Interleukin-6 Trans-Signaling Mediated Regulation of Paracellular Permeability in Human Retinal Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Measurement of Trans-Endothelial Resistance in HRECs
2.3. RNA Isolation, Library Preparation, and Sequencing
2.4. qRT-PCR
2.5. Western Blot Analyses
2.6. Statistical Analyses
3. Results
3.1. Differential Effects of IL-6 Classical and Trans-Signaling on Endothelial Barrier Function
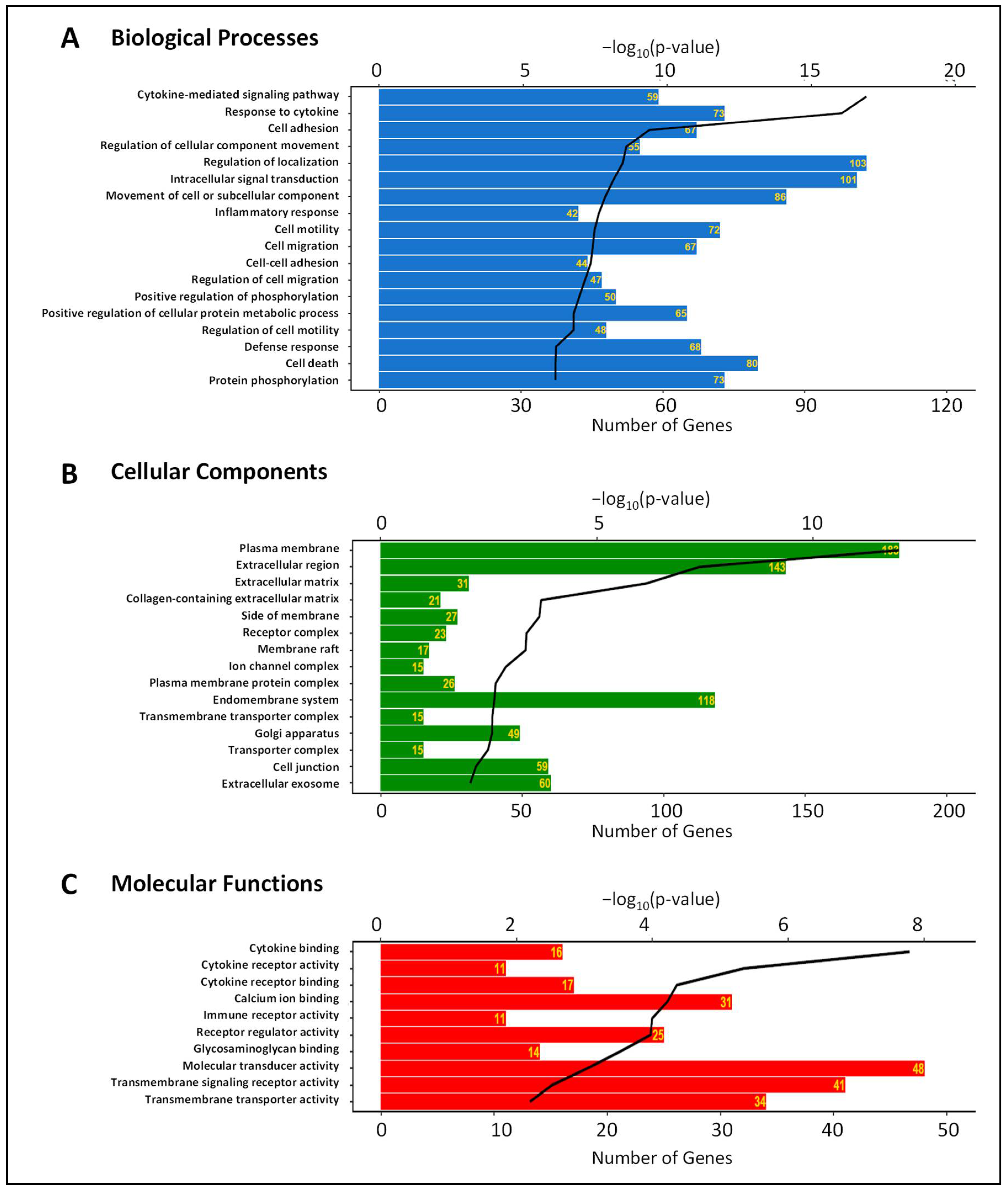
3.2. Gene Expression Changes Induced by IL-6 Classical and Trans-Signaling Activation in HRECs
3.3. Differential Expression of Adherens Junction, Tight Junction, and Gap Junction Genes Induced by IL-6 Classical and Trans-Signaling Activation
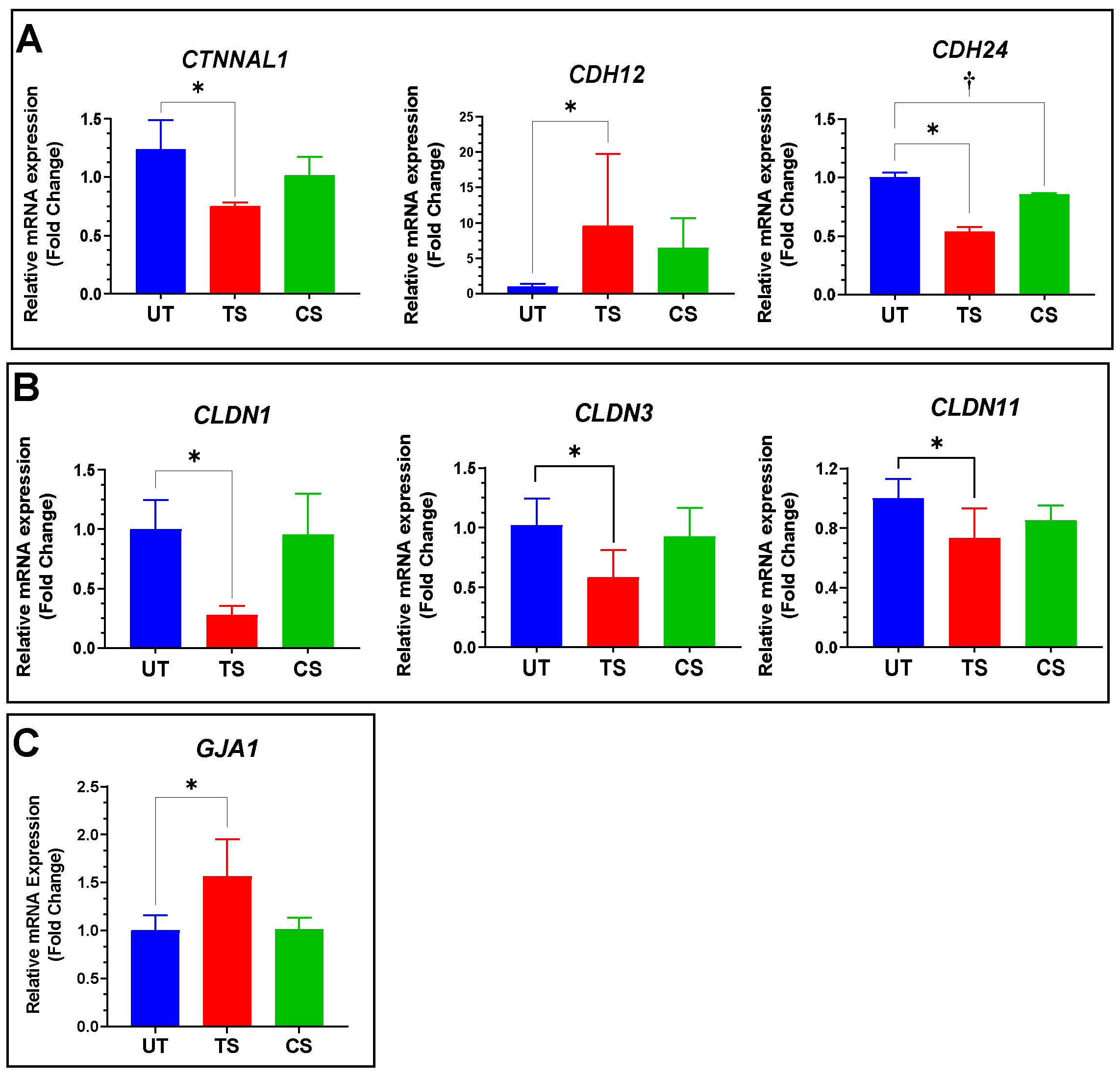
3.3.1. Adherens Junctions
3.3.2. Tight Junctions
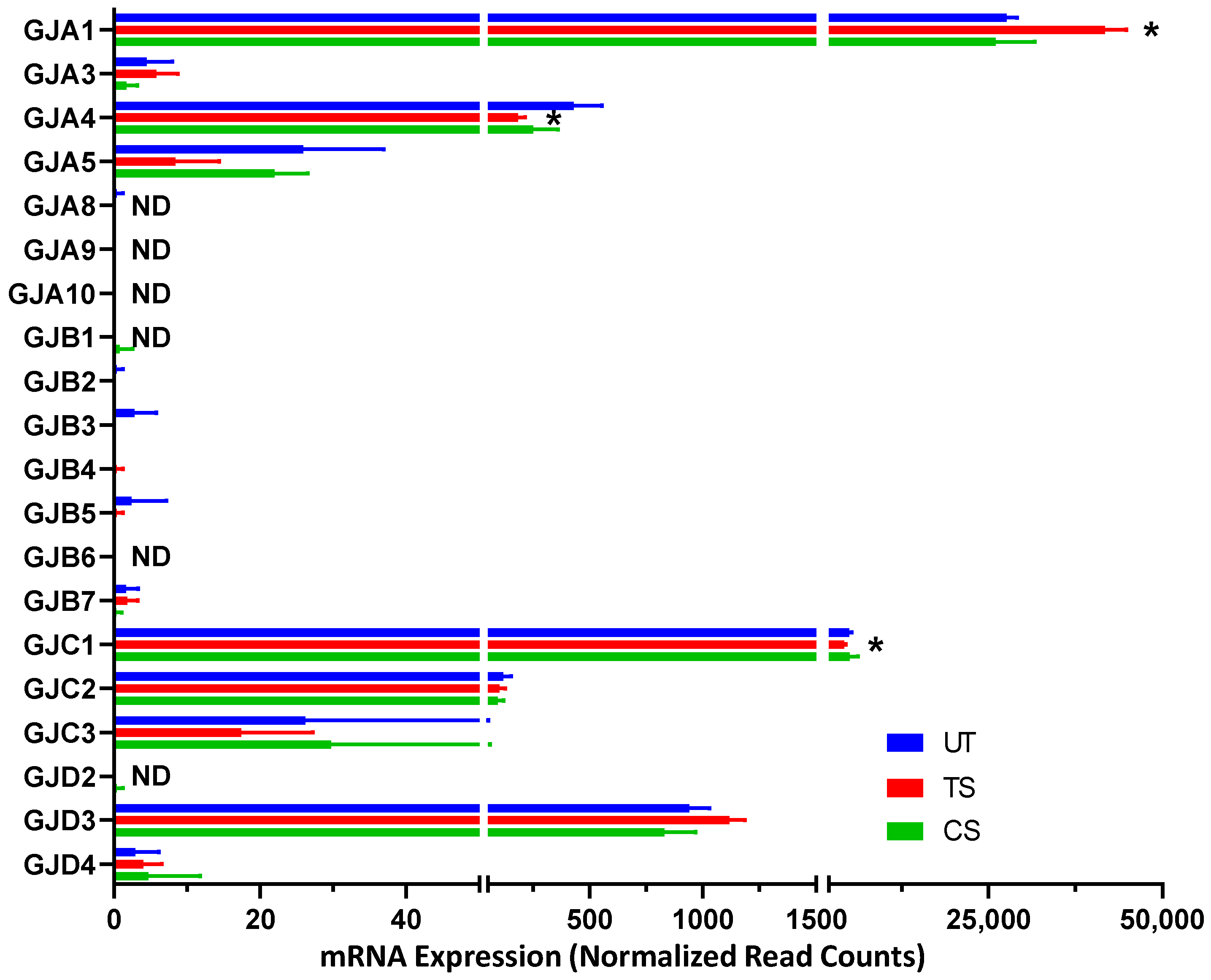
3.3.3. Gap Junctions
3.4. Validation of Selected Genes Using Quantitative RT-PCR
3.5. Confirmation Using Western Blot Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Kempen, J.H.; O’Colmain, B.J.; Leske, M.C.; Haffner, S.M.; Klein, R.; Moss, S.E.; Taylor, H.R.; Hamman, R.F.; West, S.K.; Wang, J.J. The prevalence of diabetic retinopathy among adults in the United States. Arch. Ophthalmol. 2004, 122, 552–563. [Google Scholar]
- Erickson, K.K.; Sundstrom, J.M.; Antonetti, D.A. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 2007, 10, 103–117. [Google Scholar] [CrossRef]
- Frey, T.; Antonetti, D.A. Alterations to the blood-retinal barrier in diabetes: Cytokines and reactive oxygen species. Antioxid. Redox. Signal 2011, 15, 1271–1284. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S3–S9. [Google Scholar] [CrossRef]
- Eshaq, R.S.; Aldalati, A.M.Z.; Alexander, J.S.; Harris, N.R. Diabetic retinopathy: Breaking the barrier. Pathophysiology 2017, 24, 229–241. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine mediated monocyte trafficking into the retina: Role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Smyth, N.; Niessen, C. Pathophysiology of diabetic macular edema. In Diabetic Retinopathy; Karger Publishers: Basel, Switzerland, 2007; Volume 39, pp. 1–12. [Google Scholar]
