Based Catalysts for Biodiesel Production. J. Renew. Energy 2015, 85, 94–101. [Google Scholar]
- Etim, A.O.; Eloka-Eboka, A.C.; Musonge, P. Potential of Carica papaya peels as effective biocatalyst in the optimized parametric transesterification of used vegetable oil. Environ. Eng. Res. 2021, 26, 200229. [Google Scholar] [CrossRef]
- Sarjadi, M.S.; Ling, T.C.; Khan, M.S. Analysis and comparison of olive cooking oil and palm cooking oil properties as biodiesel feedstock. J. Phys. Conf. Ser. 2019, 1358. [Google Scholar] [CrossRef]
- Marimuthu, V.; Kumaran, S.; Kumar, A.; Ganesan, S. Spectral Characterization and Vibrational Analysis of Organic Compounds. J. Mol. Struct. 2020, 1208, 127887. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Muzio, G.; Carrillo, R.; Del Campo, J. Analysis of Functional Groups in Hydrocarbon Derivatives by FTIR. J. Anal. Chem. 2020, 75, 1021–1030. [Google Scholar]
- Pascoal, J.A.R.; Souza, J.R.; Lima, L.M. FTIR Analysis of Hydroxyl and Alkane Functional Groups in Organic Compounds. J. Mater. Sci. Chem. Eng. 2016, 4, 23–31. [Google Scholar]
- Nouadjepa, N.S.; Nsob, E.; Kanaa, E.B.G.; Kapseu, C. Simplex lattice mixture design application for biodiesel production: Formulation and characterization of hybrid oil as feedstock. Fuel 2019, 252, 135–142. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Naccarato, S.; Albo, L.; De Paola, M.G.; Chakraborty, S.; Curcio, S.; Calabro, V. Enzymatic transesterification of waste vegetable oil to produce biodiesel. Ecotoxicol. Environ. Saf. 2015, 121, 229–235. [Google Scholar] [CrossRef]
- Banani, R.; Youssef, S.; Bezzarga, M.; Abderrabba, M. Waste frying oil with high levels of free fatty acids as one of the prominent sources of biodiesel production. J. Mater. Environ. Sci. 2015, 6, 1178–1185. [Google Scholar]
- Aremu, M.O.; Ibrahim, H.; Bamidele, T.O. Physicochemical characteristics of the oils extracted from some Nigerian plant foods—A review. Chem. Process Eng. Res. 2015, 32, 36–52. [Google Scholar]
- Xu, S. Predicted Residual Error Sum of Squares of Mixed Models: An Application for Genomic Prediction 2017. Available online: https://doi.org/10.1534/g3.116.038059 (accessed on 3 October 2024).
- Chicco, D.; Warrens, M.J.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef] [PubMed]
- Finch, H. Multilevel modeling in the presence of outliers: A comparison of robust estimation methods. Psicol. Int. J. Methodol. Exp. Psychol. 2017, 38, 57–92. [Google Scholar]
- Samuel, E.A.; Oladipupo, O.O. Factorial Designs Application to Study Enhanced Bioremediation of Soil Artificially Contaminated with Weathered Bonny Light Crude Oil through Biostimulation and Bioaugmentation Strategy. J. Environ. Prot. 2012, 3, 748–759. [Google Scholar]
- Sulaiman, S.; Syakirah, N.K.; Jamal, P.; Alam, M.Z. Fish bone waste as catalyst for biodiesel production. J. Trop. Resour. Sustain. Sci. JTRSS 2015, 3, 180–184. [Google Scholar] [CrossRef]
