Microplastic Migration from Food Packaging on Cheese
Abstract
1. Introduction
2. Materials and Methods
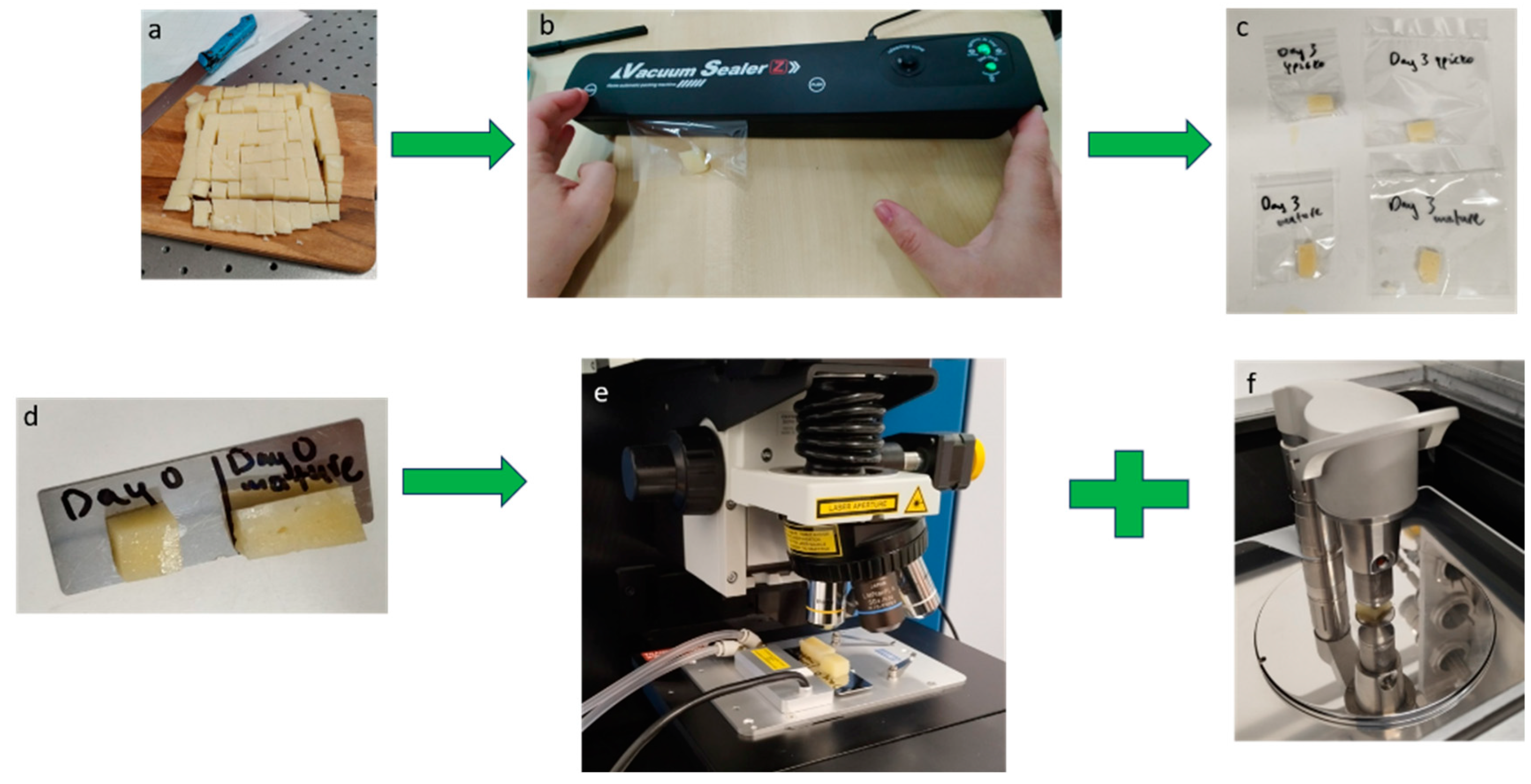
2.1. Samples and Experiment Preparation
- Cheese is cut into large slices from the “cheese head” when a specific weight is requested by the consumer, or for pre-cut slices to the shelf.
- The cheese slice is placed inside plastic containers, either LDPE or PP bags or boxes.
- For longer storage times, vacuum sealing is used, where the air is removed between the interface of the cheese surface and the packaging surface. This brings the two surfaces in strong contact.
- The packaged cheese slices are then stored in refrigerated conditions for longer preservation (2–3 weeks).
