Reduction of N-Acetylglucosaminyltransferase-I Activity Promotes Neuroblastoma Invasiveness and EGF-Stimulated Proliferation In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. 3D Cell Spheroid Formation
2.3. Whole Cell Lysates
2.4. Total Membrane Isolation and Glycosidase Treatment
2.5. Western and Lectin Blotting
2.6. Cell Dissociation Assay
2.7. 2D and 3D BrdU Proliferation
2.8. Anchorage Independent Growth
2.9. Morphology
2.10. 2D Migration and Invasion
2.11. 3D Spheroid Invasion
2.12. EGF Treatment
2.13. RhoA Activation to Examine Invasion
2.14. Data Analysis
3. Results
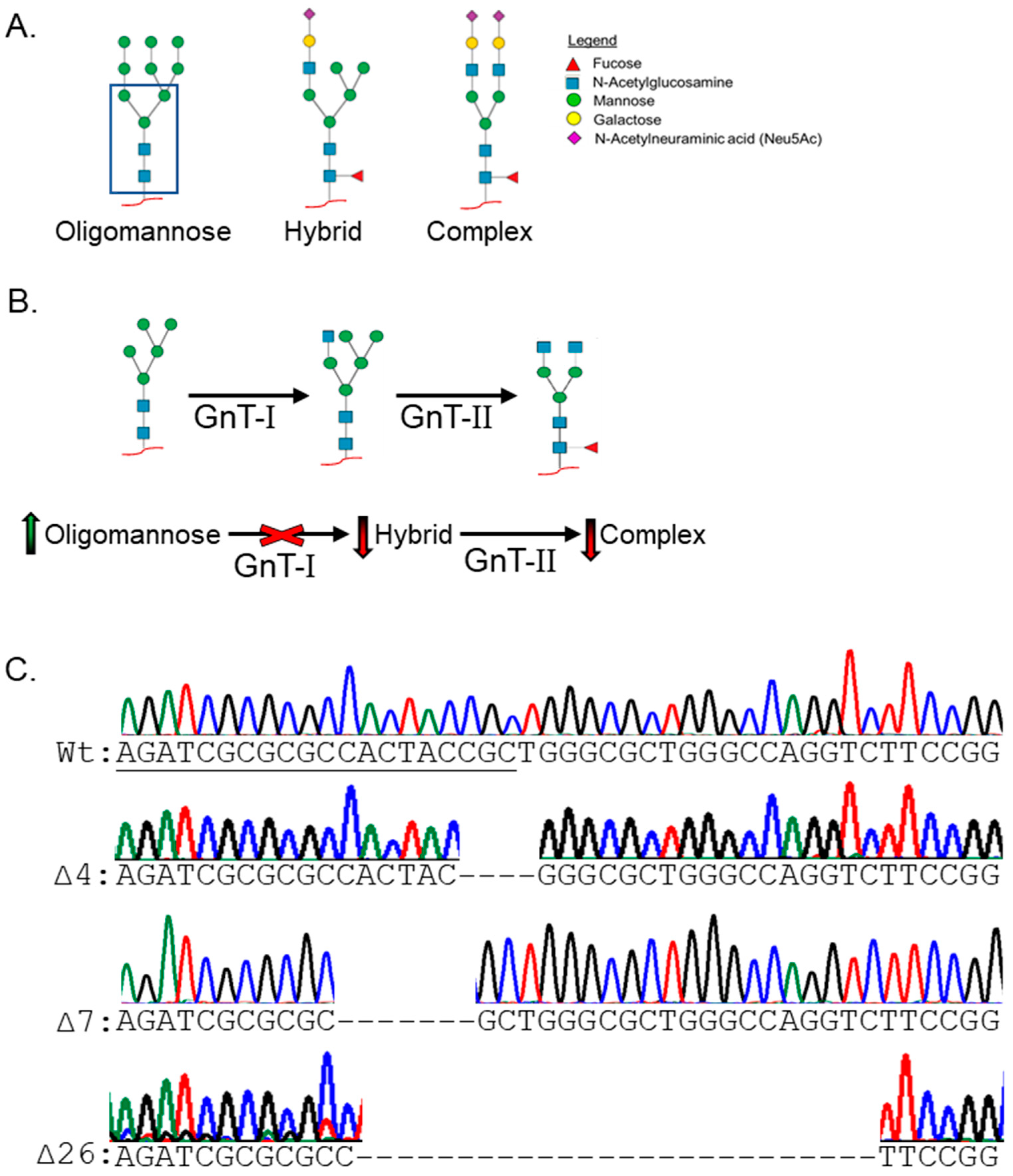
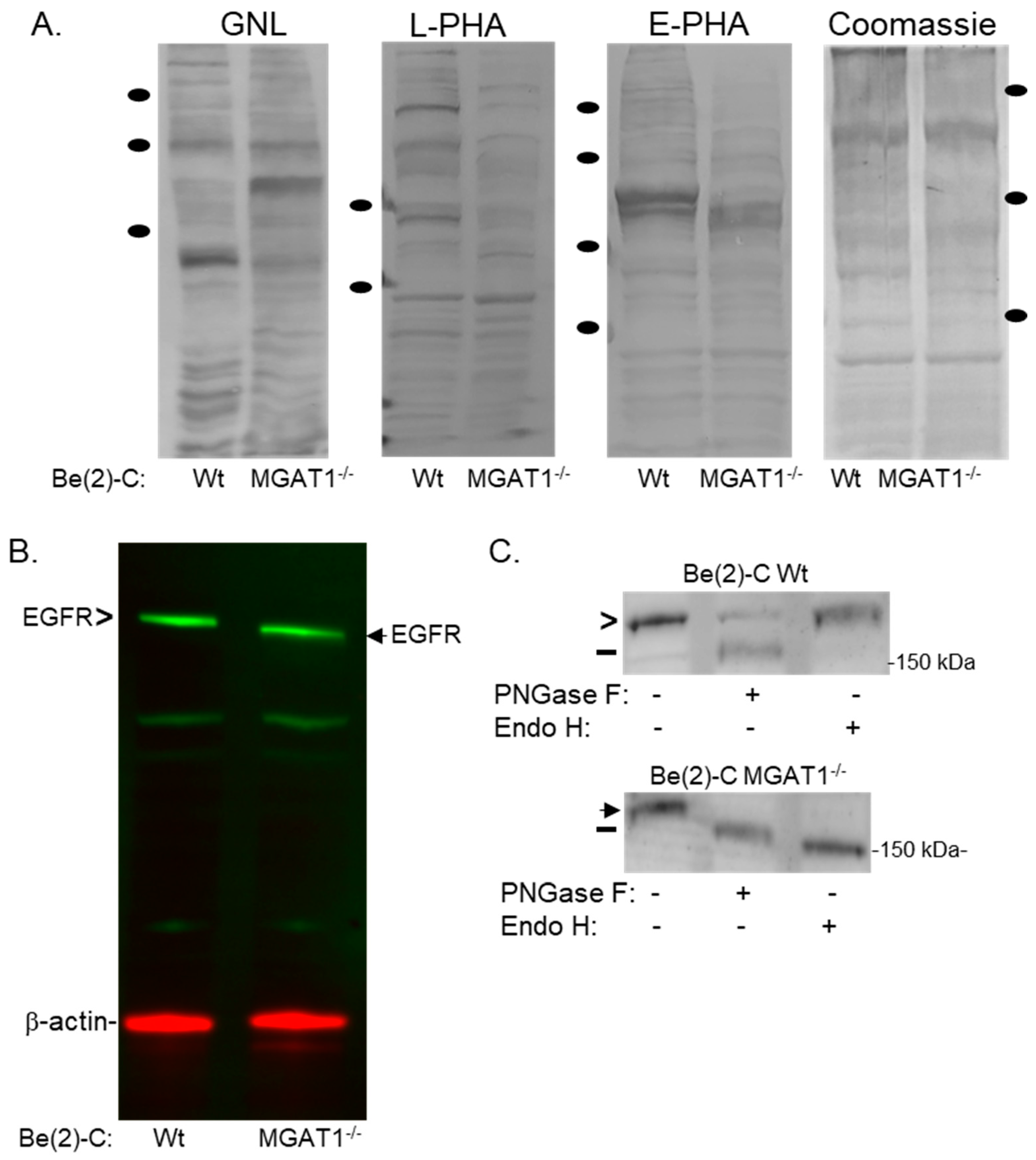
3.1. Manipulation of the N-glycan Processing Pathway to Yield Oligomannosylated EGFR
3.2. Rescue of Altered Cell–Cell Adhesion, Proliferation, and Morphology in the MGAT1 Mutant NB Cell Line
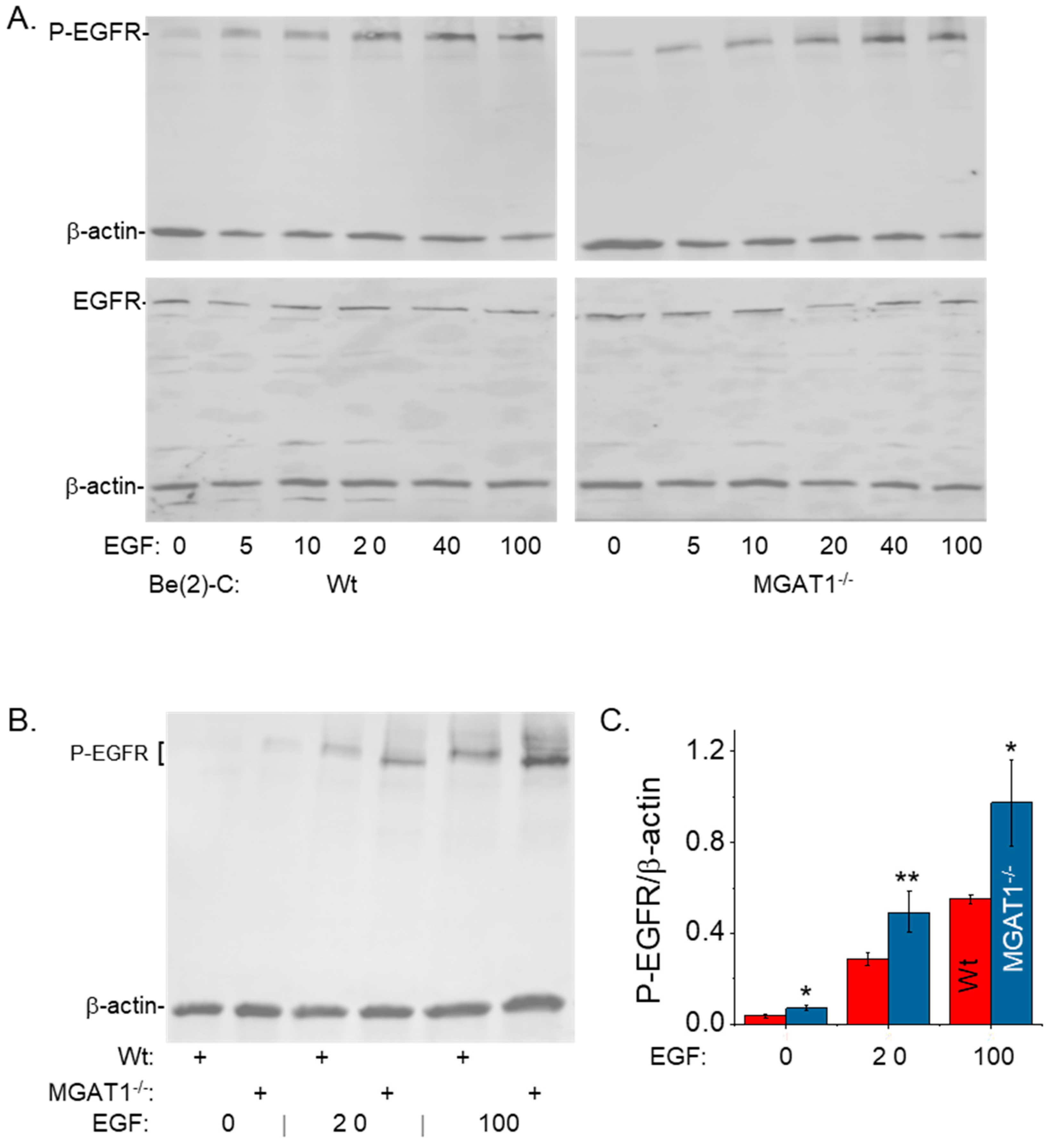
3.3. Oligomannosylated EGFRs Have an Increased Response to EGF Treatment and Sensitize NB Cells to EGF-Stimulated Proliferation
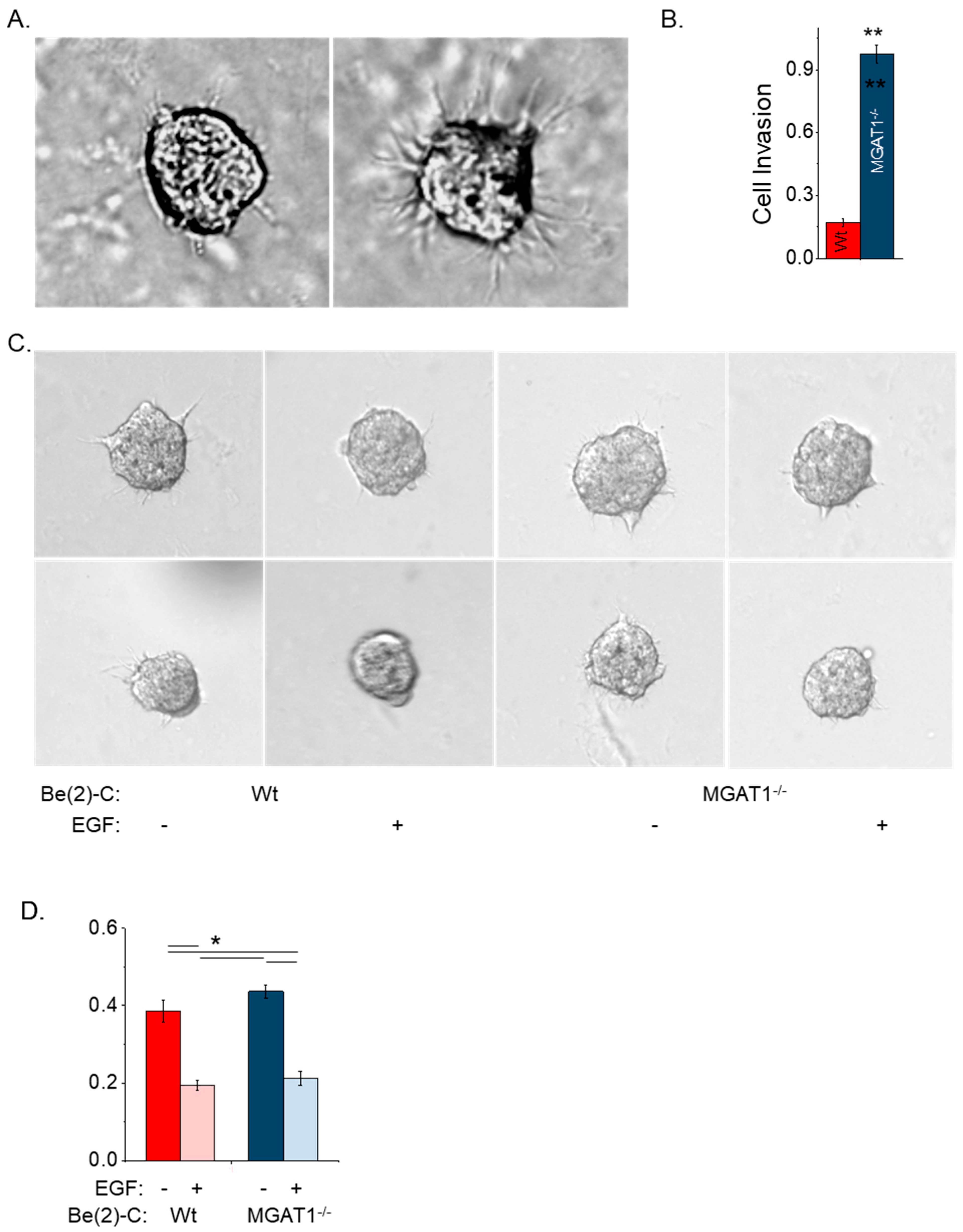
3.4. Cell Migration Is Unaltered While Cell Invasiveness Is Suppressed by Nonfunctional GnT-I Using 2D Dispersed Cell Culture
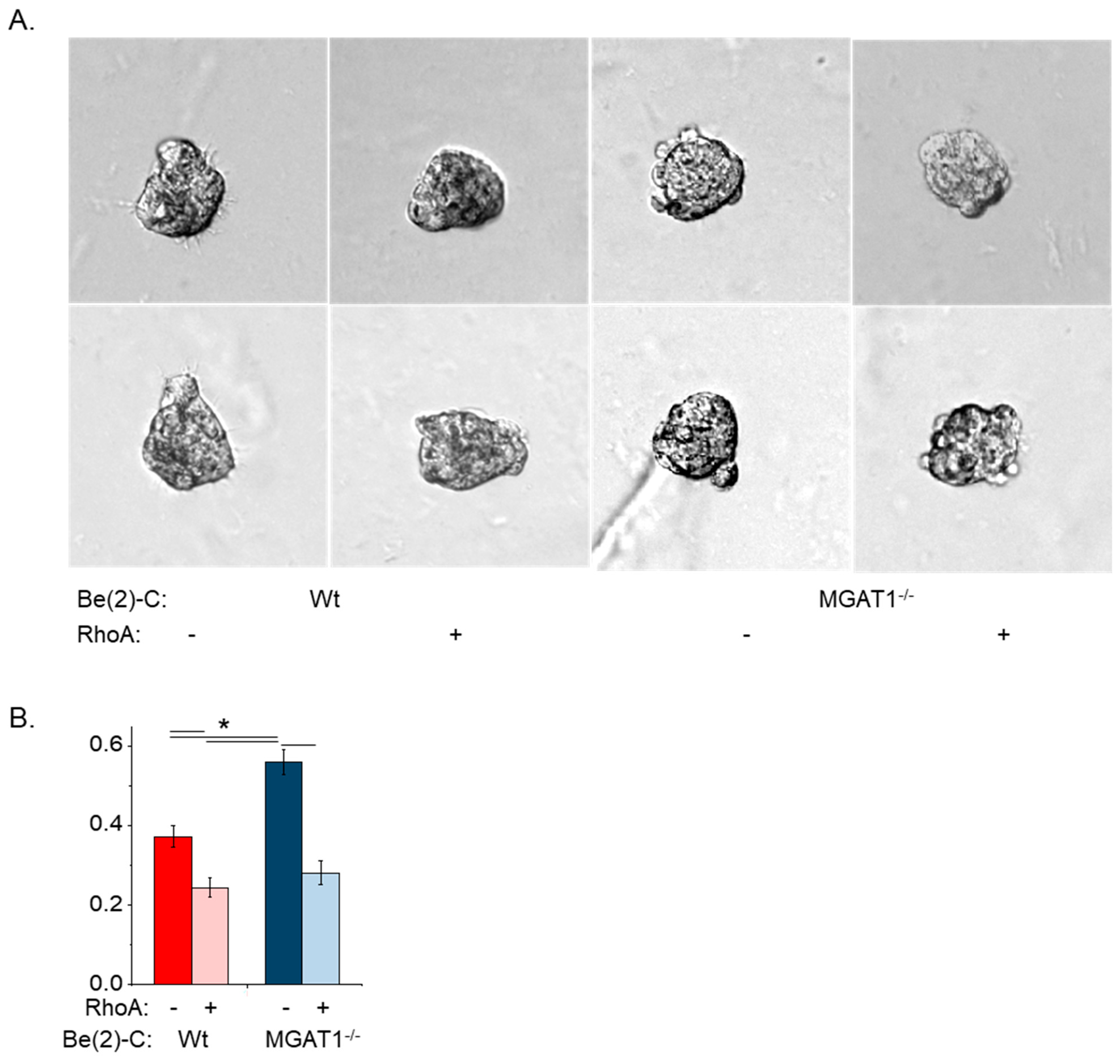
