Characterization of Microplastics and Mesoplastics and Presence of Biofilms, Collected in the Gualí Wetland Cundinamarca, Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Observation and Identification of Microplastics and Mesoplastics
2.3. Detecting Biofilms in Microplastics and Mesoplastics (Determining Their Presence)
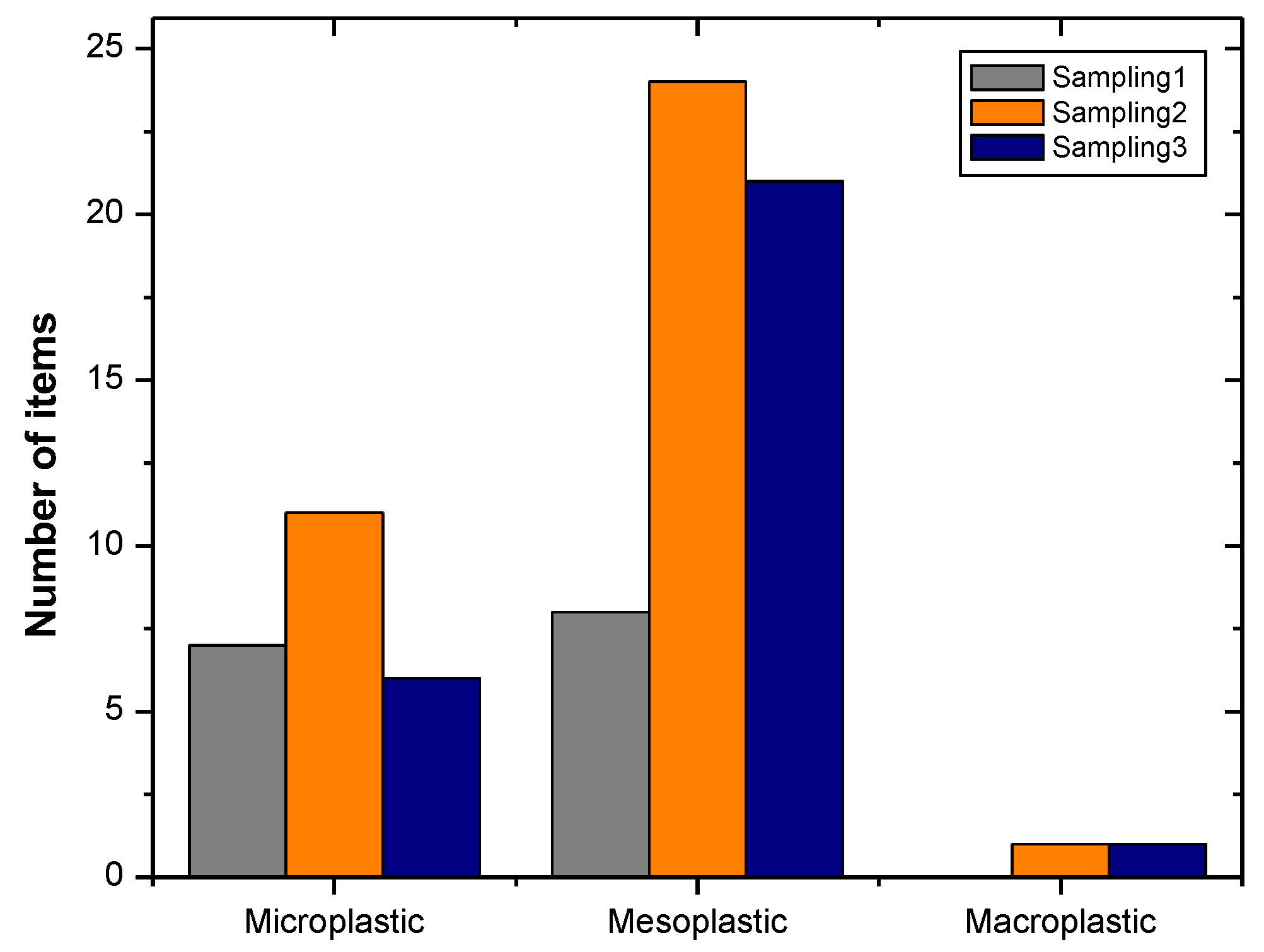
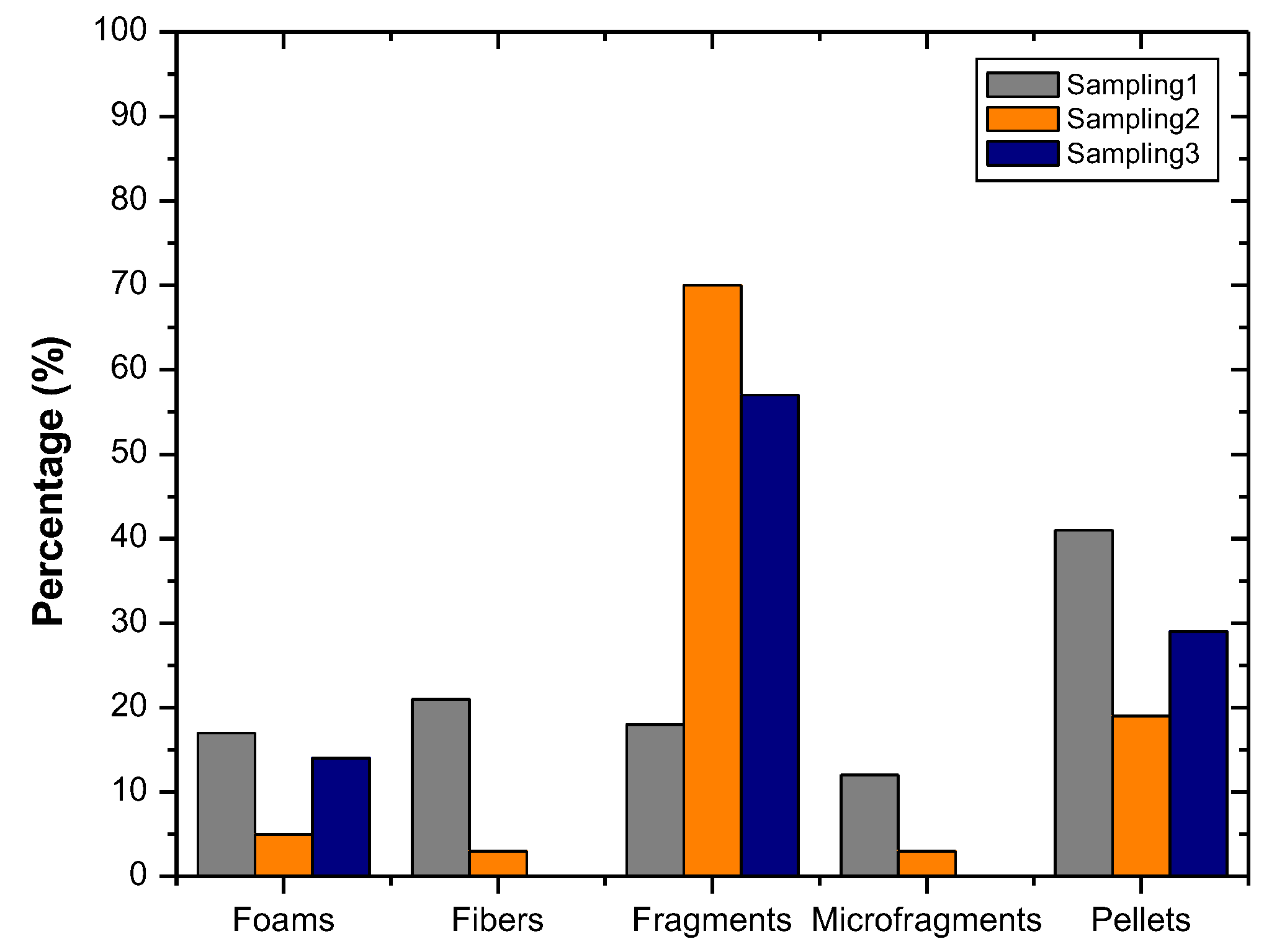
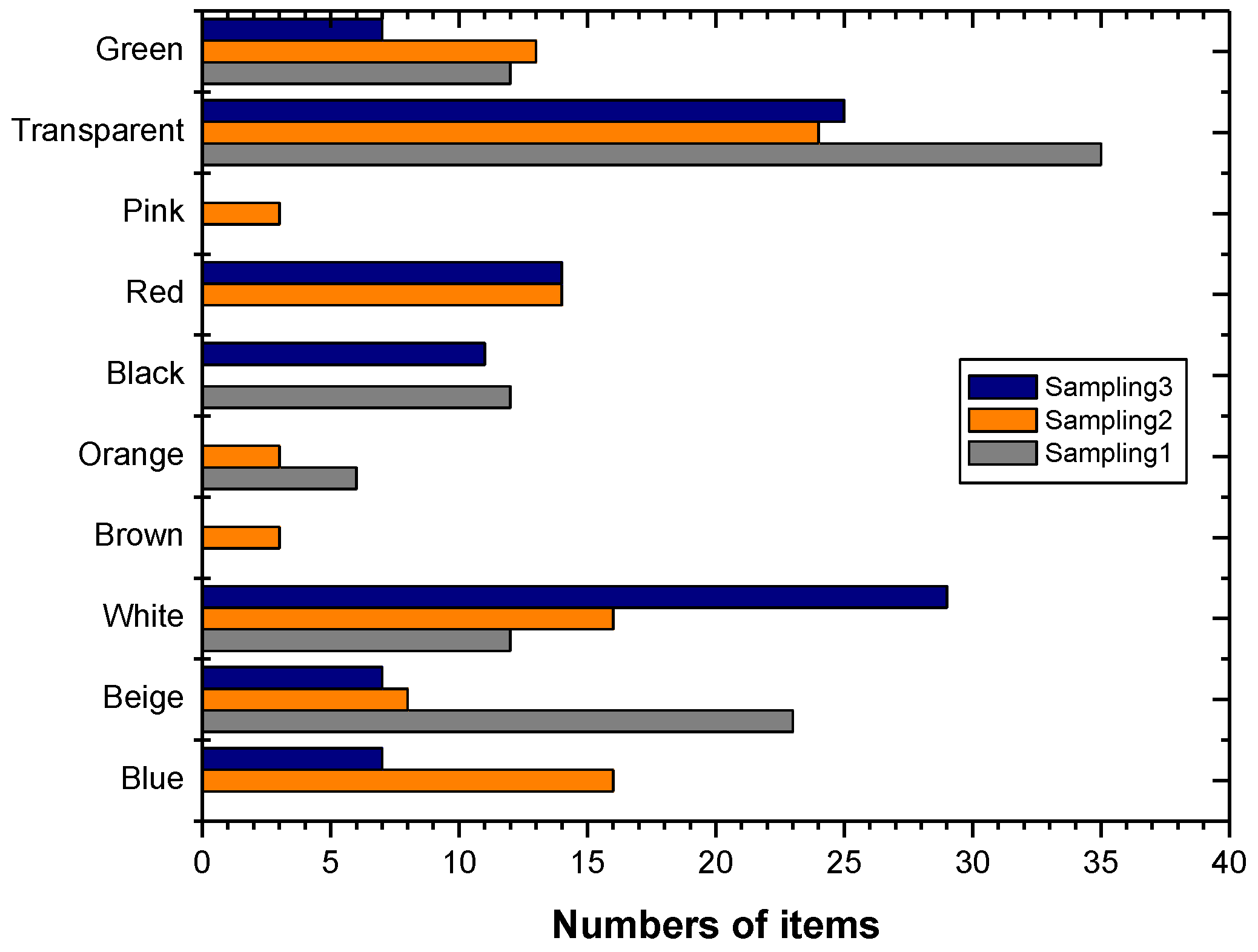
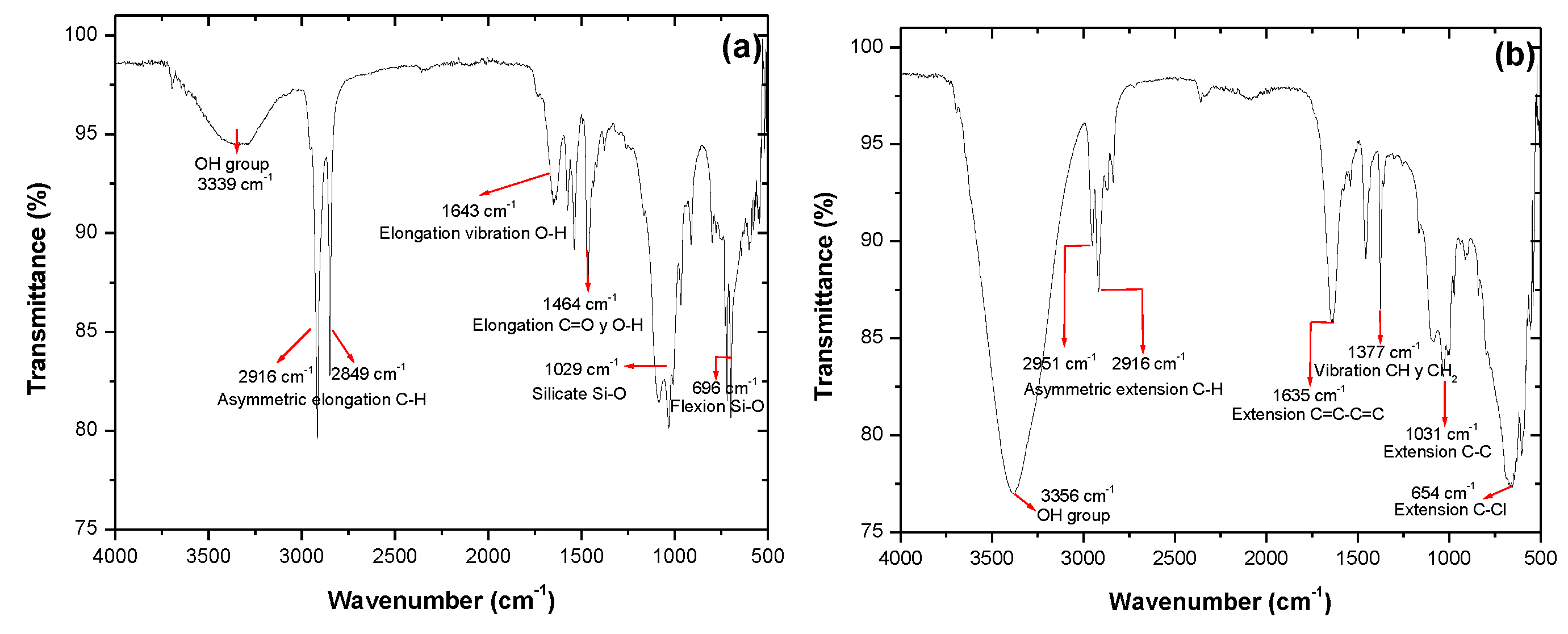
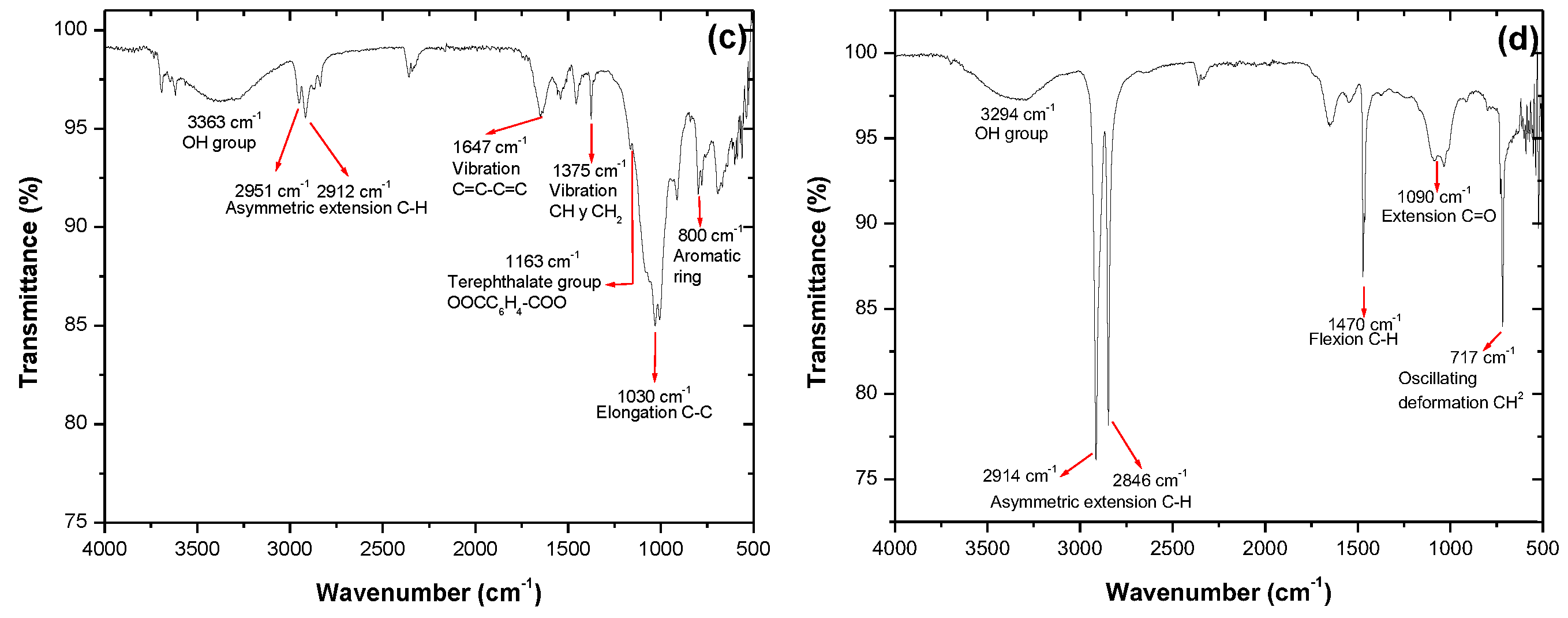
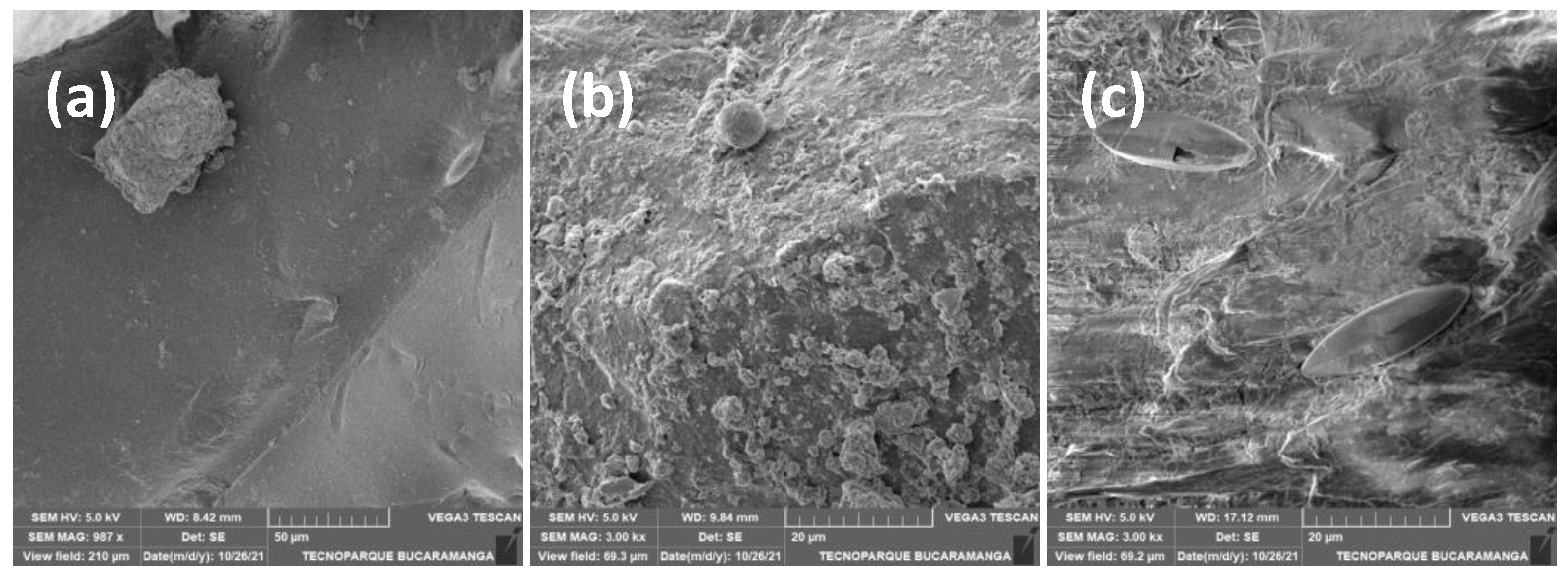
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peck, D. The Ramsar Convention Manual: A Guide to the Convention on Wetlands (Ramsar, Iran, 1971); Ramsar Convention Bureau: Gland, Switzerland, 1997. [Google Scholar]
- Estupiñán-Torres, S.; Cepeda, A.; Hurtado, A.; Vega, K. Calidad bacteriológica del agua del humedal Gualí-Tres Esquinas, Funza, Cundinamarca. Nova 2020, 18, 113–122. [Google Scholar] [CrossRef]
- Patiño, J.E.; Estupiñán-Suarez, L.M. Hotspots of Wetland Area Loss in Colombia. Wetlands 2016, 36, 935–943. [Google Scholar] [CrossRef]
- Rodriguez-Linares, J.C.; Chaparro-Herrera, S.; Sua-Becerra, A.; Echeverry-Galvis, M.A. Estado poblacional del cucarachero de pantano, Cistothorus apolinari (Passeriformes: Troglodytidae) en siete humedales de la Sabana de Bogotá, Colombia. Rev. Biol. Trop. 2019, 67, 1257–1268. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Castillo, V.; Rueda, R.; Ríos, M.; Bayona, M.; Molina, F.; Escobar, M. Diagnóstico de residuos microplásticos en las zonas marinas de Colombia. INVEMAR MinAmbiente 2017, 108–166. [Google Scholar]
