Contributing Factors for Mutagenic DNA Lesion Bypass by DNA Polymerase Eta (polη)
Abstract
1. Introduction
2. Overall and Catalytic Domain Structures of polη
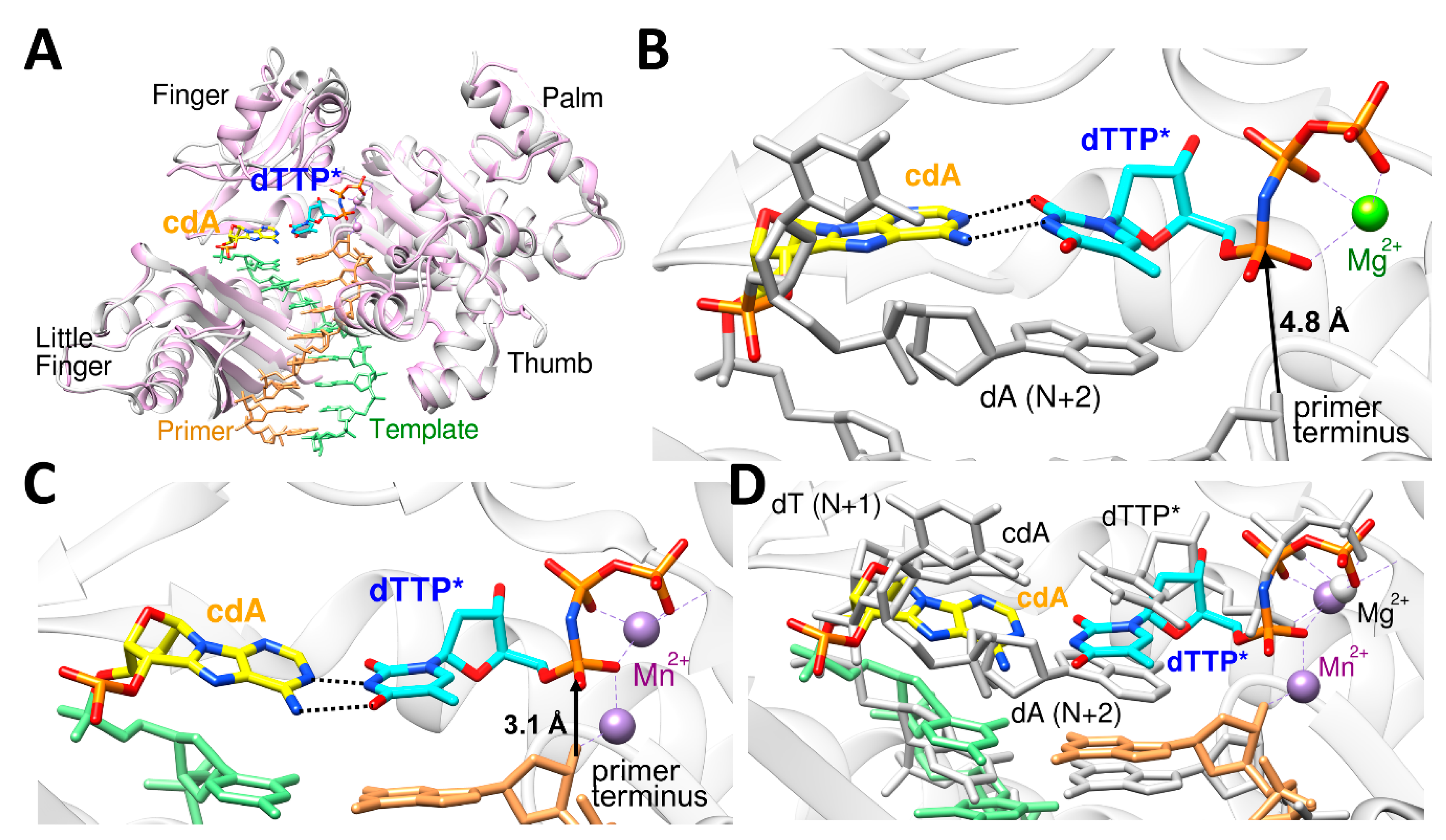
3. Catalytic Metal Cofactors—Effect of Displacement of Mg2+ by Mn2+
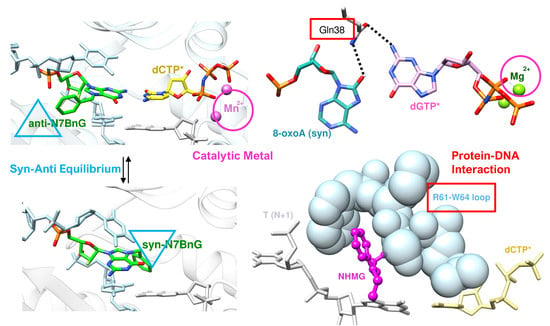
4. Syn–Anti Conformational Change
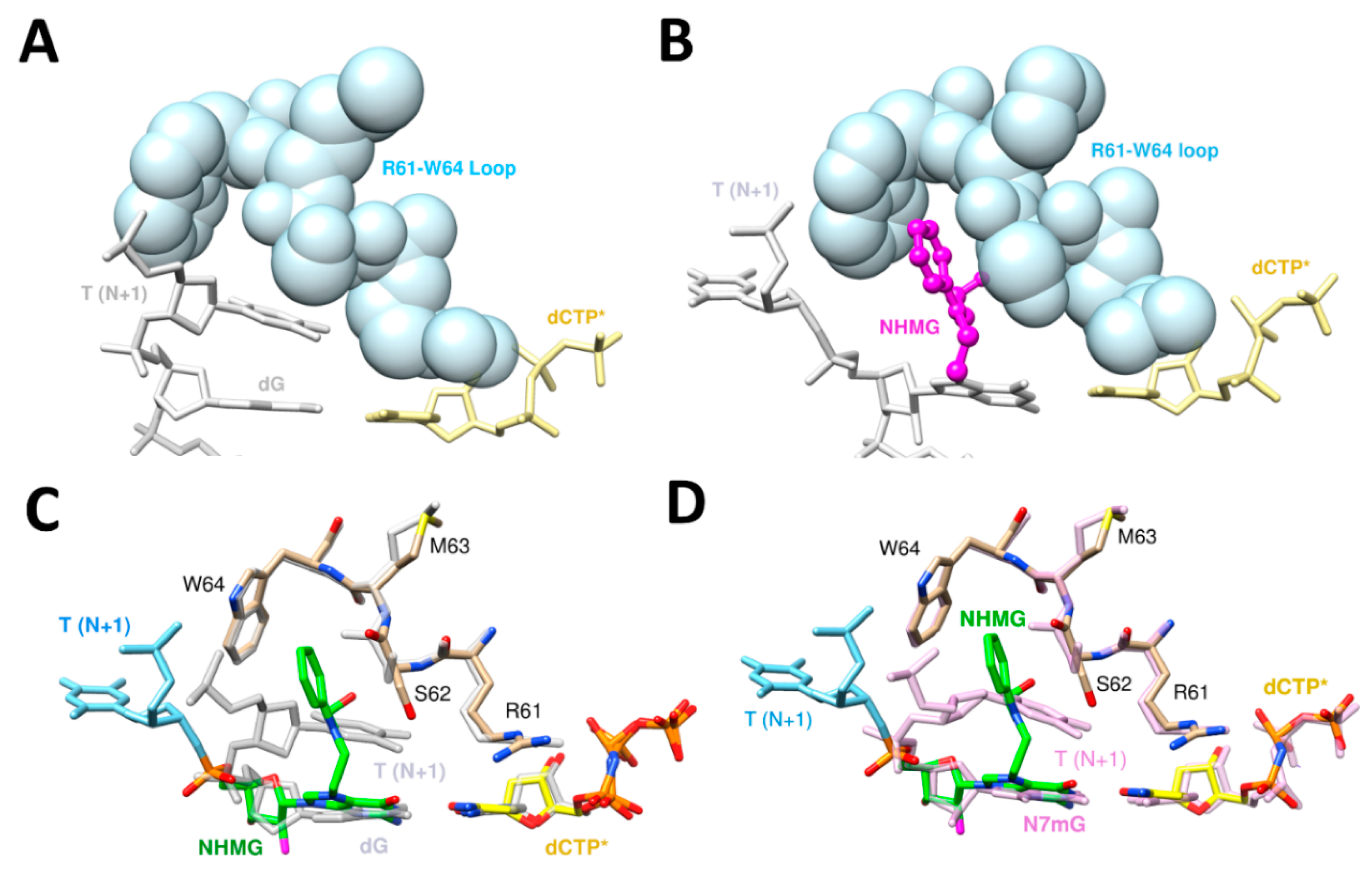
5. Enol–Keto Tautomerization and polη Residues
6. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Drablos, F.; Feyzi, E.; Aas, P.A.; Vaagbo, C.B.; Kavli, B.; Bratlie, M.S.; Pena-Diaz, J.; Otterlei, M.; Slupphaug, G.; Krokan, H.E. Alkylation damage in DNA and RNA—Repair mechanisms and medical significance. DNA Repair 2004, 3, 1389–1407. [Google Scholar] [CrossRef] [PubMed]
- Eadie, J.S.; Conrad, M.; Toorchen, D.; Topal, M.D. Mechanism of mutagenesis by O6-methylguanine. Nature 1984, 308, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Traverso, I.; Casolari, L.; Menichini, P.; Inga, A.; Ottaggio, L.; Russo, D.; Iyer, P.; Gold, B.; Fronza, G. Mutagenicity of N3-methyladenine: A multi-translesion polymerase affair. Mutat. Res. 2010, 683, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.S.; Nooner, T.; Dutta, S. Biologically relevant chemical reactions of N7-alkylguanine residues in DNA. Chem. Res. Toxicol. 2004, 17, 839–856. [Google Scholar] [CrossRef]
- Hemnani, T.; Parihar, M.S. Reactive oxygen species and oxidative DNA damage. Indian J. Physiol. Pharmacol. 1998, 42, 440–452. [Google Scholar] [PubMed]
- Ames, B.N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic. Res. Commun. 1989, 7, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, I.; Couve, S.; Ishchenko, A.A.; Kunz, C.; Schar, P.; Saparbaev, M. 7,8-Dihydro-8-oxoadenine, a highly mutagenic adduct, is repaired by Escherichia coli and human mismatch-specific uracil/thymine-DNA glycosylases. Nucleic Acids Res. 2013, 41, 912–923. [Google Scholar] [CrossRef]
- Chen, W.; Balakrishnan, K.; Kuang, Y.; Han, Y.; Fu, M.; Gandhi, V.; Peng, X. Reactive oxygen species (ROS) inducible DNA cross-linking agents and their effect on cancer cells and normal lymphocytes. J. Med. Chem. 2014, 57, 4498–4510. [Google Scholar] [CrossRef]
- Kow, Y.W. Repair of deaminated bases in DNA. Free Radic. Biol. Med. 2002, 33, 886–893. [Google Scholar] [CrossRef]
- Caulfield, J.L.; Wishnok, J.S.; Tannenbaum, S.R. Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J. Biol. Chem. 1998, 273, 12689–12695. [Google Scholar] [CrossRef]
- Wink, D.A.; Kasprzak, K.S.; Maragos, C.M.; Elespuru, R.K.; Misra, M.; Dunams, T.M.; Cebula, T.A.; Koch, W.H.; Andrews, A.W.; Allen, J.S.; et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 1991, 254, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Surova, O.; Zhivotovsky, B. Various modes of cell death induced by DNA damage. Oncogene 2013, 32, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Zhivotovsky, B. DNA damage-induced apoptosis. Oncogene 2004, 23, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Rayala, N.K.; Lee, S. Translesion synthesis of the major nitrogen mustard-induced DNA lesion by human DNA polymerase eta. Biochem. J. 2020, 477, 4543–4558. [Google Scholar] [CrossRef]