3.5. Oligomannose N-glycans Promote Cell Spheroid Invasiveness, and Both EGF and RhoA Treatment of NB Cell Lines Markedly Suppress Cell Spheroid Invasiveness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Wang, X.; Li, N.; Guo, Y.; Yang, X.; Lei, Y. Therapy resistance in neuroblastoma: Mechanisms and reversal strategies. Front. Pharmacol. 2023, 14, 1114295. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.L.; Hsu, W.M.; Huang, M.C.; Kadomatsu, K.; Nakagawara, A. Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma. J. Hematol. Oncol. 2016, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, T.; Nakagawara, A. Molecular and genetic bases of neuroblastoma. Int. J. Clin. Oncol. 2012, 17, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, W.; Hu, H.; Zhang, Y.; Li, J.; Huang, D. Factors of Recurrence After Complete Response in Children with Neuroblastoma: A 16-Year Retrospective Study of 179 Cases. Cancer Manag. Res. 2022, 14, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Matthay, K.K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.G.; Macy, M.E.; Henderson, T.O. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar] [CrossRef]
- Hall, M.K.; Burch, A.P.; Schwalbe, R.A. Functional analysis of N-acetylglucosaminyltransferase-I knockdown in 2D and 3D neuroblastoma cell cultures. PLoS ONE 2021, 16, e0259743. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.; Hatchett, C.J.; Shalygin, S.; Azadi, P.; Schwalbe, R.A. Reduction in N-Acetylglucosaminyltransferase-I Activity Decreases Survivability and Delays Development of Zebrafish. Curr. Issues Mol. Biol. 2023, 45, 9165–9180. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.T.; Condina, M.R.; Ho, Y.Y.; Everest-Dass, A.V.; Mittal, P.; Kaur, G.; Oehler, M.K.; Packer, N.H.; Hoffmann, P. MALDI Mass Spectrometry Imaging of Early- and Late-Stage Serous Ovarian Cancer Tissue Reveals Stage-Specific N-Glycans. Proteomics 2019, 19, e1800482. [Google Scholar] [CrossRef]
- Gilgunn, S.; Murphy, K.; Stöckmann, H.; Conroy, P.J.; Murphy, T.B.; Watson, R.W.; O’Kennedy, R.J.; Rudd, P.M.; Saldova, R. Glycosylation in Indolent, Significant and Aggressive Prostate Cancer by Automated High-Throughput N-Glycan Profiling. Int. J. Mol. Sci. 2020, 21, 9233. [Google Scholar] [CrossRef]
- Takayama, H.; Ohta, M.; Iwashita, Y.; Uchida, H.; Shitomi, Y.; Yada, K.; Inomata, M. Altered glycosylation associated with dedifferentiation of hepatocellular carcinoma: A lectin microarray-based study. BMC Cancer 2020, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, G.; Zhou, Y.; Zhang, X.; Sun, M.; Jiang, H.; Yu, G. Comprehensive N-Glycome Profiling of Cells and Tissues for Breast Cancer Diagnosis. J. Proteome Res. 2019, 18, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Del Grosso, F.; De Mariano, M.; Passoni, L.; Luksch, R.; Tonini, G.P.; Longo, L. Inhibition of N-linked glycosylation impairs ALK phosphorylation and disrupts pro-survival signaling in neuroblastoma cell lines. BMC