- CAR, C.A. Humedales del Territorio CAR. Consolidación de los Humedales de jurisdicción CAR; Imprenta Nacional de Colombia: Bogotá, Colombia, 2011; pp. 15–18. [Google Scholar]
- Flórez, C.; Estupiñán-Suárez, L.M.; Rojas, S.; Aponte, C.; Quiñones, M.; Acevedo, Ó.; Vilardy, S.; Jaramilo, Ú. Identificación espacial de los sistemas de humedales continentales de Colombia. Biota Colomb. 2016, 17, 44–62. [Google Scholar]
- Li, W.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of source, occurrence, and effects. Sci. Total Environ. 2016, 566, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.P.; Tsang, C.F.; Li, L.; Yu, R.M.K.; Kong, R.Y.C. Microplastics act as a carrier for wastewater-borne pathogenic bacteria in sewage. Chemosphere 2022, 301, 134692. [Google Scholar] [CrossRef]
- Wu, X.; Hou, H.; Liu, Y.; Yin, S.; Bian, S.; Liang, S.; Wan, C.; Yuan, S.; Xiao, K.; Liu, B.; et al. Microplastics affect rice (Oryza sativa L.) quality by interfering metabolite accumulation and energy expenditure pathways: A field study. J. Hazard. Mater. 2022, 422, 126834. [Google Scholar] [CrossRef] [PubMed]
- Pisani, X.G.; Lompré, J.S.; Pires, A.; Greco, L.L. Plastics in scene: A review of the effect of plastics in aquatic crustaceans. Environ. Res. 2022, 212, 113484. [Google Scholar] [CrossRef]