- Rajesh, Y.; Kolakoti, A.; Sheakar, B.C.; Bhargavi, J. Optimization of biodiesel production from waste frying palm oil using definitive screening design. Int. J. Eng. Sci. Technol. 2019, 11, 48–57. [Google Scholar] [CrossRef]
- Bharti, V.K.; Singh, G. Application of Response Surface Methodology in Optimization of Process Parameters. J. Chem. Eng. Res. 2019, 7, 15–22. [Google Scholar]
- Mbah, C.G.; Esonye, C.V.; Onukwuli, D.O.; Eze, V.C. Use of response surface methodology (RSM) in optimization of biodiesel production from cow tallow. Int. J. Innov. Eng. Res. Technol. 2021, 8, 91–102. [Google Scholar]
- Oyedoh, E.A.; Okoduwa, G.U.; Madojemu, G.O.; Akhabue, C.E. Production of Biodiesel from the Transesterification of Waste Cooking Oil using Biobased Sulphonated Catalyst prepared from Coconut Shells. J. Appl. Sci. Environ. Manag. 2022, 26, 1977–1987. [Google Scholar] [CrossRef]
- Rutto, H.L.; Enweremadu, C.C. Production of biodiesel from waste vegetable oil using impregnated diatomite as a heterogeneous catalyst. Korean J. Chem. Eng. 2013, 29, 281–289. [Google Scholar]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance, and emissions. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Mulla, M.; Patil, P.; Wagh, M. A novel review on biodiesel production from waste cooking oil. Int. J. Res. Publ. Eng. Technol. 2017, 3, 220–226. [Google Scholar]
- Foroutan, R.; Esmaeili, H.; Ghyasi, F.F. Optimization of biodiesel production from waste cooking oil by alkaline catalysts. J. Food Technol. Food Chem. Res. 2018, 1, 1–7. [Google Scholar]
- Di Serio, M.; Ledda, M.; Cozzolino, M.; Minutillo, G.; Tesser, R.; Santacesaria, E. Transesterification of soybean oil to biodiesel by using heterogeneous basic catalysts. Ind. Eng. Chem. Res. 2006, 45, 3009–3014. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Sakthivela, R.; Rameshb, K.; Purnachandrana, R.; Shameera, P.M. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]


| Name | Components | Levels | ||
|---|---|---|---|---|
| Code | Unit | Low | High | |
| CKNPA | A | % | 10 | 80 |
| CDPLA | C | % | 10 | 80 |
| CSOPA | B | % | 10 | 80 |
| S/N | Element | Concentration (%) | ||
|---|---|---|---|---|
| DPL | KNP | SOP | ||
| 1 | O | 33.83 | 29.21 | 28.90 |
| 2 | Mg | 1.18 | 7.39 | 0.13 |
| 3 | Al | 2.75 | 4.15 | 3.73 |
| 4 | Si | 7.21 | 1.64 | 4.17 |
| 5 | P | 0.59 | 0.74 | 0.31 |
| 6 | S | 2.12 | 1.98 | 0.98 |
| 7 | Cl | 2.42 | 2.03 | 1.72 |
| 8 | K | 7.39 | 31.18 | 25.18 |
| 9 | Ca | 38.31 | 17.09 | 29.53 |
| 10 | Ti | 0.22 | 0.21 | 0.40 |
| 11 | V | 0.01 | 0.01 | Nil |
| 12 | Cr | 0.01 | 0.01 | 0.01 |
| 13 | Mn | 0.30 | 0.46 | 0.19 |
| 14 | Fe | 2.93 | 2.28 | 2.71 |
| 15 | Co | 0.01 | 0.03 | 0.04 |
| 16 | Ni | 0.00 | 0.01 | 0.01 |
| 17 | Cu | 0.18 | 0.21 | 0.29 |
| 18 | Zn | 0.11 | 0.12 | 0.15 |
| 19 | Sr | 0.15 | 0.08 | 0.18 |
| 20 | Zr | 0.02 | 0.04 | 0.09 |
| 21 | Nb | 0.03 | 0.05 | 0.06 |
| 22 | Mo | 0.01 | 0.01 | 0.02 |
| 23 | Ag | 0.02 | 0.06 | 0.08 |
| 24 | Sn | 0.08 | 0.96 | 0.62 |
| 25 | Ba | 0.10 | 0.06 | 0.43 |
