Size- and Stereochemistry-Dependent Transcriptional Bypass of DNA Alkyl Phosphotriester Adducts in Mammalian Cells
Abstract
1. Introduction
2. Experimental Procedures
2.1. Construction of Transcription Templates
2.2. Cellular Transcription, RNA Extraction and Amplification
2.3. Restriction Digestion and Polyacrylamide Gel Electrophoresis (PAGE) Analysis
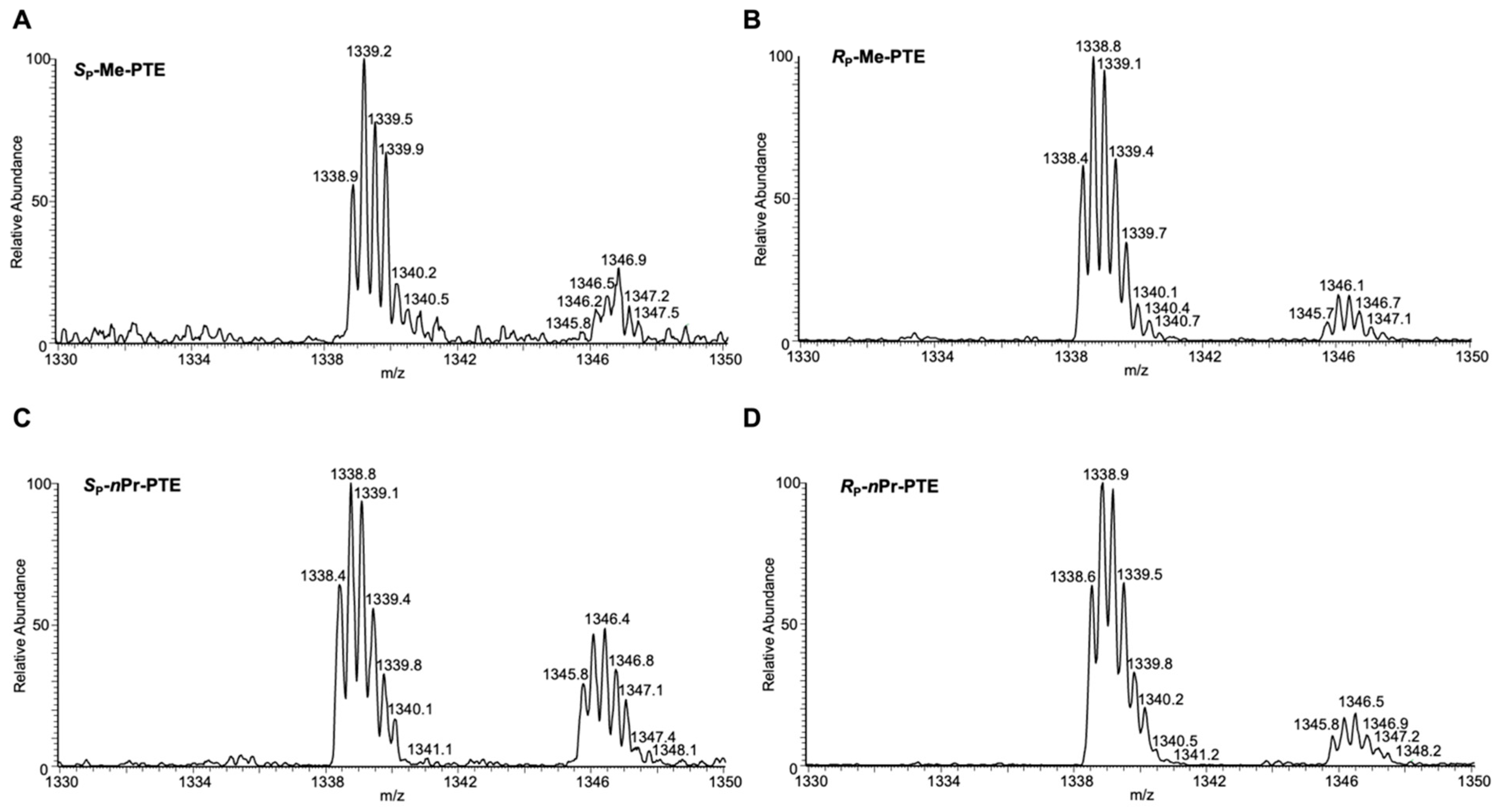
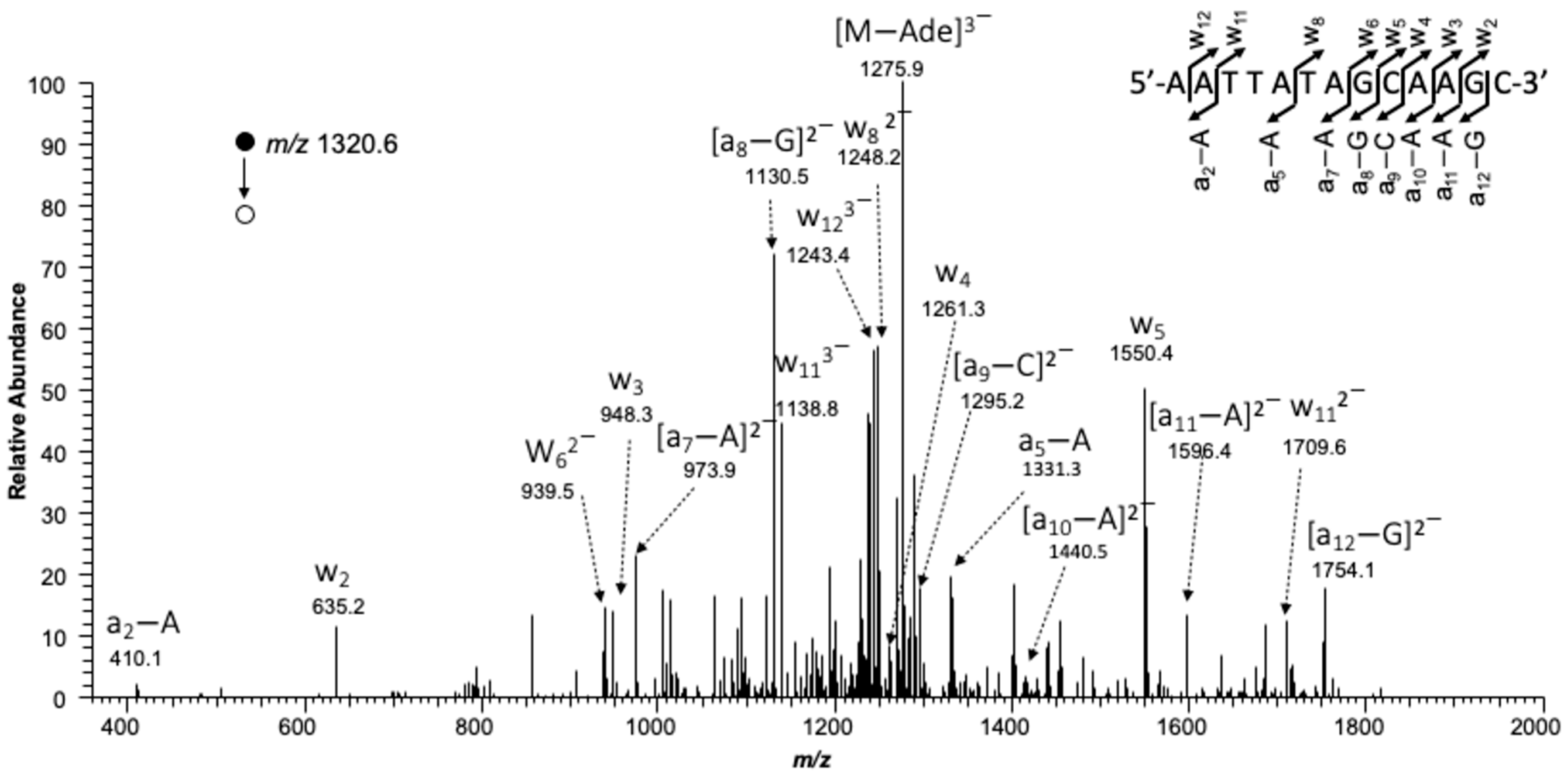
2.4. LC-MS/MS Analysis
3. Results
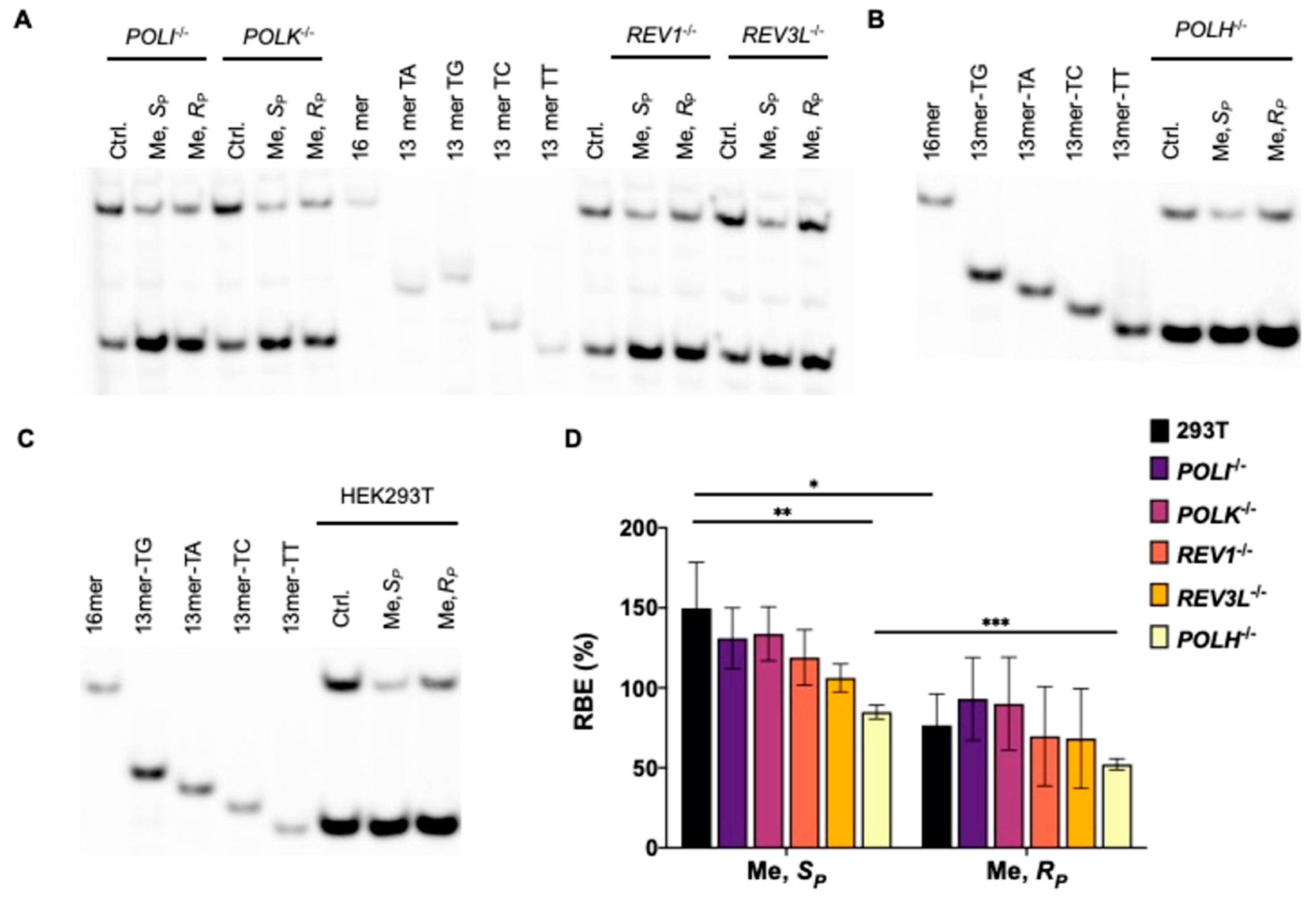
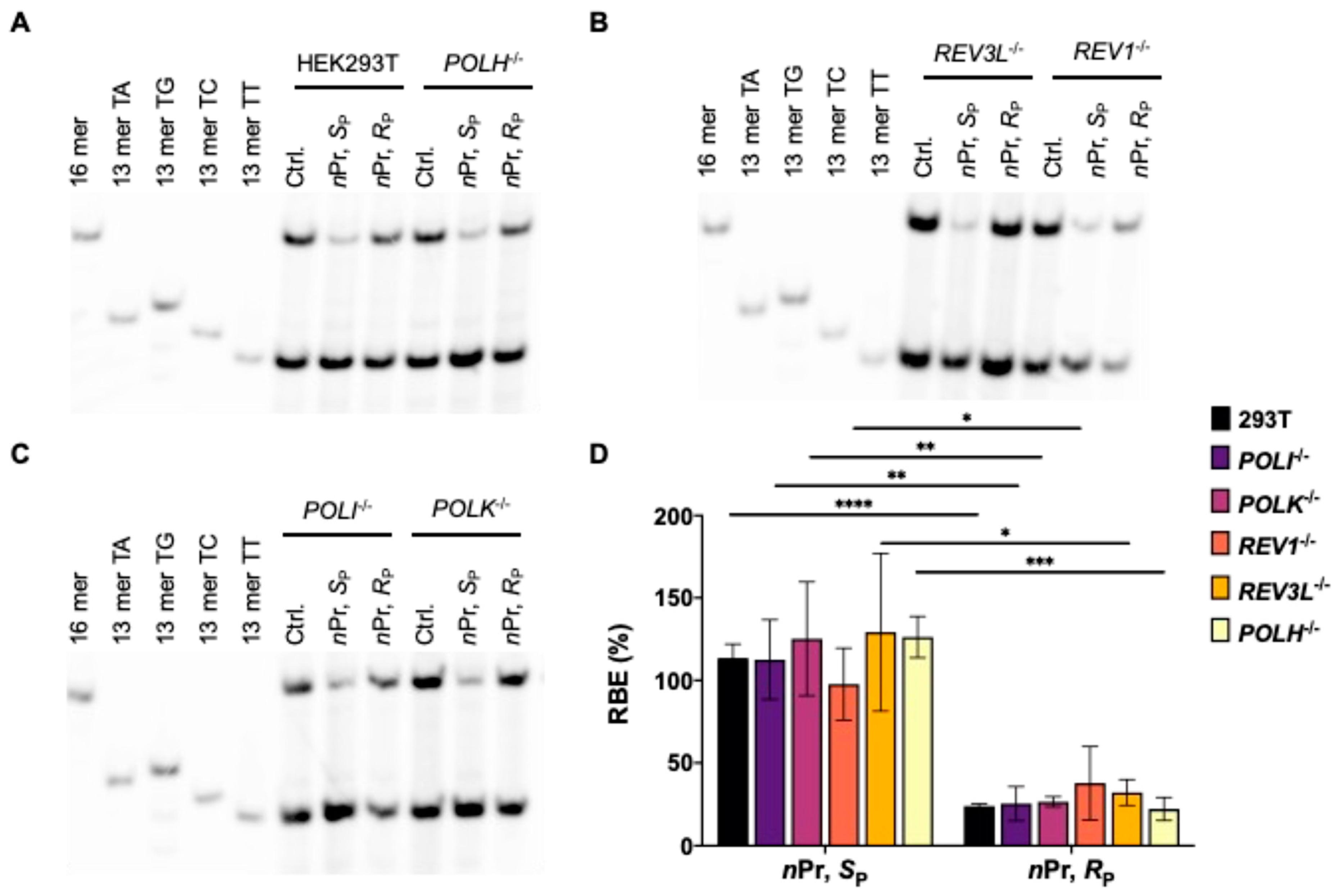
3.1. Effects of Alkyl-PTE Lesions on Transcription in Mammalian Cells
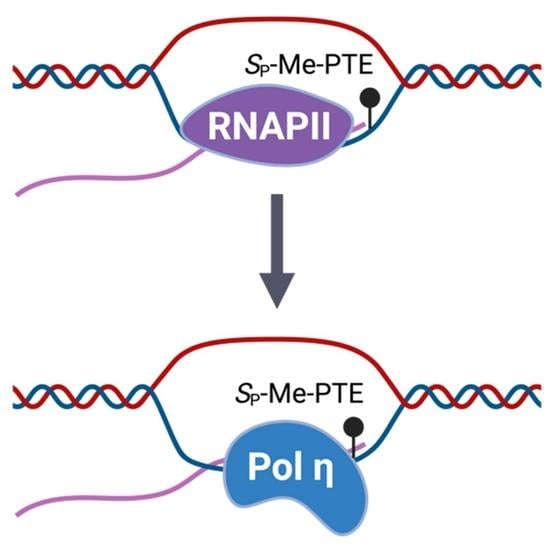
3.2. Pol η Promotes the Transcription of SP-Me-Alkyl-PTE Lesions in Mammalian Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis; ASM Press: Washington, DC, USA, 2006. [Google Scholar]
- Singer, B.; Grunberger, D. Molecular Biology of Mutagens and Carcinogens; Plenum Press: New York, NY, USA; London, UK, 1983. [Google Scholar]
- Fu, D.; Calvo, J.A.; Samson, L.D. Balancing Repair and Tolerance of DNA Damage Caused by Alkylating Agents. Nat. Rev. Cancer 2012, 12, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, B.; Bates, P.A.; Paik, J.; Jacobs, S.C.; Lindahl, T. Repair of Alkylated DNA: Recent Advances. DNA Repair Amst. 2007, 6, 429–442. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA Repair Pathways as Targets for Cancer Therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Gerson, S.L. MGMT: Its Role in Cancer Aetiology and Cancer Therapeutics. Nat. Rev. Cancer 2004, 4, 296–307. [Google Scholar] [CrossRef]