- Hanif, M.A.; Ibrahim, N.; Dahalan, F.A.; Ali, U.F.M.; Hasan, M.; Jalil, A.A. Microplastics and nanoplastics: Recent literature studies and patents on their removal from aqueous environment. Sci. Total Environ. 2022, 810, 152115. [Google Scholar] [CrossRef] [PubMed]
- Kniggendorf, A.K.; Wetzel, C.; Roth, B. Microplastics detection in streaming tap water with Raman spectroscopy. J. Sens. 2019, 19, 1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.; Arkkakadavil Valsalan, S. Presence of Microplastics in Freshwater Ecosystems: An Unheeded Emerging Concern—A Global Review. Pollution 2022, 8, 69–104. [Google Scholar]
- Battulga, B.; Kawahigashi, M.; Oyuntsetseg, B. Characterization of biofilms formed on polystyrene microplastics (PS-MPs) on the shore of the Tuul River, Mongolia. Environ. Res. 2022, 212, 113329. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, B.; Zou, L.; Xu, F.; Wang, Y.; Xin, S.; Wang, Y.; Zhang, H.; Ding, N.; Wang, R. Comparative analysis of selective bacterial bolonization by polyethylene and polyethylene terephthalate microplastics. Front. Microbiol. 2022, 13, 836052. [Google Scholar] [CrossRef]
- Rosselli, L.; Stiles, F. Wetland habitats of the Sabana de Bogotá Andean Highland Plateau and their birds. Aquat. Conserv. 2012, 22, 303–317. [Google Scholar] [CrossRef]
- Ramírez, A. Métodos de recolección. Int. J. Trop. Biol. 2010, 58, 41–50. [Google Scholar]