- Sugimoto, M.; Cutler, A.; Shen, B.; Moss, S.E.; Iyengar, S.K.; Klein, R.; Folkman, J.; Anand-Apte, B. Inhibition of EGF signaling protects the diabetic retina from insulin-induced vascular leakage. Am. J. Pathol. 2013, 183, 987–995. [Google Scholar] [CrossRef] [Green Version]
- Gardner, T.W.; Larsen, M.; Girach, A.; Zhi, X.; Protein Kinase C Diabetic Retinopathy Study (PKC-DRS2) Study Group. Diabetic macular oedema and visual loss: Relationship to location, severity and duration. Acta Ophthalmol. 2009, 87, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Coranguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.-W.; Zhang, D.-D.; Fan, Z.-G. Blood-retinal barrier as a converging pivot in understanding the initiation and development of retinal diseases. Chin. Med. J. 2020, 133, 2586. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Naggert, J.K.; Nishina, P.M. The Impact of Adherens and Tight Junctions on Physiological Function and Pathological Changes in the Retina. Adv. Exp. Med. Biol. 2018, 1074, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol. Res. 2020, 161, 105115. [Google Scholar] [CrossRef] [PubMed]
- de Wit, C.; Hoepfl, B.; Wölfle, S.E. Endothelial mediators and communication through vascular gap junctions. Biol. Chem. 2006, 387, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Usuda, H.; Tanaka, T.; Wada, K.; Shimaoka, M. The functional implications of endothelial gap junctions and cellular mechanics in vascular angiogenesis. Cancers 2019, 11, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, L.; Holloway, K.; Male, D.; Loughlin, A.; Romero, I. Amyloid-β-induced occludin down-regulation and increased permeability in human brain endothelial cells is mediated by MAPK activation. J. Cell. Mol. Med. 2010, 14, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Luo, P.-L.; Wang, Y.-J.; Yang, Y.-Y.; Yang, J.-J. Hypoxia-induced hyperpermeability of rat glomerular endothelial cells involves HIF-2α mediated changes in the expression of occludin and ZO-1. Braz. J. Med. Biol. Res. 2018, 51, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.; Cassidy, P.S.; O’Callaghan, J.; Crosbie, D.E.; Humphries, P. Manipulating ocular endothelial tight junctions: Applications in treatment of retinal disease pathology and ocular hypertension. Prog. Retin. Eye Res. 2018, 62, 120–133. [Google Scholar] [CrossRef]