| 26 | Ta | Nil | Nil | Nil |
| 27 | W | 0.01 | 0.01 | 0.01 |
| 28 | Pb | 0.02 | 0.03 | 0.05 |
| S/N | Compound | Concentration (%) | ||
|---|---|---|---|---|
| DPL | KNP | SOP | ||
| 1 | SiO2 | 16.10 | 4.05 | 10.44 |
| 2 | V2O5 | 0.00 | 0.01 | 0.00 |
| 3 | Cr2O3 | 0.01 | 0.01 | 0.01 |
| 4 | MnO | 0.35 | 0.58 | 0.25 |
| 5 | Fe2O3 | 1.64 | 1.41 | 1.70 |
| 6 | Co3O4 | 0.00 | 0.01 | 0.02 |
| 7 | NiO | 0.00 | 0.01 | 0.01 |
| 8 | CuO | 0.18 | 0.23 | 0.33 |
| 9 | Nb2O3 | 0.01 | 0.02 | 0.02 |
| 10 | MoO3 | 0.01 | 0.01 | 0.01 |
| 11 | WO3 | 0.00 | 0.00 | 0.00 |
| 12 | P2O5 | 0.59 | 0.82 | 0.35 |
| 13 | SO3 | 4.15 | 4.27 | 2.15 |
| 14 | CaO | 59.92 | 29.52 | 51.81 |
| 15 | MgO | 3.04 | 21.04 | 0.39 |
| 16 | K2O | 5.92 | 27.61 | 22.65 |
| 17 | BaO | 0.05 | 0.03 | 0.22 |
| 18 | Al2O3 | 3.19 | 5.33 | 4.86 |
| 19 | Ta2O5 | Nil | Nil | Nil |
| 20 | TiO2 | 0.28 | 0.31 | 0.59 |
| 21 | ZnO | 0.10 | 0.129 | 0.16 |
| 22 | Ag2O | 0.01 | 0.02 | 0.03 |
| 23 | Cl | 4.28 | 3.96 | 3.42 |
| 24 | ZrO2 | 0.01 | 0.03 | 0.07 |
| 25 | SnO2 | 0.04 | 0.56 | 0.37 |
| 26 | SrO | 0.12 | 0.06 | 0.14 |
| 27 | PbO | 0.01 | 0.01 | 0.02 |
| S/N | Oxide | Calcined Temperatures for DPLA (°C) | Calcined Temperatures for KNPA (°C) | Calcined Temperatures for SOPA (°C) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 | 600 | 700 | 800 | 900 | 500 | 600 | 700 | 800 | 900 | 500 | 600 | 700 | 800 | 900 | ||
| 1 | SiO2 | 21.94 | 20.19 | 24.08 | 23.32 | 23.13 | 24.39 | 27.11 | 27.08 | 27.41 | 27.53 | 23.00 | 23.28 | 22.95 | 23.93 | 23.42 |
| 2 | Al2O3 | 6.70 | 5.31 | 4.66 | 4.60 | 4.71 | 5.37 | 4.31 | 5.44 | 5.63 | 5.72 | 1.78 | 2.41 | 2.38 | 2.24 | 1.87 |
| 3 | CaO | 25.24 | 26.62 | 27.21 | 27.08 | 27.00 | 42.81 | 42.81 | 41.53 | 41.72 | 41.46 | 30.02 | 30.74 | 29.42 | 30.32 | 31.62 |
| 4 | Fe2O3 | 6.02 | 4.79 | 4.62 | 4.75 | 4.68 | 2.00 | 1.43 | 2.46 | 2.50 | 2.31 | 1.26 | 2.17 | 1.68 | 1.63 | 1.87 |
| 5 | MgO | 4.25 | 4.03 | 5.48 | 5.68 | 5.36 | 1.26 | 1.65 | 3.14 | 2.45 | 2.29 | 3.40 | 3.64 | 3.79 | 4.21 | 4.08 |
| 6 | K2O | 22.73 | 21.28 | 21.78 | 21.12 | 21.27 | 12.08 | 10.03 | 10.26 | 10.00 | 10.31 | 22.42 | 22.73 | 23.26 | 24.07 | 25.93 |
| 7 | Cl | 1.30 | 1.27 | 1.31 | 1.44 | 1.53 | 1.65 | 1.41 | 1.38 | 1.42 | 1.48 | 0.05 | 0.04 | 0.03 | 0.05 | 0.03 |
| 8 | P2O5 | 2.00 | 2.13 | 2.35 | 2.61 | 2.50 | 2.44 | 2.29 | 2.32 | 2.40 | 2.32 | 2.07 | 2.58 | 2.4 | 2.52 | 2.27 |
| 9 | SO3 | 1.32 | 1.30 | 1.63 | 1.49 | 1.32 | 4.00 | 4.21 | 2.16 | 2.03 | 2.11 | 0.3 | 0.61 | 0.53 | 0.33 | 0.86 |
| 10 | TiO2 | 2.10 | 2.10 | 2.02 | 2.00 | 2.05 | 0.85 | 0.80 | 0.63 | 0.74 | 0.61 | 0.61 | 0.59 | 0.38 | 0.35 | 0.39 |
| 11 | MnO | 0.56 | 0.24 | 0.33 | 0.31 | 0.34 | 1.40 | 1.40 | 1.51 | 1.59 | 1.38 | 0.27 | 0.29 | 0.32 | 0.30 | 0.32 |
| 12 | LOI | 5.30 | 8.34 | 4.52 | 5.54 | 6.02 | 1.70 | 1.84 | 2.06 | 2.00 | 2.02 | 13.98 | 10.54 | 12.63 | 9.97 | 7.31 |
| Element | Composition (%) |
|---|---|
| Silicon (Si) | 4.81 |
| Carbon (C) | 24.56 |
| Oxygen (O) | 7.24 |
| Calcium (Ca) | 59.03 |
| Magnesium (Mg) | 2.74 |