- Albertella, M.R.; Green, C.M.; Lehmann, A.R.; O’Connor, M.J. A Role for Polymerase Eta in the Cellular Tolerance to Cisplatin-Induced Damage. Cancer Res. 2005, 65, 9799–9806. [Google Scholar] [CrossRef]
- Doles, J.; Oliver, T.G.; Cameron, E.R.; Hsu, G.; Jacks, T.; Walker, G.C.; Hemann, M.T. Suppression of Rev3, the Catalytic Subunit of Polz, Sensitizes Drug-Resistant Lung Tumors to Chemotherapy. Proc. Natl. Acad. Sci. USA 2010, 107, 20786–20791. [Google Scholar] [CrossRef]
- Xie, K.; Doles, J.; Hemann, M.T.; Walker, G.C. Error-Prone Translesion Synthesis Mediates Acquired Chemoresistance. Proc. Natl. Acad. Sci. USA 2010, 107, 20792–20797. [Google Scholar] [CrossRef]
- Ma, B.; Zarth, A.T.; Carlson, E.S.; Villalta, P.W.; Upadhyaya, P.; Stepanov, I.; Hecht, S.S. Methyl DNA Phosphate Adduct Formation in Rats Treated Chronically with 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone and Enantiomers of Its Metabolite 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol. Chem. Res. Toxicol. 2018, 31, 48–57. [Google Scholar] [CrossRef]
- Jones, G.D.; Le Pla, R.C.; Farmer, P.B. Phosphotriester Adducts (PTEs): DNA’s Overlooked Lesion. Mutagenesis 2010, 25, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Beranek, D.T. Distribution of Methyl and Ethyl Adducts Following Alkylation with Monofunctional Alkylating Agents. Mutat. Res. 1990, 231, 11–30. [Google Scholar] [PubMed]