Cancer 2011, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, H.; Becker, C.; Glüer, S.; Rösner, H.; Gerardy-Schahn, R.; Rahmann, H. Polysialic acid on the neural cell adhesion molecule correlates with expression of polysialyltransferases and promotes neuroblastoma cell growth. Cancer Res. 1998, 58, 779–784. [Google Scholar] [PubMed]
- Feduska, J.M.; Garcia, P.L.; Brennan, S.B.; Bu, S.; Council, L.N.; Yoon, K.J. N-glycosylation of ICAM-2 is required for ICAM-2-mediated complete suppression of metastatic potential of SK-N-AS neuroblastoma cells. BMC Cancer 2013, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Valentiner, U.; Mühlenhoff, M.; Lehmann, U.; Hildebrandt, H.; Schumacher, U. Expression of the neural cell adhesion molecule and polysialic acid in human neuroblastoma cell lines. Int. J. Oncol. 2011, 39, 417–424. [Google Scholar] [CrossRef]
- Qin, W.; Pei, H.; Li, X.; Li, J.; Yao, X.; Zhang, R. Serum Protein N-Glycosylation Signatures of Neuroblastoma. Front. Oncol. 2021, 11, 603417. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jiang, G.; Blume-Jensen, P.; Hunter, T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell Biol. 2001, 21, 4016–4031. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 (Suppl. S4), S9–S15. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Peckys, D.B.; Gaa, D.; de Jonge, N. Quantification of EGFR-HER2 Heterodimers in HER2-Overexpressing Breast Cancer Cells Using Liquid-Phase Electron Microscopy. Cells 2021, 10, 3244. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hasegawa, Y.; Gao, C.; Kuroki, Y.; Taniguchi, N. N-glycans of growth factor receptors: Their role in receptor function and disease implications. Clin. Sci. 2016, 130, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.J.; van de Wouwer, M.; André, S.; Villalobo, A. Down-regulation of the epidermal growth factor receptor by altering N-glycosylation: Emerging role of β1,4-galactosyltransferases. Anticancer Res. 2012, 32, 1565–1572. [Google Scholar] [PubMed]
- Liu, Y.C.; Yen, H.Y.; Chen, C.Y.; Chen, C.H.; Cheng, P.F.; Juan, Y.H.; Chen, C.H.; Khoo, K.H.; Yu, C.J.; Yang, P.C.; et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11332–11337. [Google Scholar] [CrossRef] [PubMed]
- Partridge, E.A.; Le Roy, C.; Di Guglielmo, G.M.; Pawling, J.; Cheung, P.; Granovsky, M.; Nabi, I.R.; Wrana, J.L.; Dennis, J.W. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 2004, 306, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Soderquist, A.M.; Carpenter, G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J. Biol. Chem. 1984, 259, 12586–12594. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.; Minturn, J.E.; Hishiki, T.; Zhao, H.; Wang, Q.; Cnaan, A.; Maris, J.; Evans, A.E.; Brodeur, G.M. Proliferation of human neuroblastomas mediated by the epidermal growth factor receptor. Cancer Res. 2005, 65, 9868–9875. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shen, R.; Li, K.; Zheng, N.; Zong, Y.; Ye, D.; Wang, Q.; Wang, Z.; Chen, L.; Ma, Y. Epidermal growth factor receptor is overexpressed in neuroblastoma tissues and cells. Acta Biochim. Biophys. Sin. 2016, 48, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Bucci, C. Role of EGFR in the Nervous System. Cells 2020, 9, 1887. [Google Scholar] [CrossRef]
- Kawashima, N.; Yoon, S.J.; Itoh, K.; Nakayama, K. Tyrosine kinase activity of epidermal growth factor receptor is regulated by GM3 binding through carbohydrate to carbohydrate interactions. J. Biol. Chem. 2009, 284, 6147–6155. [Google Scholar] [CrossRef]
- Hall, M.K.; Shajahan, A.; Burch, A.P.; Hatchett, C.J.; Azadi, P.; Schwalbe, R.A. Limited N-Glycan Processing Impacts Chaperone Expression Patterns, Cell Growth and Cell Invasiveness in Neuroblastoma. Biology 2023, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Labun, K.; Montague, T.G.; Gagnon, J.A.; Thyme, S.B.; Valen, E. CHOPCHOP v2: A web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016, 44, W272–W276. [Google Scholar] [CrossRef] [PubMed]
- Labun, K.; Montague, T.G.; Krause, M.; Torres Cleuren, Y.N.; Tjeldnes, H.; Valen, E. CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019, 47, W171–W174. [Google Scholar] [CrossRef] [PubMed]