| Sodium (Na) | 1.29 |
| Run | Component (%) | Response | ||
|---|---|---|---|---|
| A: KNPA | B: SOPA | C: DPLA | Biodiesel Yield (%) | |
| 1 | 45.00 | 10.00 | 45.00 | 16.0 |
| 2 | 21.67 | 56.67 | 21.67 | 32.0 |
| 3 | 21.67 | 21.67 | 56.67 | 61.0 |
| 4 | 10.00 | 10.00 | 80.00 | 47.0 |
| 5 | 33.33 | 33.33 | 33.33 | 65.3 |
| 6 | 80.00 | 10.00 | 10.00 | 52.0 |
| 7 | 10.00 | 45.00 | 45.00 | 54.0 |
| 8 | 45.00 | 45.00 | 10.00 | 36.0 |
| 9 | 80.00 | 10.00 | 10.00 | 44.0 |
| 10 | 10.00 | 10.00 | 80.00 | 60.0 |
| 11 | 56.67 | 21.67 | 21.67 | 37.3 |
| 12 | 10.00 | 80.00 | 10.00 | 44.0 |
| 13 | 10.00 | 80.00 | 10.00 | 47.7 |
| 14 | 45.00 | 45.00 | 10.00 | 30.7 |
| Source | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | PRESS |
|---|---|---|---|---|---|
| Linear | 0.0127 | 0.0445 | −0.1292 | −0.3848 | 0.0026 |
| Quadratic | 0.0102 | 0.5495 | 0.2679 | −1.3850 | 0.0044 |
| Special cubic | 0.0068 | 0.8269 | 0.6785 | −0.6881 | 0.0031 |
| Cubic | 0.0026 | 0.9824 | 0.9542 | 0.7417 | 0.0005 * |
| Special quartic | 0.0026 | 0.9824 | 0.9542 | 0.7417 | 0.0005 |
| Quartic | 0.0027 | 0.9840 | 0.9481 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.0018 | 8 | 0.0002 | 34.88 | 0.0006 * |
| Linear mixture | 0.0001 | 2 | 0.0000 | 6.32 | 0.0428 * |
| AB | 0.0001 | 1 | 0.0001 | 15.84 | 0.0105 * |
| AC | 0.0015 | 1 | 0.0015 | 224.22 | <0.0001 * |
| BC | 2.348 × 10−6 | 1 | 2.348 × 10−6 | 0.3575 | 0.5760 |
| A2BC | 0.0001 | 1 | 0.0001 | 21.62 | 0.0056 * |
| AB2C | 0.0001 | 1 | 0.0001 | 11.39 | 0.0198 * |
| ABC2 | 0.0003 | 1 | 0.0003 | 49.93 | 0.0009 * |
| Residual | 0.0000 | 5 | 6.567 × 10−6 | ||
| Lack of fit | 3.045 × 10−6 | 1 | 3.045 × 10−6 | 0.4088 | 0.5573 |
| Pure error | 0.0000 | 4 | 7.448 × 10−6 | ||
| Cor total | 0.0019 | 13 |
| Properties | Biodiesel Yield |
|---|---|
| Standard deviation | 0.0026 |
| Mean | 0.0253 |
| C.V | 10.11 |
| PRESS | 0.0005 |
| R2 | 0.9824 |
| Adjusted R2 | 0.9542 |
| Predicted R2 | 0.7417 |
| Adequate precision | 22.9195 |
| Properties | Values Obtained |
|---|---|
| Density (g/cm3) | 910.4 |
| Kinematic viscosity (mm2/s) at 40 °C | 32.83 |
| Cloud point (°C) | −6 |
| Pour point (°C) | −11 |
| Flash point (°C) | 164 |
| Saponification value (mgKOH/g) | 186.27 |
| Acid value (mg/g) | 3.48 |
| Free fatty acid (mg/g) | 1.74 |
| Properties | RUN 5 | RUN 3 | RUN 10 |
|---|---|---|---|
| Specify gravity @40 °C | 0.8752 | 0.8770 | 0.8804 |
| Kinematic viscosity (@40 °C (mm2s−1) | 4.32 | 4.90 | 3.75 |
| Moisture content (%) | 0.02 | 0.02 | 0.02 |
| Saponification (mgKOH/g) | 82.46 | 80.26 | 80.18 |
| Iodine value (g I2/100 g) | 0.41 | 5.70 | 3.95 |
| Peroxide value (meq. O2 kg−1) | 7.4 | 6.8 | 6.2 |
| Refractive index | 1.46 | 1.46 | 1.45 |
| Flashpoint (°C) | 137 | 160 | 172 |
| Pour point (°C) | 6 | 8 | 6 |
| Cloud point (°C) | −4 | −2 | −2 |
| Cetane number (Ignition quality) | 46 | 45 | 45 |
| Calorific value (MJ/kg) | 37.42 | 37.38 | 37.33 |
| Dependent Variable: Observation | |||||
|---|---|---|---|---|---|