- Feng, S.; Lu, H.; Yao, T.; Xue, Y.; Yin, C.; Tang, M. Spatial characteristics of microplastics in the high-altitude area on the Tibetan Plateau. J. Hazard. Mater. 2021, 417, 126034. [Google Scholar] [CrossRef]
- Beckingham, B.; Apintiloaiei, A.; Moore, C.; Brandes, J. Hot or not: Systematic review and laboratory evaluation of the hot needle test for microplastic identification. Microplast. Nanoplast. 2023, 3, 8. [Google Scholar] [CrossRef]
- Crawford, C.; Quinn, B. Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; Volume 10, pp. 219–267. [Google Scholar]
- Gómez-Méndez, L.D.; Moreno-Bedoya, D.A.; Poutou-Piñales, R.A.; Salcedo-Reyes, J.C.; Pedroza-Rodríguez, A.M.; Vargas, A.; Bogoyá, J. Biodeterioration of plasma pretreated LDPE sheets by Pleurotus ostreatus. PLoS ONE 2018, 13, e0203786. [Google Scholar] [CrossRef] [Green Version]
- Dehaut, A.; Hermabessiere, L.; Duflos, G. Current frontiers and recommendations for the study of microplastics in seafood. Trends Anal. Chem. 2019, 116, 346–359. [Google Scholar] [CrossRef]
- INVEMAR—Instituto de Investigaciones Marinas y Costeras. Protocolo de Muestreo Y análisis de microplásticos en Aguas Marinas Superficiales, Sedimentos de Playas Y Tracto Digestivo de Peces. Componente 5: Diagnóstico de Microplásticos en Zonas Costeras de Colombia. Resolución 646 MinAmbiente; INVEMAR: Santa Marta, Colombia, 2017; 21p. [Google Scholar]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Miao, L.; Jou, J.; Young, G.; Liu, Z.; Liu, S.; Li, T.; Mo, Y.; Guo, S.; Qu, H. Acute effects of nanoplastics and microplastics on periphytic biofilms depending on particle size, concentration, and surface modification. Environ. Pollut. 