2.2. Data Acquisition
2.2.1. Optical Microscopy and Raman Spectroscopy
2.2.2. FTIR-ATR Experiments
2.3. Spectral Processing and Analysis
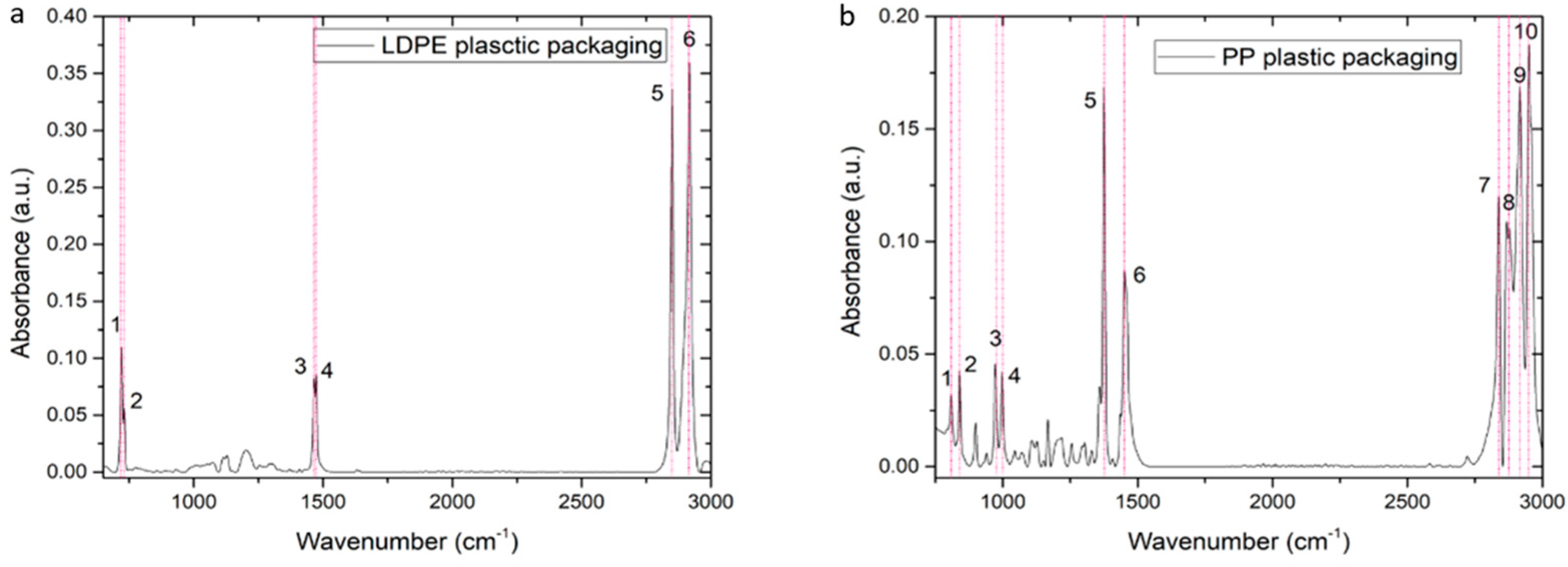
2.4. Spectral Reference Data and Analysis
2.4.1. Raman Spectral References
2.4.2. FTIR-ATR Spectral References
3. Results and Discussion