- Gregory, M.T.; Park, G.Y.; Johnstone, T.C.; Lee, Y.S.; Yang, W.; Lippard, S.J. Structural and mechanistic studies of polymerase eta bypass of phenanthriplatin DNA damage. Proc. Natl. Acad. Sci. USA 2014, 111, 9133–9138. [Google Scholar] [CrossRef]
- Hegde, M.L.; Hazra, T.K.; Mitra, S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008, 18, 27–47. [Google Scholar] [CrossRef]
- Kusakabe, M.; Onishi, Y.; Tada, H.; Kurihara, F.; Kusao, K.; Furukawa, M.; Iwai, S.; Yokoi, M.; Sakai, W.; Sugasawa, K. Mechanism and regulation of DNA damage recognition in nucleotide excision repair. Genes Environ. 2019, 41, 2. [Google Scholar] [CrossRef]
- Pecina-Slaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch Repair Pathway, Genome Stability and Cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef]
- Wright, W.D.; Shah, S.S.; Heyer, W.D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10524–10535. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Li, X.; Heyer, W.D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Knobel, P.A.; Marti, T.M. Translesion DNA synthesis in the context of cancer research. Cancer Cell Int. 2011, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Sale, J.E.; Lehmann, A.R.; Woodgate, R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat. Rev. Mol. Cell Biol. 2012, 13, 141–152. [Google Scholar] [CrossRef]
- Yang, W. An overview of Y-Family DNA polymerases and a case study of human DNA polymerase eta. Biochemistry 2014, 53, 2793–2803. [Google Scholar] [CrossRef]
- Kraemer, K.H.; Slor, H. Xeroderma pigmentosum. Clin. Dermatol. 1985, 3, 33–69. [Google Scholar] [CrossRef]
- Setlow, R.B.; Regan, J.D.; German, J.; Carrier, W.L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc. Natl. Acad. Sci. USA 1969, 64, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, J.E. Defective repair replication of DNA in xeroderma pigmentosum. Nature 1968, 218, 652–656. [Google Scholar] [CrossRef]
- Epstein, J.H.; Fukuyama, K.; Reed, W.B.; Epstein, W.L. Defect in DNA synthesis in skin of patients with xeroderma pigmentosum demonstrated in vivo. Science 1970, 168, 1477–1478. [Google Scholar] [CrossRef]
- Johnson, R.E.; Prakash, S.; Prakash, L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science 1999, 283, 1001–1004. [Google Scholar] [CrossRef]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 1999, 399, 700–704. [Google Scholar] [CrossRef]