- Valle, M.L.; Dworshak, J.; Sharma, A.; Ibrahim, A.S.; Al-Shabrawey, M.; Sharma, S. Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells. Exp. Eye Res. 2019, 178, 27–36. [Google Scholar] [CrossRef]
- Mesquida, M.; Drawnel, F.; Lait, P.J.; Copland, D.A.; Stimpson, M.L.; Llorenc, V.; Sainz de la Maza, M.; Adan, A.; Widmer, G.; Strassburger, P.; et al. Modelling Macular Edema: The Effect of IL-6 and IL-6R Blockade on Human Blood-Retinal Barrier Integrity In Vitro. Transl. Vis. Sci. Technol. 2019, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Tonade, D.; Liu, H.; Palczewski, K.; Kern, T.S. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia 2017, 60, 2111–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237. [Google Scholar] [CrossRef]
- Rabe, B.; Chalaris, A.; May, U.; Waetzig, G.H.; Seegert, D.; Williams, A.S.; Jones, S.A.; Rose-John, S.; Scheller, J. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood J. Am. Soc. Hematol. 2008, 111, 1021–1028. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting interleukin-6 signaling in clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Heinrich, P.C. Soluble receptors for cytokines and growth factors: Generation and biological function. Biochem. J. 1994, 300, 281–290. [Google Scholar] [CrossRef]
- Yun, J.H.; Park, S.W.; Kim, K.J.; Bae, J.S.; Lee, E.H.; Paek, S.H.; Kim, S.U.; Ye, S.; Kim, J.H.; Cho, C.H. Endothelial STAT3 Activation Increases Vascular Leakage Through Downregulating Tight Junction Proteins: Implications for Diabetic Retinopathy. J. Cell Physiol. 2017, 232, 1123–1134. [Google Scholar] [CrossRef]