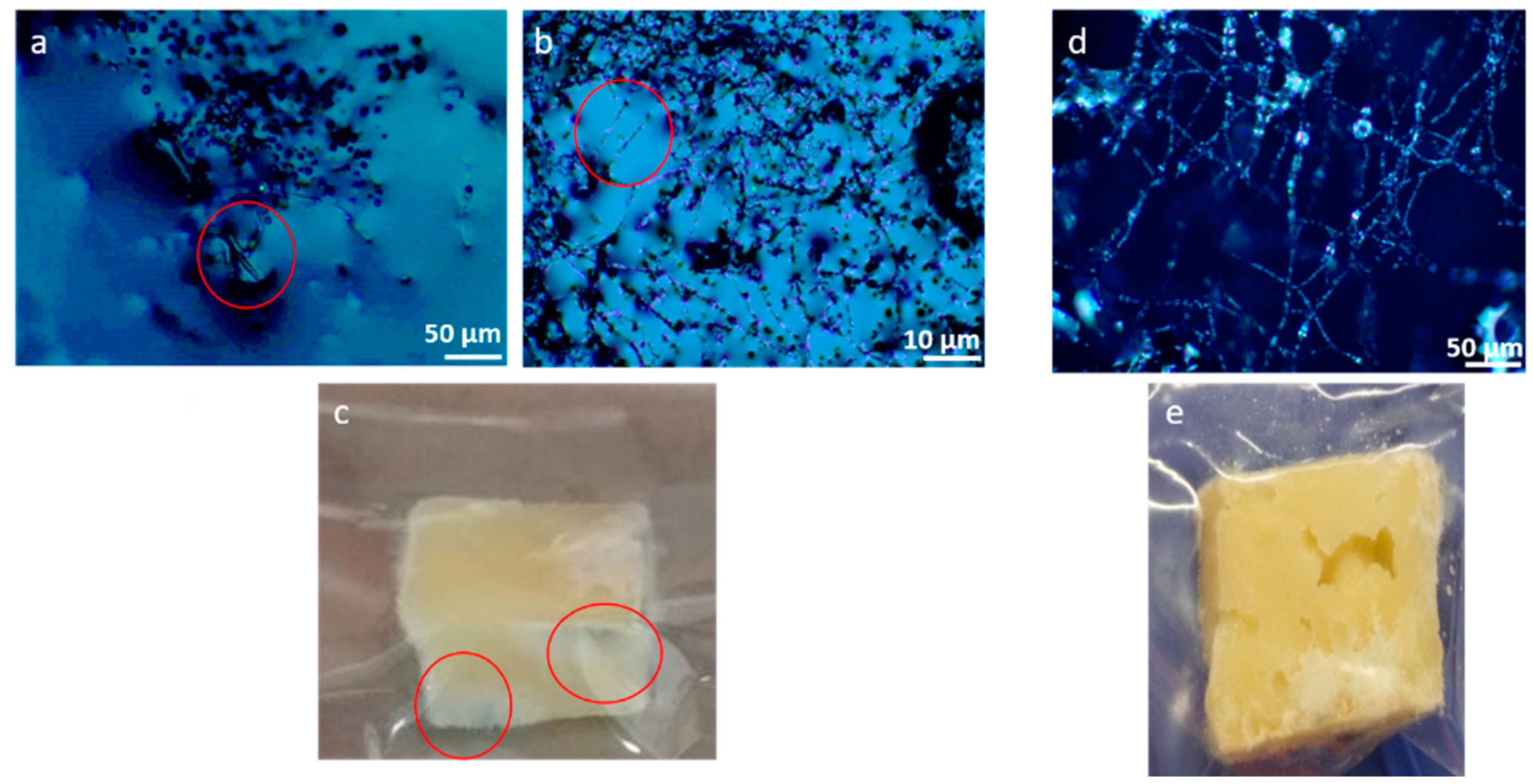
3.1. Microscopic Analysis of Graviera Cheese-Microbial Growth
3.2. Raman Spectroscopic Analysis
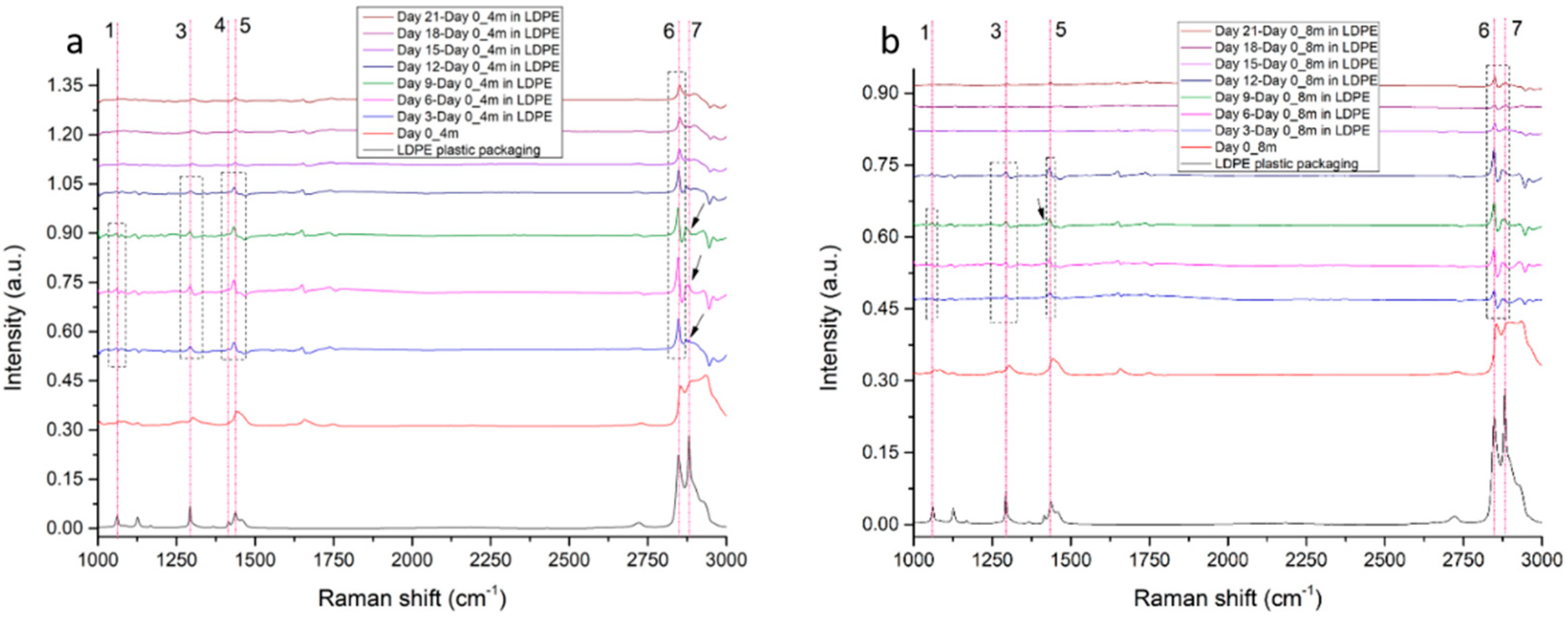
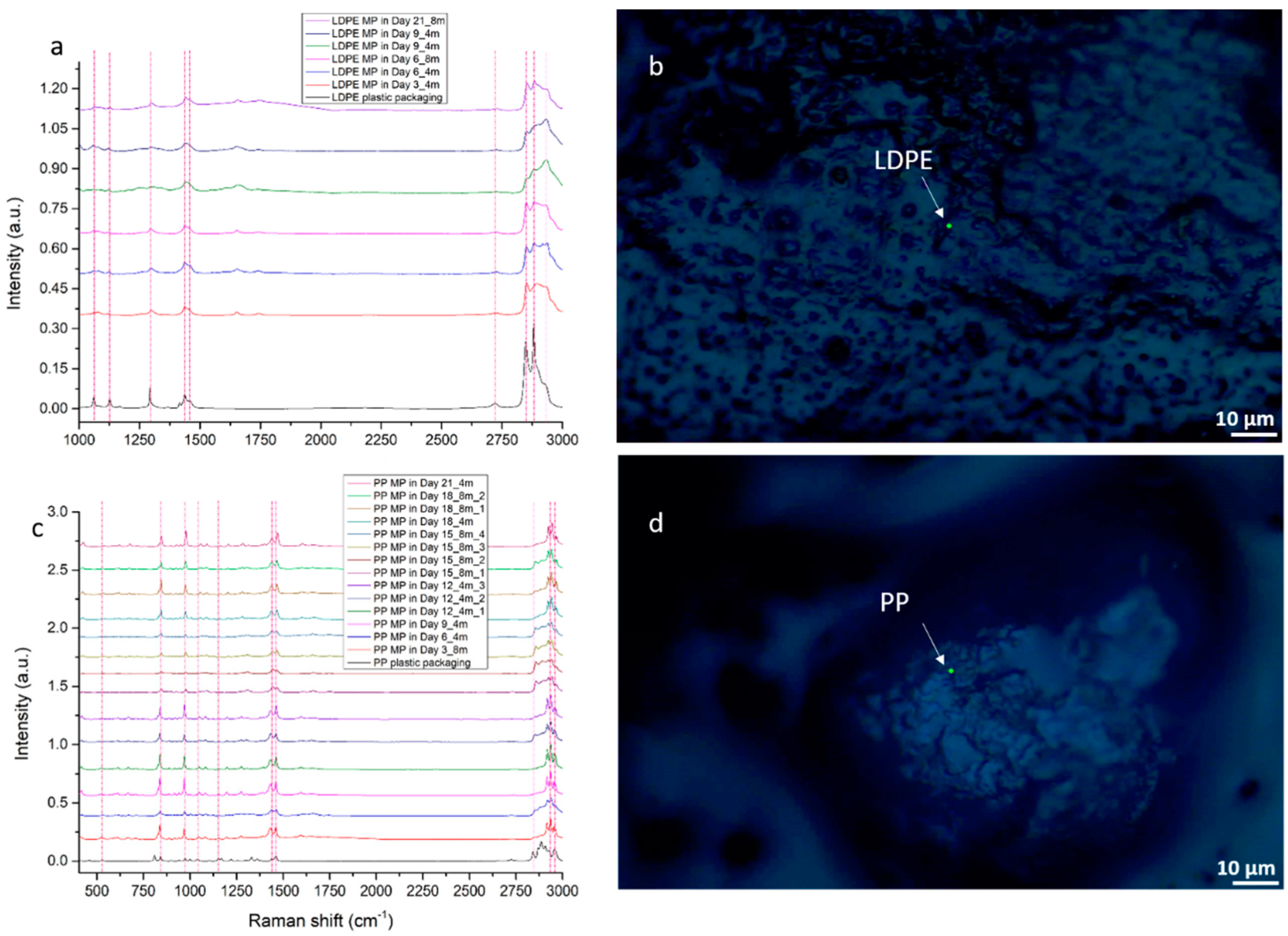
3.2.1. LDPE Migration Traces in 4-Month-Old and 8-Month-Old Graviera Cheese