- Matsuda, T.; Bebenek, K.; Masutani, C.; Hanaoka, F.; Kunkel, T.A. Low fidelity DNA synthesis by human DNA polymerase-eta. Nature 2000, 404, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Masutani, C.; Araki, M.; Yamada, A.; Kusumoto, R.; Nogimori, T.; Maekawa, T.; Iwai, S.; Hanaoka, F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999, 18, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Biertumpfel, C.; Zhao, Y.; Kondo, Y.; Ramon-Maiques, S.; Gregory, M.; Lee, J.Y.; Masutani, C.; Lehmann, A.R.; Hanaoka, F.; Yang, W. Structure and mechanism of human DNA polymerase eta. Nature 2010, 465, 1044–1048. [Google Scholar] [CrossRef]
- Su, Y.; Patra, A.; Harp, J.M.; Egli, M.; Guengerich, F.P. Roles of Residues Arg-61 and Gln-38 of Human DNA Polymerase eta in Bypass of Deoxyguanosine and 7,8-Dihydro-8-oxo-2′-deoxyguanosine. J. Biol. Chem. 2015, 290, 15921–15933. [Google Scholar] [CrossRef]
- Koag, M.C.; Jung, H.; Lee, S. Mutagenic Replication of the Major Oxidative Adenine Lesion 7,8-Dihydro-8-oxoadenine by Human DNA Polymerases. J. Am. Chem. Soc. 2019, 141, 4584–4596. [Google Scholar] [CrossRef] [PubMed]
- Koag, M.C.; Jung, H.; Lee, S. Mutagenesis mechanism of the major oxidative adenine lesion 7,8-dihydro-8-oxoadenine. Nucleic Acids Res. 2020, 48, 5119–5134. [Google Scholar] [CrossRef]
- Patra, A.; Zhang, Q.; Lei, L.; Su, Y.; Egli, M.; Guengerich, F.P. Structural and kinetic analysis of nucleoside triphosphate incorporation opposite an abasic site by human translesion DNA polymerase eta. J. Biol. Chem. 2015, 290, 8028–8038. [Google Scholar] [CrossRef]
- Zhao, Y.; Biertumpfel, C.; Gregory, M.T.; Hua, Y.J.; Hanaoka, F.; Yang, W. Structural basis of human DNA polymerase eta-mediated chemoresistance to cisplatin. Proc. Natl. Acad. Sci. USA 2012, 109, 7269–7274. [Google Scholar] [CrossRef]
- Koag, M.C.; Jung, H.; Kou, Y.; Lee, S. Bypass of the Major Alkylative DNA Lesion by Human DNA Polymerase eta. Molecules 2019, 24, 3928. [Google Scholar] [CrossRef]
- Jung, H.; Rayala, N.K.; Lee, S. Effects of N7-Alkylguanine Conformation and Metal Cofactors on the Translesion Synthesis by Human DNA Polymerase eta. Chem. Res. Toxicol. 2022, 35, 512–521. [Google Scholar] [CrossRef]
- Jung, H.; Hawkins, M.; Lee, S. Structural insights into the bypass of the major deaminated purines by translesion synthesis DNA polymerase. Biochem. J. 2020, 477, 4797–4810. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Cleaver, J.E.; Hatahet, Z.; Honkanen, R.E.; Chang, J.Y.; Yen, Y.; Chou, K.M. Human DNA polymerase eta activity and translocation is regulated by phosphorylation. Proc. Natl. Acad. Sci. USA 2008, 105, 16578–16583. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; You, C.; Wang, Y. The Functions of Serine 687 Phosphorylation of Human DNA Polymerase eta in UV Damage Tolerance. Mol. Cell. Proteom. 2016, 15, 1913–1920. [Google Scholar] [CrossRef]
- Kannouche, P.L.; Wing, J.; Lehmann, A.R. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: A possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 2004, 14, 491–500. [Google Scholar] [CrossRef]