| Source | Type III Sum of Squares | Df | Mean Square | F | Sig. |
| Corrected Model | 74751.601 * | 13 | 5750.123 | 211.335 | 0.000 |
| Intercept | 29043.124 | 1 | 29043.124 | 1067.425 | 0.000 |
| Sample | 59.338 | 2 | 29.669 | 1.090 | 0.354 |
| Properties | 74692.263 | 11 | 6790.206 | 249.561 | 0.000 |
| Error | 598.589 | 22 | 27.209 | ||
| Total | 104393.314 | 36 | |||
| Corrected Total | 75350.190 | 35 | |||
| (I) Sample | (J) Sample | Mean Diff. (I – J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| R5 | R3 | −2.41932 | 2.129498 | 0.268 | −6.8356 | 1.9970 |
| R10 | −2.94960 | 2.129498 | 0.180 | −7.3659 | 1.4667 | |
| R3 | R5 | 2.41932 | 2.129498 | 0.268 | −1.9970 | 6.8356 |
| R10 | −0.53028 | 2.129498 | 0.806 | −4.9466 | 3.8860 | |
| R10 | R5 | 2.94960 | 2.129498 | 0.180 | −1.4667 | 7.3659 |
| R3 | 0.53028 | 2.129498 | 0.806 | −3.88603 | 4.94659 | |
| Properties | Biodiesel Standards | Present Work (WFOB) | |||
|---|---|---|---|---|---|
| ASTM D6751 | ASTM D975 | EN 14214 | EN590 | ||
| Physical state | Liquid | Liquid | Liquid | Liquid | Liquid |
| Specify gravity @15 °C | 0.88 | NA | 0.86–0.9 | NA | 0.8752 |
| Kinematic viscosity (@40 °C (mm2s−1) | 1.9–6.0 | 1.3–4.1 | 3.5–5.0 | 2.0–4.5 | 4.32 |
| Moisture content (%) | 0.050 | 0.52% | 0.5 | 0.02 | 0.02 |
| Saponification (mgKOH/g) | <500 | NA | NA | NA | 82.46 |
| Iodine value (g I2/100 g) | <115 | NA | Max 120 | NA | 0.41 |
| Peroxide value (meq. O2 kg−1) | NA | NA | NA | NA | 7.4 |
| Refractive index | NA | NA | NA | NA | 1.46 |
| Flashpoint (°C) | 100 to 170 | 60–80 | Min 120 | 55 | 137 |
| Pour point (°C) | −15 to 10 | −35 to −15 | NA | NA | 6 |
| Cloud point (°C) | −3 to 12 | −15 to 5 | L and S dependent | L and S dependent | −4 |
| Cetane number (Ignition quality) | 48–65 | 40–45 | Min 51.0 | 51.0 min | 46 |
| Calorific value (MJ/kg) | 42 | NA | 35 | NA | 37.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyedele, O.A.; Jekayinfa, S.O.; Alade, A.O.; Enweremadu, C.C. Catalytic Evaluation of an Optimized Heterogeneous Composite Catalyst Derived from Fusion of Tri-Biogenic Residues. Biomass 2024, 4, 1219-1237. https://doi.org/10.3390/biomass4040068
Oyedele OA, Jekayinfa SO, Alade AO, Enweremadu CC. Catalytic Evaluation of an Optimized Heterogeneous Composite Catalyst Derived from Fusion of Tri-Biogenic Residues. Biomass. 2024; 4(4):1219-1237. https://doi.org/10.3390/biomass4040068
Chicago/Turabian StyleOyedele, Oyelayo Ajamu, Simeon Olatayo Jekayinfa, Abass O. Alade, and Christopher Chintua Enweremadu. 2024. "Catalytic Evaluation of an Optimized Heterogeneous Composite Catalyst Derived from Fusion of Tri-Biogenic Residues" Biomass 4, no. 4: 1219-1237. https://doi.org/10.3390/biomass4040068
APA StyleOyedele, O. A., Jekayinfa, S. O., Alade, A. O., & Enweremadu, C. C. (2024). Catalytic Evaluation of an Optimized Heterogeneous Composite Catalyst Derived from Fusion of Tri-Biogenic Residues. Biomass, 4(4), 1219-1237. https://doi.org/10.3390/biomass4040068