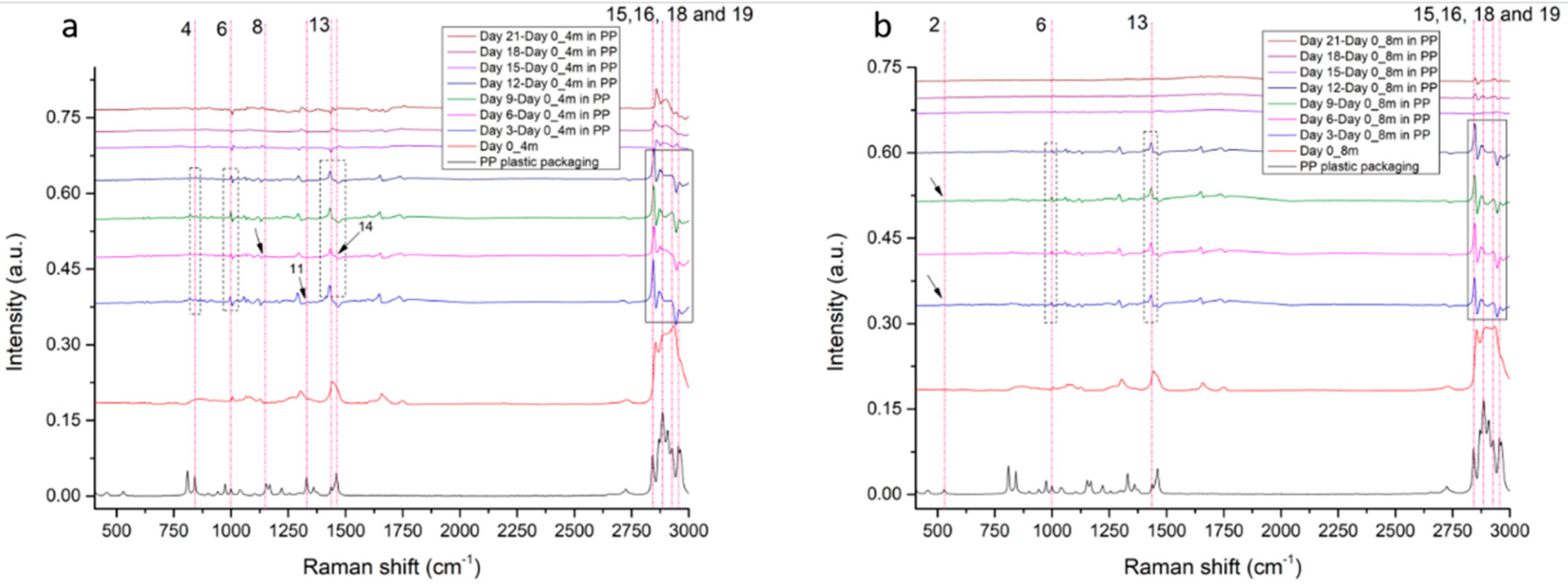
3.2.2. PP Migration Traces in 4-Month-Old and 8-Month-Old Graviera Cheese
3.2.3. MP Detection in Graviera Cheese Samples by Raman Spectroscopy
3.3. FTIR-ATR Spectroscopic Analysis
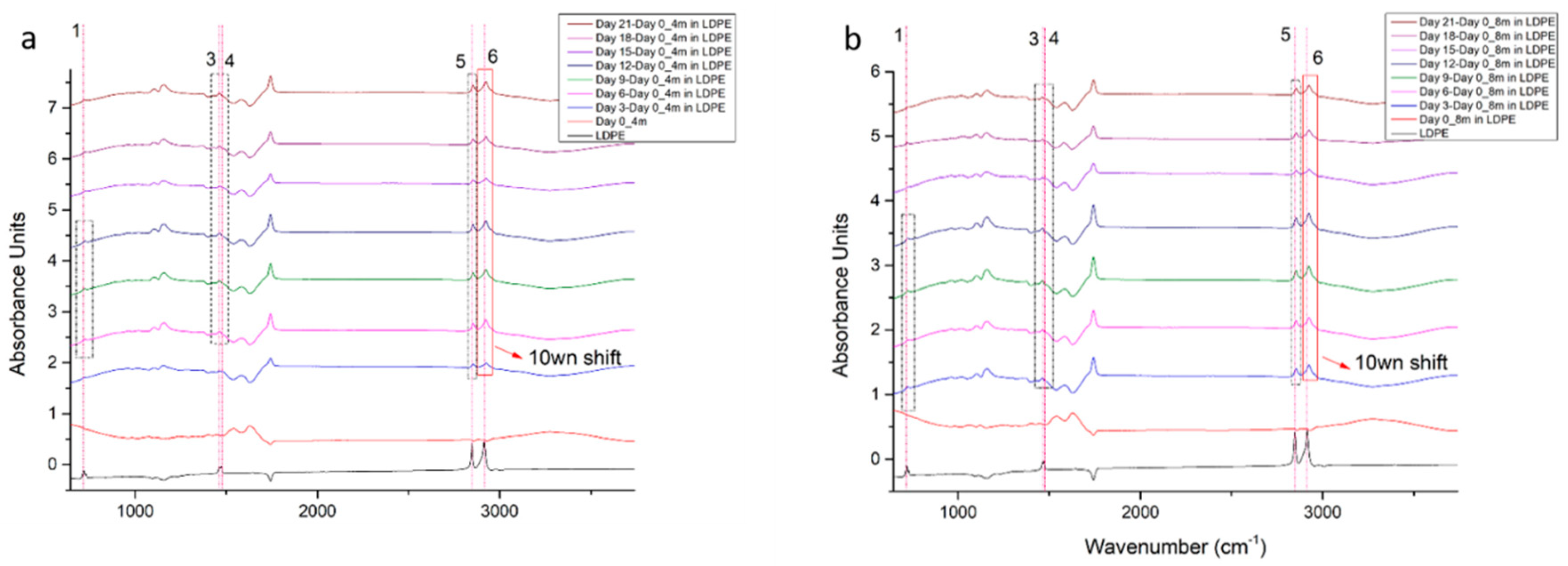
3.3.1. LDPE Migration Traces in 4-Month-Old and 8-Month-Old Graviera Cheese
3.3.2. PP Migration Traces in 4-Month-Old and 8-Month-Old Graviera Cheese
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kajla, P.; Sharma, A. Polymer Migration. 2018. Available online: https://www.researchgate.net/publication/327822021_Polymer_Migration (accessed on 26 January 2025).