- Bienko, M.; Green, C.M.; Sabbioneda, S.; Crosetto, N.; Matic, I.; Hibbert, R.G.; Begovic, T.; Niimi, A.; Mann, M.; Lehmann, A.R.; et al. Regulation of translesion synthesis DNA polymerase eta by monoubiquitination. Mol. Cell 2010, 37, 396–407. [Google Scholar] [CrossRef]
- Bomar, M.G.; Pai, M.T.; Tzeng, S.R.; Li, S.S.; Zhou, P. Structure of the ubiquitin-binding zinc finger domain of human DNA Y-polymerase eta. EMBO Rep. 2007, 8, 247–251. [Google Scholar] [CrossRef]
- Pozhidaeva, A.; Pustovalova, Y.; D’Souza, S.; Bezsonova, I.; Walker, G.C.; Korzhnev, D.M. NMR structure and dynamics of the C-terminal domain from human Rev1 and its complex with Rev1 interacting region of DNA polymerase eta. Biochemistry 2012, 51, 5506–5520. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Ouzon-Shubeita, H.; Baker, M.; Koag, M.C.; Lee, S. Structural basis for the bypass of the major oxaliplatin-DNA adducts by human DNA polymerase eta. Biochem. J. 2019, 476, 747–758. [Google Scholar] [CrossRef]
- Patra, A.; Zhang, Q.; Guengerich, F.P.; Egli, M. Mechanisms of Insertion of dCTP and dTTP Opposite the DNA Lesion O6-Methyl-2′-deoxyguanosine by Human DNA Polymerase eta. J. Biol. Chem. 2016, 291, 24304–24313. [Google Scholar] [CrossRef]
- Zhao, Y.; Gregory, M.T.; Biertumpfel, C.; Hua, Y.J.; Hanaoka, F.; Yang, W. Mechanism of somatic hypermutation at the WA motif by human DNA polymerase eta. Proc. Natl. Acad. Sci. USA 2013, 110, 8146–8151. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Nagy, L.D.; Zhang, Q.; Su, Y.; Muller, L.; Guengerich, F.P.; Egli, M. Kinetics, structure, and mechanism of 8-Oxo-7,8-dihydro-2′-deoxyguanosine bypass by human DNA polymerase eta. J. Biol. Chem. 2014, 289, 16867–16882. [Google Scholar] [CrossRef] [PubMed]
- Weng, P.J.; Gao, Y.; Gregory, M.T.; Wang, P.; Wang, Y.; Yang, W. Bypassing a 8,5′-cyclo-2′-deoxyadenosine lesion by human DNA polymerase eta at atomic resolution. Proc. Natl. Acad. Sci. USA 2018, 115, 10660–10665. [Google Scholar] [CrossRef]
- Patra, A.; Banerjee, S.; Johnson Salyard, T.L.; Malik, C.K.; Christov, P.P.; Rizzo, C.J.; Stone, M.P.; Egli, M. Structural Basis for Error-Free Bypass of the 5-N-Methylformamidopyrimidine-dG Lesion by Human DNA Polymerase eta and Sulfolobus solfataricus P2 Polymerase IV. J. Am. Chem. Soc. 2015, 137, 7011–7014. [Google Scholar] [CrossRef]
- Su, Y.; Egli, M.; Guengerich, F.P. Mechanism of Ribonucleotide Incorporation by Human DNA Polymerase eta. J. Biol. Chem. 2016, 291, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Beese, L.S.; Steitz, T.A. Structural basis for the 3′-5′ exonuclease activity of Escherichia coli DNA polymerase I: A two metal ion mechanism. EMBO J. 1991, 10, 25–33. [Google Scholar] [CrossRef]