- Ma, B.; Zarth, A.T.; Carlson, E.S.; Villalta, P.W.; Upadhyaya, P.; Stepanov, I.; Hecht, S.S. Identification of More than One Hundred Structurally Unique DNA-Phosphate Adducts Formed during Rat Lung Carcinogenesis by the Tobacco-Specific Nitrosamine 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone. Carcinogenesis 2017, 39, 232–241. [Google Scholar] [CrossRef]
- Den Engelse, L.; De Graaf, A.; De Brij, R.J.; Menkveld, G.J. O2- and O4-Ethylthymine and the Ethylphosphotriester DTp(Et)DT Are Highly Persistent DNA Modifications in Slowly Dividing Tissues of the Ethylnitrosourea-Treated Rat. Carcinogenesis 1987, 8, 751–757. [Google Scholar] [CrossRef]
- Shooter, K.V.; Slade, T.A. The Stability of Methyl and Ethyl Phosphotriesters in DNA in Vivo. Chem. Biol. Interact. 1977, 19, 353–361. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Aguilera, A.; Gellert, M.; Hanawalt, P.C.; Hays, J.B.; Lehmann, A.R.; Lindahl, T.; Lowndes, N.; Sarasin, A.; Wood, R.D. DNA Repair: From Molecular Mechanism to Human Disease. DNA Repair Amst. 2006, 5, 986–996. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, S.; Wu, J.; Du, H.; Li, L.; You, C.; Wang, Y. DNA Polymerase η Promotes the Transcriptional Bypass of N2-Alkyl-2′-Deoxyguanosine Adducts in Human Cells. J. Am. Chem. Soc. 2021, 143, 16197–16205. [Google Scholar] [CrossRef]