2019, 255, 113300. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Marisa, R.; Sáez-Zamacona, I.; Silva, D.; Rodrigues, S.; Pereira, R.; Ramos, S. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water 2023, 15, 1382. [Google Scholar] [CrossRef]
- Xia, F.; Liu, H.; Zhang, J.; Wang, D. Migration characteristics of microplastics based on source-sink investigation in a typical urban wetland. Water Res. 2022, 213, 118154. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Drapper, D.; Hornbuckle, A.; Rintoul, L.; Leusch, F.D.L. Microplastic pollution in a stormwater floating treatment wetland: Detection of tyre particles in sediment. Sci. Total Environ. 2020, 713, 136356. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.; Cheng-Hsuan, L.; Sharley, D.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Zellers, A.; Amato, S. Microplastic pollution in the Surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, P.; Norris, T.; Ceccanese, T.; Walzak, M.; Helm, P.; Marvin, C. Hidden plastics of Humber Bay, Canada, and their potential preservation in the sediment record. Environ. Pollut. 2015, 204, 17–25. [Google Scholar] [CrossRef]
- Free, C.; Jensen, O.; Mason, A.; Eriksen, M.; Williamson, N.; Boldgiv, B. High levels of microplastic pollution in a large, remote mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Wang, Z.; Wu, C. Microplastics in freshwater sediment; A review on methods, occurrence, sources. Sci. Total Environ. 2020, 754, 141948. [Google Scholar]