- Arvanitoyannis, I.S.; Bosnea, L. Migration of substances from food packaging materials to foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Montoille, L.; Morales Vicencio, C.; Fontalba, D.; Ortiz, J.A.; Moreno-Serna, V.; Peponi, L.; Matiacevich, S.; Zapata, P.A. Study of the effect of the addition of plasticizers on the physical properties of biodegradable films based on kefiran for potential application as food packaging. Food Chem. 2021, 360, 129966. [Google Scholar] [CrossRef]
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef]
- Katsara, K.; Kenanakis, G.; Viskadourakis, Z.; Papadakis, V.M. Polyethylene Migration from Food Packaging on Cheese Detected by Raman and Infrared (ATR/FT-IR) Spectroscopy. Materials 2021, 14, 3872. [Google Scholar] [CrossRef]
- Katsara, K.; Kenanakis, G.; Alissandrakis, E.; Papadakis, V.M. Honey Quality and Microplastic Migration from Food Packaging: A Potential Threat for Consumer Health? Microplastics 2022, 1, 406–427. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Katsara, K.; Viskadourakis, Z.; Alissandrakis, E.; Kountourakis, N.; Kenanakis, G.; Papadakis, V.M. Microplastics’ Detection in Honey: Development of Protocols in a Simulation. Appl. Sci. 2024, 14, 4720. [Google Scholar] [CrossRef]
- Katsara, K.; Kenanakis, G.; Alissandrakis, E.; Papadakis, V.M. Low-Density Polyethylene Migration from Food Packaging on Cured Meat Products Detected by Micro-Raman Spectroscopy. Microplastics 2022, 1, 428–439. [Google Scholar] [CrossRef]
- MATERIALS, I.A. Cheese Manufacturing and Processing Materials (NAICS 311513). Available online: https://interstateam.com/cheese-manufacturing-processing-materials (accessed on 27 June 2024).
- Govari, M.; Iliadis, S.; Papageorgiou, D.; Fletouris, D. Lipid and protein oxidation of grated Kefalotyri cheese packaged in vacuum or modified atmosphere and stored under retail display conditions. Int. Dairy J. 2022, 131, 105369. [Google Scholar] [CrossRef]
- Giri, S.; Lamichhane, G.; Khadka, D.; Devkota, H.P. Microplastics contamination in food products: Occurrence, analytical techniques and potential impacts on human health. Curr. Res. Biotechnol. 2024, 7, 100190. [Google Scholar] [CrossRef]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef]
- Yu, C.; Takhistov, P.; Alocilja, E.; de Corcuera, J.R.; Frey, M.W.; Gomes, C.L.; Mao, Y.J.; McLamore, E.S.; Lin, M.; Tsyusko, O.V.; et al. Bioanalytical approaches for the detection, characterization, and risk assessment of micro/nanoplastics in agriculture and food systems. Anal. Bioanal. Chem. 2022, 414, 4591–4612. [Google Scholar] [CrossRef]
- Aly, S.S.H. Studying the Migration of Plasticizers from Plastic Packaging to Local Processed Cheese. Middle East J. Appl. Sci. 2016, 6, 957–963. [Google Scholar]
- Chiesa, L.M.; Panseri, S.; Soncin, S.; Vallone, L.; Dragoni, I. Determination of styrene content in Gorgonzola PDO cheese by headspace solid phase micro-extraction (HS-SPME) and gas-chromatography mass-spectrometry (GC-MS). Veter- Res. Commun. 2010, 34 (Suppl. S1), S167–S170. [Google Scholar] [CrossRef]
- Wang, Q.; Storm, B. Migration Study of Polypropylene (PP) Oil Blends in Food Simulants. Macromol. Symp. 2006, 242, 307–314. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E. Descriptive Characteristics and Cheesemaking Technology of Greek Cheeses Not Listed in the EU Geographical Indications Registers. Dairy 2023, 4, 43–67. [Google Scholar] [CrossRef]
- Hartmann, K.; Licitra, G.; Eugster-Meier, E.; Fröhlich-Wyder, M.-T.; Jakob, E.; Wechsler, D.; Maubois, J.L.; Karatzas, K.-A.G.; Bintsis, T.; Alichanidis, E.; et al. Hard Cheeses. In Global Cheesemaking Technology; Wiley: Hoboken, NJ, USA, 2017; pp. 204–246. [Google Scholar]