- Wu, J.; Wang, P.; Wang, Y. Cytotoxic and Mutagenic Properties of Alkyl Phosphotriester Lesions in Escherichia Coli Cells. Nucleic Acids Res. 2018, 46, 4013–4021. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Wang, P.; You, C.; Williams, N.L.; Wang, Y. Translesion Synthesis of O4-Alkylthymidine Lesions in Human Cells. Nucleic Acids Res. 2016, 44, 9256–9265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, J.; Du, H.; Li, L.; Price, N.E.; Liu, X.; Wang, Y. The Impact of Minor-Groove N2-Alkyl-2′-Deoxyguanosine Lesions on DNA Replication in Human Cells. ACS Chem. Biol. 2019, 14, 1708–1716. [Google Scholar] [CrossRef]
- You, C.; Wang, Y. Quantitative Measurement of Transcriptional Inhibition and Mutagenesis Induced by Site-Specifically Incorporated DNA Lesions in Vitro and in Vivo. Nat. Protoc. 2015, 10, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; You, C.; Park, J.; Kim, H.S.; Guo, S.; Schärer, O.D.; Wang, Y. Transcriptional Perturbations of 2,6-Diaminopurine and 2-Aminopurine. ACS Chem. Biol. 2022, 17, 1672–1676. [Google Scholar] [CrossRef]
- Bregeon, D.; Doetsch, P.W. Transcriptional Mutagenesis: Causes and Involvement in Tumour Development. Nat. Rev. Cancer 2011, 11, 218–227. [Google Scholar] [CrossRef]
- Saxowsky, T.T.; Doetsch, P.W. RNA Polymerase Encounters with DNA Damage: Transcription-Coupled Repair or Transcriptional Mutagenesis? Chem. Rev. 2006, 106, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Morreall, J.F.; Petrova, L.; Doetsch, P.W. Transcriptional Mutagenesis and Its Potential Roles in the Etiology of Cancer and Bacterial Antibiotic Resistance. J. Cell. Physiol. 