- Barboza, L.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from Northeast Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Bray, L.; Digka, N.; Tsangaris, C.; Camedda, A.; Gambaiani, D.; Giuseppe, L.; Matiddi, M.; Miaud, C.; Palazzo, L.; Olmo, A.; et al. Determining suitable fish to monitor plastic ingestion trends in the Mediterranean Sea. Environ. Pollut. 2019, 247, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, X.; Lingchen, L.; Chen, L.; Teng, J.; Zhu, X.; Zhao, J.; Wang, Q. Spatial and seasonal variations in biofilm formation on microplastics in coastal waters. Sci. Total Environ. 2021, 770, 145303. [Google Scholar] [CrossRef]
- Pereira, A.P.D.S.; Silva, M.H.P.D.; Lima, É.P.; Paula, A.D.S.; Tommasini, F.J. Processing and characterization of PET composites reinforced with geopolymer concrete waste. Mater. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Scopetani, C.; Chelazzi, D.; Martellini, T.; Pellinen, J.; Ugolini, A.; Sarti, C.; Cinicinelli, A. Occurrence and characterization of microplastic and mesoplastic pollution in the Migliarino San Rossore, Massaciuccoli, Nature Park (Italy). Mar. Pollut. Bull. 2021, 171, 112712. [Google Scholar] [CrossRef]