- Yun, J.H.; Han, M.H.; Jeong, H.S.; Lee, D.H.; Cho, C.H. Angiopoietin 1 attenuates interleukin-6-induced endothelial cell permeability through SHP-1. Biochem. Biophys. Res. Commun. 2019, 518, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, H.; Martino, N.; Garrett, J.P.; Adam, A.P. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am. J. Physiol. Cell Physiol. 2018, 314, C589–C602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, R.; Brown, D.; Churchwell, L.; Lee, T.-J.; Kodeboyina, S.K.; Bloom, J.; Sharma, A.; Sharma, S. RNA-Seq analysis reveals gene expression changes induced by IL-6 trans-signaling activation in retinal endothelial cells. Cytokine 2021, 139, 155375. [Google Scholar] [CrossRef] [PubMed]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014, 28, 51300. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 20 July 2020).
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [Green Version]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org (accessed on 20 July 2020).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. Available online: https://rdrr.io/cran/gplots/ (accessed on 20 July 2020).
- Devarakonda, A.K.; Rafalovsky, E.G.; Lee, T.J.; Sharma, A. GOFIG: A tool for Gene Ontology Enrichment Analysis and Visualization. 2020. Available online: https://github.com/adidevara/GOFIG (accessed on 20 July 2020).
- Matsuda, M.; Kubo, A.; Furuse, M.; Tsukita, S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 2004, 117, 1247–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonetti, D.A.; Barber, A.J.; Khin, S.; Lieth, E.; Tarbell, J.M.; Gardner, T.W. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 1998, 47, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Jiang, H.; Yan, S.; Fu, Y.; Liu, C.; Sun, X.; Chai, M.; Gao, Y.; Yuan, B.; Chen, C. RNA-seq analysis provide new insights into mapk signaling of apolipoproteinciii-induced inflammation in porcine vascular endothelial cells. Cell Cycle 2017, 16, 2230–2238. [Google Scholar] [CrossRef] [Green Version]