- Kaushik, N.; Pandey, V.N.; Modak, M.J. Significance of the O-helix residues of Escherichia coli DNA polymerase I in DNA synthesis: Dynamics of the dNTP binding pocket. Biochemistry 1996, 35, 7256–7266. [Google Scholar] [CrossRef]
- Doublie, S.; Tabor, S.; Long, A.M.; Richardson, C.C.; Ellenberger, T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 A resolution. Nature 1998, 391, 251–258. [Google Scholar] [CrossRef]
- Ling, H.; Boudsocq, F.; Woodgate, R.; Yang, W. Crystal structure of a Y-family DNA polymerase in action: A mechanism for error-prone and lesion-bypass replication. Cell 2001, 107, 91–102. [Google Scholar] [CrossRef]
- Jung, H.; Lee, S. Promutagenic bypass of 7,8-dihydro-8-oxoadenine by translesion synthesis DNA polymerase Dpo4. Biochem. J. 2020, 477, 2859–2871. [Google Scholar] [CrossRef]
- Franklin, M.C.; Wang, J.; Steitz, T.A. Structure of the replicating complex of a pol alpha family DNA polymerase. Cell 2001, 105, 657–667. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Prasad, R.; Wilson, S.H.; Kraut, J.; Pelletier, H. Crystal structures of human DNA polymerase beta complexed with gapped and nicked DNA: Evidence for an induced fit mechanism. Biochemistry 1997, 36, 11205–11215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pence, M.G.; Christov, P.P.; Wawrzak, Z.; Choi, J.Y.; Rizzo, C.J.; Egli, M.; Guengerich, F.P. Basis of miscoding of the DNA adduct N2,3-ethenoguanine by human Y-family DNA polymerases. J. Biol. Chem. 2012, 287, 35516–35526. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Zhao, Y.; Yamagata, Y.; Hua, Y.J.; Yang, W. Watching DNA polymerase eta make a phosphodiester bond. Nature 2012, 487, 196–201. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S.; Downey, K.M.; So, A.G. Molecular mechanisms of manganese mutagenesis. Proc. Natl. Acad. Sci. USA 1984, 81, 7378–7382. [Google Scholar] [CrossRef]
- Beckman, R.A.; Mildvan, A.S.; Loeb, L.A. On the fidelity of DNA replication: Manganese mutagenesis in vitro. Biochemistry 1985, 24, 5810–5817. [Google Scholar] [CrossRef]
- Miyaki, M.; Murata, I.; Osabe, M.; Ono, T. Effect of metal cations on misincorporation by E. coli DNA polymerases. Biochem. Biophys. Res. Commun. 1977, 77, 854–860. [Google Scholar] [CrossRef]
- Sirover, M.A.; Loeb, L.A. On the fidelity of DNA replication. Effect of metal activators during synthesis with avian myeloblastosis virus DNA polymerase. J. Biol. Chem. 1977, 252, 3605–3610. [Google Scholar] [CrossRef]
- Hays, H.; Berdis, A.J. Manganese substantially alters the dynamics of translesion DNA synthesis. Biochemistry 2002, 41, 4771–4778. [Google Scholar] [CrossRef]
- Tabor, S.; Richardson, C.C. Effect of manganese ions on the incorporation of dideoxynucleotides by bacteriophage T7 DNA polymerase and Escherichia coli DNA polymerase I. Proc. Natl. Acad. Sci. USA 1989, 86, 4076–4080. [Google Scholar] [CrossRef]