- Danezis, G.; Theodorou, C.; Massouras, T.; Zoidis, E.; Hadjigeorgiou, I.; Georgiou, C.A. Greek Graviera Cheese Assessment through Elemental Metabolomics—Implications for Authentication, Safety and Nutrition. Molecules 2019, 24, 670. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kargaki, G.K.; Hadjichristodoulou, C. Effect of several MAP compositions on the microbiological and sensory properties of Graviera cheese. Anaerobe 2011, 17, 310–314. [Google Scholar] [CrossRef]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Quality Evaluation of Grated Graviera Cheese Stored at 4 and 12 °C using Active and Modified Atmosphere Packaging. Packag. Technol. Sci. 2011, 24, 15–29. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Ioannidou, M.D.; Maggira, M.; Samouris, G. Physicochemical Characteristics, Fatty Acids Profile and Lipid Oxidation during Ripening of Graviera Cheese Produced with Raw and Pasteurized Milk. Foods 2022, 11, 2138. [Google Scholar] [CrossRef] [PubMed]
- Solomakos, N.; Govari, M.; Botsoglou, E.; Pexara, A. Effect of modified atmosphere packaging on physicochemical and microbiological characteristics of Graviera Agraphon cheese during refrigerated storage. J. Dairy Res. 2019, 86, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Holroyd, S.E.; Reid, D.C.W.; Gordon, K.C. Raman imaging processed cheese and its components. J. Raman Spectrosc. 2016, 48, 374–383. [Google Scholar] [CrossRef]
- Burdikova, Z.; Svindrych, Z.; Hickey, C.; Wilkinson, M.G.; Auty, M.A.E.; Samek, O.; Bernatova, S.; Krzyzanek, V.; Periasamy, A.; Sheehan, J.J. Application of advanced light microscopic techniques to gain deeper insights into cheese matrix physico-chemistry. Dairy Sci. Technol. 2015, 95, 687–700. [Google Scholar] [CrossRef]
- Oliveira Kde, S.; Callegaro Lde, S.; Stephani, R.; Almeida, M.R.; de Oliveira, L.F. Analysis of spreadable cheese by Raman spectroscopy and chemometric tools. Food Chem. 2016, 194, 441–446. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I.u. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- da Silva, D.J.; Parra, D.F.; Wiebeck, H. Applying confocal Raman spectroscopy and different linear multivariate analyses to sort polyethylene residues. Chem. Eng. J. 2021, 426, 131344. [Google Scholar] [CrossRef]
- Sato, H.; Shimoyama, M.; Kamiya, T.; Amari, T.; Aic, S.; Ninomiya, T.; Siesler, H.W.; Ozaki, Y. Raman spectra of high-density, low-density, and linear low-density polyethylene pellets and prediction of their physical properties by multivariate data analysis. J. Appl. Polym. Sci. 2002, 86, 443–448. [Google Scholar] [CrossRef]
- Banquet-Teran, J.; Johnson-Restrepo, B.; Hernandez-Morelo, A.; Ropero, J.; Fontalvo-Gomez, M.; Romanach, R.J. Linear and Nonlinear Calibration Methods for Predicting Mechanical Properties of Polypropylene Pellets Using Raman Spectroscopy. Appl. Spectrosc. 2016, 70, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Dorota, P.; Malgorzata, C. Raman Spectroscopy in the Analysis of Textile Structures. In Recent Developments in Atomic Force Microscopy and Raman Spectroscopy for Materials Characterization; Chandra Shakher, P., Samir, K., Eds.; IntechOpen: Rijeka, Croatia, 2021; Chapter 9. [Google Scholar]
- Ferchichi, S.; Sheibat-Othman, N.; Boyron, O.; Bonnin, C.; Norsic, S.; Rey-Bayle, M.; Monteil, V. In situ dissolved polypropylene prediction by Raman and ATR-IR spectroscopy for its recycling. Anal. Methods 2024, 16, 3109–3117. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Kita, Y.; Matsukawa, K.; Yamaguchi, H.; Ochiai, S.; Siesler, H.; Ozaki, Y. Molecular Structure, Crystallinity and Morphology of Polyethylene/Polypropylene Blends Studied by Raman Mapping, Scanning Electron Microscopy, Wide Angle X-Ray Diffraction, and Differential Scanning Calorimetry. Polym. J. 2006, 38, 1127–1136. [Google Scholar] [CrossRef]
- Gopanna, A.; Mandapati, R.N.; Thomas, S.P.; Rajan, K.; Chavali, M. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polym. Bull. 2019, 76, 4259–4274. [Google Scholar] [CrossRef]
- Guo, X.; Lin, Z.; Wang, Y.; He, Z.; Wang, M.; Jin, G. In-Line Monitoring the Degradation of Polypropylene under Multiple Extrusions Based on Raman Spectroscopy. Polymers 2019, 11, 1698. [Google Scholar] [CrossRef]
- Xiang, T.; Sun, Y.; Ding, D.; Yao, W.; Yu, Z.; Xie, Y. Microscopic Raman-based rapid detection of submicron/nano polypropylene plastics in tea and tea beverages. Food Chem. 2024, 454, 139657. [Google Scholar] [CrossRef]