- Tőkés, A.-M.; Szász, A.M.; Juhász, É.; Schaff, Z.; Harsányi, L.; Molnár, I.A.; Baranyai, Z.; Besznyák, I.; Zaránd, A.; Salamon, F. Expression of tight junction molecules in breast carcinomas analysed by array PCR and immunohistochemistry. Pathol. Oncol. Res. 2012, 18, 593–606. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, M.; Yang, S.; Liu, F.; Sun, L. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. BioMed Res. Int. 2013, 2013, 987064. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Hu, Y.; Ding, L.; Chen, Y.; Takahashi, Y.; Mott, R.; Ma, J.-x. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes 2012, 61, 2948–2957. [Google Scholar] [CrossRef] [Green Version]
- Morcos, Y.; Hosie, M.J.; Bauer, H.C.; Chan-Ling, T. Immunolocalization of occludin and claudin-1 to tight junctions in intact CNS vessels of mammalian retina. J. Neurocytol. 2001, 30, 107–123. [Google Scholar] [CrossRef]
- Liebner, S.; Kniesel, U.; Kalbacher, H.; Wolburg, H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur. J. Cell Biol. 2000, 79, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier a lesson from claudin-1–deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Katsuno, T.; Hoshimoto, A.; Hirano, N.; Saito, Y.; Suzuki, Y. Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intestinal epithelial cell monolayers. Exp. Cell Res. 2004, 298, 1–8. [Google Scholar] [CrossRef]
- Kojima, T.; Sawada, N.; Chiba, H.; Kokai, Y.; Yamamoto, M.; Urban, M.; Lee, G.-H.; Hertzberg, E.L.; Mochizuki, Y.; Spray, D.C. Induction of tight junctions in human connexin 32 (hCx32)-transfected mouse hepatocytes: Connexin 32 interacts with occludin. Biochem. Biophys. Res. Commun. 1999, 266, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Spray, D.C.; Kokai, Y.; Chiba, H.; Mochizuki, Y.; Sawada, N. Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp. Cell Res. 2002, 276, 40–51. [Google Scholar] [CrossRef]
- Meşe, G.; Richard, G.; White, T.W. Gap junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2516–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruo, N.; Morita, I.; Shirao, M.; Murota, S. IL-6 increases endothelial permeability in vitro. Endocrinology 1992, 131, 710–714. [Google Scholar] [PubMed]
- Bao, B.; Jiang, J.; Yanase, T.; Nishi, Y.; Morgan, J.R. Connexon-mediated cell adhesion drives microtissue self-assembly. FASEB J. 2011, 25, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene Name | Gene Symbol | Designed qRT-PCR Primers | |