- Pelletier, H.; Sawaya, M.R.; Wolfle, W.; Wilson, S.H.; Kraut, J. A structural basis for metal ion mutagenicity and nucleotide selectivity in human DNA polymerase beta. Biochemistry 1996, 35, 12762–12777. [Google Scholar] [CrossRef]
- Villani, G.; Tanguy Le Gac, N.; Wasungu, L.; Burnouf, D.; Fuchs, R.P.; Boehmer, P.E. Effect of manganese on in vitro replication of damaged DNA catalyzed by the herpes simplex virus type-1 DNA polymerase. Nucleic Acids Res. 2002, 30, 3323–3332. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, O.; Ruiz, J.F.; Lain de Lera, T.; Garcia-Diaz, M.; Gonzalez, M.A.; Kirchhoff, T.; Martinez, A.C.; Bernad, A.; Blanco, L. DNA polymerase mu (Pol mu), homologous to TdT, could act as a DNA mutator in eukaryotic cells. EMBO J. 2000, 19, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, A.; Ling, H.; Woodgate, R.; Yang, W. Fidelity of Dpo4: Effect of metal ions, nucleotide selection and pyrophosphorolysis. EMBO J. 2005, 24, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Baruch-Torres, N.; Iwai, S.; Herrmann, G.K.; Brieba, L.G.; Yin, Y.W. Human Mitochondrial DNA Polymerase Metal Dependent UV Lesion Bypassing Ability. Front. Mol. Biosci. 2022, 9, 808036. [Google Scholar] [CrossRef]
- Garcia-Diaz, M.; Bebenek, K.; Krahn, J.M.; Pedersen, L.C.; Kunkel, T.A. Role of the catalytic metal during polymerization by DNA polymerase lambda. DNA Repair 2007, 6, 1333–1340. [Google Scholar] [CrossRef]
- Frank, E.G.; Woodgate, R. Increased catalytic activity and altered fidelity of human DNA polymerase iota in the presence of manganese. J. Biol. Chem. 2007, 282, 24689–24696. [Google Scholar] [CrossRef]
- Park, J.W.; Ames, B.N. 7-Methylguanine adducts in DNA are normally present at high levels and increase on aging: Analysis by HPLC with electrochemical detection. Proc. Natl. Acad. Sci. USA 1988, 85, 7467–7470. [Google Scholar] [CrossRef]
- Hu, G.; Tsai, A.L.; Quiocho, F.A. Insertion of an N7-methylguanine mRNA cap between two coplanar aromatic residues of a cap-binding protein is fast and selective for a positively charged cap. J. Biol. Chem. 2003, 278, 51515–51520. [Google Scholar] [CrossRef]
- Haschemeyer, A.E.; Rich, A. Nucleoside conformations: An analysis of steric barriers to rotation about the glycosidic bond. J. Mol. Biol. 1967, 27, 369–384. [Google Scholar] [CrossRef]
- Wilson, H.R.; Rahman, A. Nucleoside conformation and non-bonded interactions. J. Mol. Biol. 1971, 56, 129–142. [Google Scholar] [CrossRef]
- Son, T.D.; Guschlbauer, W.; Gueron, M. Flexibility and conformations of guanosine monophosphates by the Overhauser effect. J. Am. Chem. Soc. 1972, 94, 7903–7911. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, G.; Vojtechovsky, J.; Clowney, L.; Brunger, A.T.; Berman, H.M. New parameters for the refinement of nucleic acid-containing structures. Acta Crystallogr. D Biol. Crystallogr. 