- Ballent, A.; Cocoran, P.; Madden, O.; Longstaffe, F. Sources and Sinks of Microplastics in Canadian Lake Ontario Nearshore, tributary and beach sediments. Earth Sci. Publ. 2016, 110, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Sadri, S.; Thompson, R. On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England. Mar. Pollut. Bull. 2014, 81, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Jiwarungrueangkul, T.; Phaksopa, J.; Sompongchaiyakul, P.; Tipmanee, D. Seasonal microplastic variations in estuarine sediments from urban canals on the west coast of Thailand: A case study in Phuket province. Mar. Pollut. Bull. 2021, 168, 112452. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, T.; Monisha, V.; Sivanesan, S.; Vasanthy, M.; Omine, K.; Sivasankar, V. Micro-plastic pollution along the Bay of Bengal coastal strech of Tamil Nadu, South India. Sci. Total Environ. 2020, 756, 144073. [Google Scholar] [CrossRef] [PubMed]




| Point | Sampling 1 (28 March 2021) | Sampling 2 (25 June 2021) | Sampling 3 (9 October 2021) | Total |
|---|---|---|---|---|
| Point 1 | 5 | 24 | 11 | 40 |
| Point 2 | 2 | 3 | 3 | 8 |
| Point 3 | 8 | 9 | 14 | 31 |
| Total | 15 | 36 | 28 | 79 |
| Identification | Polymer | Classification (Size) | Absorbance (595 nm) |
|---|---|---|---|
| 1 | PE + Cl | mesoplastic | 1.901 |
| 2 | PET | mesoplastic | 0.894 |
| 3 | HDPE | mesoplastic | 1.019 |
| 4 | CPVC | microplastic | 0.572 |
| 5 | PE | microplastic | 0.522 |
| 6 | EPS | mesoplastic | 2.276 |
| 7 | HDPE | microplastic | 0.541 |
| 8 | PPS | microplastic | 0.451 |
| 9 | HDPE | mesoplastic | 0.977 |