- Zhang, Z.-Y. Rapid Discrimination of Cheese Products Based on Probabilistic Neural Network and Raman Spectroscopy. J. Spectrosc. 2020, 2020, 8896535. [Google Scholar] [CrossRef]
- Koca, N.; Rodriguez-Saona, L.E.; Harper, W.J.; Alvarez, V.B. Application of Fourier transform infrared spectroscopy for monitoring short-chain free fatty acids in Swiss cheese. J. Dairy Sci. 2007, 90, 3596–3603. [Google Scholar] [CrossRef]
- Leite, A.I.N.; Pereira, C.G.; Andrade, J.; Vicentini, N.M.; Bell, M.J.V.; Anjos, V. FTIR-ATR spectroscopy as a tool for the rapid detection of adulterations in butter cheeses. LWT 2019, 109, 63–69. [Google Scholar] [CrossRef]
- Pax, A.P.; Ong, L.; Vongsvivut, J.; Tobin, M.J.; Kentish, S.E.; Gras, S.L. The characterisation of Mozzarella cheese microstructure using high resolution synchrotron transmission and ATR-FTIR microspectroscopy. Food Chem. 2019, 291, 214–222. [Google Scholar] [CrossRef]
- Martín-Del-Campo, S.; Picque, D.; Cosío-Ramírez, R.; Corrieu, G. Middle infrared spectroscopy characterization of ripening stages of Camembert-type cheeses. Int. Dairy J. 2007, 17, 835–845. [Google Scholar]
- Phan, S.; Padilla-Gamiño, J.L.; Luscombe, C.K. The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. polyethylene and polypropylene. Polym. Test. 2022, 116, 107752. [Google Scholar] [CrossRef]
- Cheruthazhekatt, S.; Pijpers, T.F.; Mathot, V.B.; Pasch, H. Combination of TREF, high-temperature HPLC, FTIR and HPer DSC for the comprehensive analysis of complex polypropylene copolymers. Anal. Bioanal. Chem. 2013, 405, 8995–9007. [Google Scholar] [CrossRef]
- Lin, J.H.; Pan, Y.J.; Liu, C.F.; Huang, C.L.; Hsieh, C.T.; Chen, C.K.; Lin, Z.I.; Lou, C.W. Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Materials 2015, 8, 8850–8859. [Google Scholar] [CrossRef]
- Tian, N.; Lv, R.; Na, B.; Xu, W.; Li, Z. Molecular origin of enhanced strength in the monofilament of syndiotactic polypropylene because of annealing: A micro-FTIR study. J. Phys. Chem. B 2009, 113, 14920–14924. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Harper, W.J.; Rodriguez-Saona, L.E. Cheddar cheese classification based on flavor quality using a novel extraction method and Fourier transform infrared spectroscopy. J. Dairy Sci. 2009, 92, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Pinzone, M.; Nordøy, E.; Eppe, G.; Malherbe, C.; Das, K.; Collard, F. First record of plastic debris in the stomach of a hooded seal pup from the Greenland Sea. Mar. Pollut. Bull. 2021, 167, 112350. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Bleicher, A.; Delbes-Paus, C.; Kakouri, A.; Neuhaus, K.; Montel, M.C. FTIR-based polyphasic identification of lactic acid bacteria isolated from traditional Greek Graviera cheese. Food Microbiol. 2011, 28, 76–83. [Google Scholar] [CrossRef]
- Ryu, S.R.; Noda, I.; Jung, Y.M. What is the origin of positional fluctuation of spectral features: True frequency shift or relative intensity changes of two overlapped bands? Appl. Spectrosc. 2010, 64, 1017–1021. [Google Scholar] [CrossRef]
- Blinco, J. The Synthesis and Application of Novel Profluorescent Nitroxides as Probes for Polymer Degradation. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2008. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsara, K.; Viskadourakis, Z.; Kenanakis, G.; Papadakis, V.M. Microplastic Migration from Food Packaging on Cheese. Microplastics 2025, 4, 17. https://doi.org/10.3390/microplastics4020017
Katsara K, Viskadourakis Z, Kenanakis G, Papadakis VM. Microplastic Migration from Food Packaging on Cheese. Microplastics. 2025; 4(2):17. https://doi.org/10.3390/microplastics4020017
Chicago/Turabian StyleKatsara, Klytaimnistra, Zacharias Viskadourakis, George Kenanakis, and Vassilis M. Papadakis. 2025. "Microplastic Migration from Food Packaging on Cheese" Microplastics 4, no. 2: 17. https://doi.org/10.3390/microplastics4020017
APA StyleKatsara, K., Viskadourakis, Z., Kenanakis, G., & Papadakis, V. M. (2025). Microplastic Migration from Food Packaging on Cheese. Microplastics, 4(2), 17. https://doi.org/10.3390/microplastics4020017