1996, 52, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Koag, M.C.; Kou, Y.; Ouzon-Shubeita, H.; Lee, S. Transition-state destabilization reveals how human DNA polymerase beta proceeds across the chemically unstable lesion N7-methylguanine. Nucleic Acids Res. 2014, 42, 8755–8766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reed, A.J.; Suo, Z. Time-Dependent Extension from an 8-Oxoguanine Lesion by Human DNA Polymerase Beta. J. Am. Chem. Soc. 2017, 139, 9684–9690. [Google Scholar] [CrossRef]
- Kimsey, I.J.; Szymanski, E.S.; Zahurancik, W.J.; Shakya, A.; Xue, Y.; Chu, C.C.; Sathyamoorthy, B.; Suo, Z.; Al-Hashimi, H.M. Dynamic basis for dG*dT misincorporation via tautomerization and ionization. Nature 2018, 554, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lawley, P.D.; Brookes, P. Acidic dissociation of 7:9-dialkylguanines and its possible relation to mutagenic properties of alkylating agents. Nature 1961, 192, 1081–1082. [Google Scholar] [CrossRef]
- Kou, Y.; Koag, M.C.; Lee, S. N7 methylation alters hydrogen-bonding patterns of guanine in duplex DNA. J. Am. Chem. Soc. 2015, 137, 14067–14070. [Google Scholar] [CrossRef]
- Yasui, M.; Suzuki, N.; Miller, H.; Matsuda, T.; Matsui, S.; Shibutani, S. Translesion synthesis past 2′-deoxyxanthosine, a nitric oxide-derived DNA adduct, by mammalian DNA polymerases. J. Mol. Biol. 2004, 344, 665–674. [Google Scholar] [CrossRef]
- Yasui, M.; Suenaga, E.; Koyama, N.; Masutani, C.; Hanaoka, F.; Gruz, P.; Shibutani, S.; Nohmi, T.; Hayashi, M.; Honma, M. Miscoding properties of 2′-deoxyinosine, a nitric oxide-derived DNA Adduct, during translesion synthesis catalyzed by human DNA polymerases. J. Mol. Biol. 2008, 377, 1015–1023. [Google Scholar] [CrossRef]





| DNA Lesion. | Mutagenic Property | References |
|---|---|---|
| Cyclobutane pyrimidine dimer (CPD) | Error-free insertion of dATP across TT | [33] |
| Cisplatin intrastrand crosslink GpG | Error-free insertion of dCTP | [38] |
| T:G Mismatch | Error-prone insertion of dTTP | [51] |
| 8-Oxoguanine (8-oxoG) | Error-prone insertion with 3.5:1 ratio of dCTP:dATP | [52] |
| 8-Oxoadenine (8-oxoA) | Error-prone insertion with 2:1 ratio of dTTP:dGTP | [35] |
| 8,5′-cyclo-2′-deoxyadenosine (cdA) | Error-free insertion of dTTP across cdA (Mg2+) | [53] |
| Abasic (AP) site | Purine nucleotide insertion across AP next to dT/dC | [37] |
| 5-N-methylformamidopyrimidine dG | Error-free insertion of dCTP across FapydG | [54] |
| Ribonucleotide insertion | dCTP:rCTP ratio is 1:0.005 across dG | [55] |
| N7-methylguanine (N7mG) | Error-free insertion with 14:1 ratio of dCTP:dTTP | [39] |
| N7-benzylguanine (N7BnG) | Error-free insertion with 10:1 ratio of dCTP:dTTP | [40] |
| O6-methylguanine (O6mG) | Error-prone insertion with 1:1 ratio of dTTP:dCTP | [50] |
| Xanthine (XT) | Error-prone insertion with 3:1 ratio of dCTP:dTTP | [41] |
| Hypoxanthine (HX) | Exclusive error-prone insertion of dCTP | [41] |