|---|---|---|---|
| Forward Primer (5′→3′) | Reverse Primer (5′→3′) | ||
| Cadherin 12 | CDH12 | GGACAGTCGTCAGCTTTCTCT | CTTTCTGCCACTGACCACCT |
| Cadherin 24 | CDH24 | AGAGCTCGACAGTTCTGCAC | GTATCGTAGGGCTCAGCCAG |
| Catenin alpha like 1 | CTNNAL1 | TCTGACGCTGACTGCGAAAT | ACCCTCTGCAGCAAAAACCT |
| Claudin 1 | CLDN1 | CCCAGTCAATGCCAGGTACG | CAAAGTAGGGCACCTCCCAG |
| Claudin 3 | CLDN3 | GTCTAAGGGACAGACGCAGG | AAGTATTGGCGGTCACCCAG |
| Claudin 11 | CLDN11 | TTGACTGCCTGCTTTGTGCTAC | CTCACGATGGTGGTCTCACG |
| Gap junction protein alpha 1 | GJA1 | CTGAGTGCCTGAACTTGCCT | CTGGGCACCACTCTTTTGC |
| Glyceraldeyhde 3-phosphate dehydrogenase | GAPDH | AATGAAGGGGTCATTGATGG | AAGGTGAAGGTCGGAGTCAA |
| Symbol | Description | FC (TS vs. UT) | Adj. p-Value (TS vs. UT) | FC (CS vs. UT) | Adj. p-Value (CS vs. UT) |
|---|---|---|---|---|---|
| Adherens Junctions | |||||
| CTNNA1 | Catenin alpha 1 | −1.04 | 0.525 | −1.24 | 0.213 |
| CTNNAL1 | Catenin alpha-like 1 | −1.21 * | 4.36 × 10−5 | −1.16 | 0.404 |
| CTNNB1 | Catenin beta 1 | 1.07 | 0.235 | −1.17 | 0.380 |
| CTNNBIP1 | Catenin beta interacting protein 1 | −1.29 * | 6.05 × 10−4 | −1.37 | 0.086 |
| CTNNBL1 | Catenin beta-like 1 | −1.08 | 0.352 | −1.35 | 0.078 |
| CTNND1 | Catenin delta 1 | 1.72 | 0.089 | 1.06 | 0.889 |
| CDH2 | Cadherin 2 | −1.09 | 0.112 | −1.13 | 0.474 |
| CDH5 | Cadherin 5 | −1.08 | 0.138 | −1.14 | 0.467 |
| CDH6 | Cadherin 6 | −1.66 * | 5.96 × 10−20 | −1.10 | 0.637 |
| CDH11 | Cadherin 11 | 1.74 * | 3.08 × 10−14 | −1.06 | 0.774 |
| CDH12 | Cadherin 12 | 1.97 * | 2.35 × 10−4 | −1.11 | NA |
| CDH13 | Cadherin 13 | −1.12 | 0.076 | −1.21 | 0.290 |
| CDH15 | Cadherin 15 | 1.38 | 0.108 | −1.48 | NA |
| CDH24 | Cadherin 24 | −1.67 * | 8.42 × 10−15 | −1.21 | 0.202 |
| Tight Junctions | |||||
| CLDN1 | Claudin 1 | −3.27 * | 6.91 × 10−10 | −1.52 | NA |
| CLDN3 | Claudin 3 | −4.44 * | 3.91 × 10−8 | −1.45 | NA |
| CLDN4 | Claudin 4 | −1.26 | 0.368 | 1.75 | NA |
| CLDN5 | Claudin 5 | −1.04 | 0.723 | −1.39 | 0.052 |
| CLDN7 | Claudin 7 | −1.13 | 0.131 | −1.30 | 0.155 |