2013, 228, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y. Replication of Pyridyloxobutyl Phosphotriester Lesions in Cells. Chem. Res. Toxicol. 2020, 33, 308–311. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, J.; Price, N.E.; Wang, Y. Ada Protein- and Sequence Context-Dependent Mutagenesis of Alkyl Phosphotriester Lesions in Escherichia Coli Cells. J. Biol. Chem. 2020, 295, 8775–8783. [Google Scholar] [CrossRef] [PubMed]
- Koike, G.; Maki, H.; Takeya, H.; Hayakawa, H.; Sekiguchi, M. Purification, Structure, and Biochemical Properties of Human O6-Methylguanine-DNA Methyltransferase. J. Biol. Chem. 1990, 265, 14754–14762. [Google Scholar] [CrossRef]
- Viswanathan, A.; Doetsch, P.W. Effects of Nonbulky DNA Base Damages on Escherichia Coli RNA Polymerase-Mediated Elongation and Promoter Clearance. J. Biol. Chem. 1998, 273, 21276–21281. [Google Scholar] [CrossRef] [PubMed]
- Saxowsky, T.T.; Meadows, K.L.; Klungland, A.; Doetsch, P.W. 8-Oxoguanine-Mediated Transcriptional Mutagenesis Causes Ras Activation in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 18877–18882. [Google Scholar] [CrossRef]
- Lans, H.; Hoeijmakers, J.H.J.; Vermeulen, W.; Marteijn, J.A. The DNA Damage Response to Transcription Stress. Nat. Rev. Mol. Cell Biol. 2019, 20, 766–784. [Google Scholar] [CrossRef]