| N7-nitrogen half-mustard (NHMG) | Error-free insertion with 10:1 ratio of dCTP:dTTP | [14] |
| Template:dNTP | Km (μM) | kcat (min−1) | kcat/Km (min−1 μM−1) | f a |
|---|---|---|---|---|
| dA:dTTP (Mg2+) | 5.4 ± 0.7 | 109 ± 13 | 20 | 1 |
| cdA:dTTP (Mg2+) | 570 ± 70 | 8.6 ± 0.5 | 0.015 | 6.7 × 10−4 |
| dA:dTTP (Mn2+) | 0.44 ± 0.04 | 82 ± 5 | 186 | 1 |
| cdA:dTTP (Mn2+) | 0.49 ± 0.07 | 10.1 ± 0.2 | 21 | 0.11 |
| Template:dNTP | Km (μM) | kcat (10−3 s−1) | kcat/Km (10−3 s−1 μM−1) | f a |
|---|---|---|---|---|
| dG:dCTP | 2.7 ± 0.3 | 120.6 ± 6.1 | 46 | 1 |
| dG:dTTP | 159.3 ± 2.7 | 74.8 ± 0.9 | 0.5 | 0.01 |
| N7mG:dCTP | 4.3 ± 0.4 | 56.4 ± 2.7 | 13 | 1 |
| N7mG:dTTP | 52.5 ± 1.7 | 49.3 ± 0.1 | 0.9 | 0.07 |
| N7BnG:dCTP | 10.2 ± 2.4 | 20.6 ± 3.6 | 2.1 | 1 |
| N7BnG:dTTP | 51.7 ± 5.3 | 11.5 ± 0.3 | 0.2 | 0.1 |
| N7BnG:dCTP (Mn2+) | 5.6 ± 0.9 | 38.7 ± 4.4 | 6.9 | 1 |
| N7BnG:dTTP (Mn2+) | 18.6 ± 1.9 | 17.8 ± 2.1 | 1.0 | 0.14 |
| NHMG:dCTP | 113.4 ± 1.4 | 40.9 ± 1.3 | 0.36 | 1 |
| NHMG:dTTP | 146.3 ± 5.1 | 5.5 ± 0.1 | 0.037 | 0.1 |
| Template:dNTP | Km (μM) | kcat (10−3 s−1) | kcat/Km (10−3 s−1 μM−1) | f a |
|---|---|---|---|---|
| dG:dCTP | 1.3 ± 0.2 | 1330 ± 50 | 1000 | 1 |
| dG:dATP | 92 ± 23 | 100 ± 10 | 1.1 | 0.001 |
| oxoG:dCTP | 2.3 ± 0.2 | 1200 ± 30 | 520 | 1 |
| oxoG:dATP | 5.4 ± 0.6 | 780 ± 30 | 150 | 0.28 |
| dA:dTTP | 5.4 ± 0.2 | 90.9 ± 5.8 | 17 | 1 |
| dA:dGTP | 76.3 ± 4.8 | 6.3 ± 0.5 | 0.08 | 0.005 |
| oxoA:dTTP | 3.6 ± 0.3 | 37.3 ± 2.3 | 11 | 1 |
| oxoA:dGTP | 4.9 ± 0.3 | 24.8 ± 1.3 | 5.1 | 0.46 |
| oxoA:dTTP (Q38A) | 71.3 ± 4.4 | 172.6 ± 5.6 | 2.4 | 1 |
| oxoA:dGTP (Q38A) | 113.8 ± 2.8 | 10.5 ± 0.1 | 0.092 | 0.037 |
| Template:dNTP | Km (μM) | kcat (10−3 s−1) | kcat/Km (10−3 s−1 μM−1) | f a |
|---|---|---|---|---|
| dG:dCTP | 2.7 ± 0.3 | 120.6 ± 6.1 | 46 | 1 |
| dG:dTTP | 159.3 ± 2.7 | 74.8 ± 0.9 | 0.5 | 0.01 |
| XT:dCTP | 10.7 ± 0.9 | 123.3 ± 3.6 | 11.5 | 1 |
| XT:dTTP | 20.6 ± 0.9 | 82.2 ± 4.2 | 4.0 | 0.34 |
| dA:dTTP | 5.4 ± 0.2 | 90.9 ± 5.8 | 17 | 1 |
| dA:dCTP | 80.3 ± 3.2 | 15.2 ± 2.5 | 0.19 | 0.011 |
| HX:dTTP | 21.9 ± 1.4 | 11.7 ± 0.2 | 0.54 | 1 |
| HX:dCTP | 4.6 ± 0.4 | 170.5 ± 4.1 | 37.4 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H. Contributing Factors for Mutagenic DNA Lesion Bypass by DNA Polymerase Eta (polη). DNA 2022, 2, 205-220. https://doi.org/10.3390/dna2040015
Jung H. Contributing Factors for Mutagenic DNA Lesion Bypass by DNA Polymerase Eta (polη). DNA. 2022; 2(4):205-220. https://doi.org/10.3390/dna2040015
Chicago/Turabian StyleJung, Hunmin. 2022. "Contributing Factors for Mutagenic DNA Lesion Bypass by DNA Polymerase Eta (polη)" DNA 2, no. 4: 205-220. https://doi.org/10.3390/dna2040015
APA StyleJung, H. (2022). Contributing Factors for Mutagenic DNA Lesion Bypass by DNA Polymerase Eta (polη). DNA, 2(4), 205-220. https://doi.org/10.3390/dna2040015