| CLDN11 | Claudin 11 | −1.85 * | 2.79 × 10−52 | −1.25 | 0.181 |
| CLDN12 | Claudin 12 | 1.25 * | 0.003 | 1.02 | 0.936 |
| CLDN14 | Claudin 14 | −1.36 * | 2.17 × 10−4 | −1.15 | NA |
| CLDN15 | Claudin 15 | 1.04 | 0.823 | 1.48 * | 0.048 |
| CLDND1 | Claudin domain containing 1 | 1.08 | 0.217 | −1.19 | 0.373 |
| TJAP1 | Tight junction associated protein 1 | −1.06 | 0.473 | −1.06 | 0.732 |
| TJP1 | Tight junction protein 1 | 1.17 * | 0.013 | 1.04 | 0.866 |
| TJP2 | Tight junction protein 2 | −1.31 * | 9.83 × 10−8 | −1.12 | 0.548 |
| Gap Junctions | |||||
| GJA1 | Gap junction protein alpha 1 | 1.49 * | 1.75 × 10−18 | −1.18 | 0.366 |
| GJA4 | Gap junction protein alpha 4 | −2.41 * | 2.36 × 10−9 | −1.89 | NA |
| GJC1 | Gap junction protein gamma 1 | −1.19 * | 0.002 | −1.09 | 0.661 |
| GJC2 | Gap junction protein gamma 2 | −1.19 | 0.438 | −1.33 | NA |
| GJD3 | Gap junction protein delta 3 | 1.16 | 0.056 | −1.24 | 0.207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glass, J.; Robinson, R.; Lee, T.-J.; Sharma, A.; Sharma, S. Interleukin-6 Trans-Signaling Mediated Regulation of Paracellular Permeability in Human Retinal Endothelial Cells. Int. J. Transl. Med. 2021, 1, 137-153. https://doi.org/10.3390/ijtm1020010
Glass J, Robinson R, Lee T-J, Sharma A, Sharma S. Interleukin-6 Trans-Signaling Mediated Regulation of Paracellular Permeability in Human Retinal Endothelial Cells. International Journal of Translational Medicine. 2021; 1(2):137-153. https://doi.org/10.3390/ijtm1020010
Chicago/Turabian StyleGlass, Joshua, Rebekah Robinson, Tae-Jin Lee, Ashok Sharma, and Shruti Sharma. 2021. "Interleukin-6 Trans-Signaling Mediated Regulation of Paracellular Permeability in Human Retinal Endothelial Cells" International Journal of Translational Medicine 1, no. 2: 137-153. https://doi.org/10.3390/ijtm1020010
APA StyleGlass, J., Robinson, R., Lee, T.-J., Sharma, A., & Sharma, S. (2021). Interleukin-6 Trans-Signaling Mediated Regulation of Paracellular Permeability in Human Retinal Endothelial Cells. International Journal of Translational Medicine, 1(2), 137-153. https://doi.org/10.3390/ijtm1020010