- Tornaletti, S.; Maeda, L.S.; Lloyd, D.R.; Reines, D.; Hanawalt, P.C. Effect of Thymine Glycol on Transcription Elongation by T7 RNA Polymerase and Mammalian RNA Polymerase II. J. Biol. Chem. 2001, 276, 45367–45371. [Google Scholar] [CrossRef]
- Charlet-Berguerand, N.; Feuerhahn, S.; Kong, S.E.; Ziserman, H.; Conaway, J.W.; Conaway, R.; Egly, J.M. RNA Polymerase II Bypass of Oxidative DNA Damage Is Regulated by Transcription Elongation Factors. EMBO J. 2006, 25, 5481–5491. [Google Scholar] [CrossRef] [PubMed]
- Clauson, C.L.; Oestreich, K.J.; Austin, J.W.; Doetsch, P.W. Abasic Sites and Strand Breaks in DNA Cause Transcriptional Mutagenesis in Escherichia Coli. Proc. Natl. Acad. Sci. USA 2010, 107, 3657–3662. [Google Scholar] [CrossRef]
- Tornaletti, S.; Reines, D.; Hanawalt, P.C. Structural Characterization of RNA Polymerase II Complexes Arrested by a Cyclobutane Pyrimidine Dimer in the Transcribed Strand of Template DNA. J. Biol. Chem. 1999, 274, 24124–24130. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.C.; Lippard, S.J. Inhibition of Transcription by Platinum Antitumor Compounds. Metallomics 2009, 1, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, M.; Masutani, C.; Eki, T.; Hanaoka, F. Genomic Structure, Chromosomal Localization and Identification of Mutations in the Xeroderma Pigmentosum Variant (XPV) Gene. Oncogene 2000, 19, 4721–4728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biertümpfel, C.; Zhao, Y.; Kondo, Y.; Ramón-Maiques, S.; Gregory, M.; Lee, J.Y.; Masutani, C.; Lehmann, A.R.; Hanaoka, F.; Yang, W. Structure