| 10 | HDPE | microplastic | 0.442 |
| 11 | PE + Cl | mesoplastic | 2.098 |
| 12 | EPS | mesoplastic | 1.342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porras-Rojas, M.A.; Charry-Vargas, C.; Muñoz-Yustres, J.L.; Martínez-Silva, P.; Gómez-Méndez, L.D. Characterization of Microplastics and Mesoplastics and Presence of Biofilms, Collected in the Gualí Wetland Cundinamarca, Colombia. Microplastics 2023, 2, 255-267. https://doi.org/10.3390/microplastics2030021
Porras-Rojas MA, Charry-Vargas C, Muñoz-Yustres JL, Martínez-Silva P, Gómez-Méndez LD. Characterization of Microplastics and Mesoplastics and Presence of Biofilms, Collected in the Gualí Wetland Cundinamarca, Colombia. Microplastics. 2023; 2(3):255-267. https://doi.org/10.3390/microplastics2030021
Chicago/Turabian StylePorras-Rojas, Maria Alejandra, Cristina Charry-Vargas, Jorge Leonardo Muñoz-Yustres, Paula Martínez-Silva, and Luis David Gómez-Méndez. 2023. "Characterization of Microplastics and Mesoplastics and Presence of Biofilms, Collected in the Gualí Wetland Cundinamarca, Colombia" Microplastics 2, no. 3: 255-267. https://doi.org/10.3390/microplastics2030021
APA StylePorras-Rojas, M. A., Charry-Vargas, C., Muñoz-Yustres, J. L., Martínez-Silva, P., & Gómez-Méndez, L. D. (2023). Characterization of Microplastics and Mesoplastics and Presence of Biofilms, Collected in the Gualí Wetland Cundinamarca, Colombia. Microplastics, 2(3), 255-267. https://doi.org/10.3390/microplastics2030021