and Mechanism of Human DNA Polymerase η. Nature 2010, 465, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Egli, M.; Guengerich, F.P. Mechanism of Ribonucleotide Incorporation by Human DNA Polymerase η. J. Biol. Chem. 2016, 291, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Egli, M.; Guengerich, F.P. Human DNA Polymerase η Accommodates RNA for Strand Extension. J. Biol. Chem. 2017, 292, 18044–18051. [Google Scholar] [CrossRef]
- Mentegari, E.; Crespan, E.; Bavagnoli, L.; Kissova, M.; Bertoletti, F.; Sabbioneda, S.; Imhof, R.; Sturla, S.J.; Nilforoushan, A.; Hübscher, U.; et al. Ribonucleotide Incorporation by Human DNA Polymerase η Impacts Translesion Synthesis and RNase H2 Activity. Nucleic Acids Res. 2017, 45, 2600–2614. [Google Scholar] [CrossRef] [PubMed]
- Meroni, A.; Nava, G.M.; Bianco, E.; Grasso, L.; Galati, E.; Bosio, M.C.; Delmastro, D.; Muzi-Falconi, M.; Lazzaro, F. RNase H Activities Counteract a Toxic Effect of Polymerase η in Cells Replicating with Depleted DNTP Pools. Nucleic Acids Res. 2019, 47, 4612–4623. [Google Scholar] [CrossRef]
- Gali, V.K.; Balint, E.; Serbyn, N.; Frittmann, O.; Stutz, F.; Unk, I. Translesion Synthesis DNA Polymerase η Exhibits a Specific RNA Extension Activity and a Transcription-Associated Function. Sci. Rep. 2017, 7, 13055. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Belluscio, L.; van Cappellen, W.A.; Kanaar, R.; Essers, J.; Gottifredi, V. DNA Damage Induced Pol η Recruitment Takes Place Independently of the Cell Cycle Phase. Cell Cycle 2009, 8, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Crespan, E.; Furrer, A.; Rösinger, M.; Bertoletti, F.; Mentegari, E.; Chiapparini, G.; Imhof, R.; Ziegler, N.; Sturla, S.J.; Hübscher, U.; et al. Impact of Ribonucleotide Incorporation by DNA Polymerases β and λ on Oxidative Base Excision Repair. Nat. Commun. 2016, 7, 10805. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Wu, J.; Clabaugh, G.; Li, L.; Du, H.; Wang, Y. Size- and Stereochemistry-Dependent Transcriptional Bypass of DNA Alkyl Phosphotriester Adducts in Mammalian Cells. DNA 2022, 2, 221-230. https://doi.org/10.3390/dna2040016
Tan Y, Wu J, Clabaugh G, Li L, Du H, Wang Y. Size- and Stereochemistry-Dependent Transcriptional Bypass of DNA Alkyl Phosphotriester Adducts in Mammalian Cells. DNA. 2022; 2(4):221-230. https://doi.org/10.3390/dna2040016
Chicago/Turabian StyleTan, Ying, Jiabin Wu, Garrit Clabaugh, Lin Li, Hua Du, and Yinsheng Wang. 2022. "Size- and Stereochemistry-Dependent Transcriptional Bypass of DNA Alkyl Phosphotriester Adducts in Mammalian Cells" DNA 2, no. 4: 221-230. https://doi.org/10.3390/dna2040016
APA StyleTan, Y., Wu, J., Clabaugh, G., Li, L., Du, H., & Wang, Y. (2022). Size- and Stereochemistry-Dependent Transcriptional Bypass of DNA Alkyl Phosphotriester Adducts in Mammalian Cells. DNA, 2(4), 221-230. https://doi.org/10.3390/dna2040016