- Montague, T.G.; Cruz, J.M.; Gagnon, J.A.; Church, G.M.; Valen, E. CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014, 42, W401–W407. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.; Weidner, D.A.; Whitman, A.A.; Schwalbe, R.A. Lack of complex type N-glycans lessens aberrant neuronal properties. PLoS ONE 2018, 13, e0199202. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.K.; Stanley, P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 2006, 416, 159–182. [Google Scholar] [PubMed]
- Kumar, R.; Yang, J.; Larsen, R.D.; Stanley, P. Cloning and expression of N-acetylglucosaminyltransferase I, the medial Golgi transferase that initiates complex N-linked carbohydrate formation. Proc. Natl. Acad. Sci. USA 1990, 87, 9948–9952. [Google Scholar] [CrossRef] [PubMed]
- Bojar, D.; Meche, L.; Meng, G.; Eng, W.; Smith, D.F.; Cummings, R.D.; Mahal, L.K. A Useful Guide to Lectin Binding: Machine-Learning Directed Annotation of 57 Unique Lectin Specificities. ACS Chem. Biol. 2022, 17, 2993–3012. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.; Chen, M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases 2013, 4, 141–147. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef]
- Cummings, R.D.; Soderquist, A.M.; Carpenter, G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J. Biol. Chem. 1985, 260, 11944–11952. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yokoe, S.; Asahi, M.; Lee, S.H.; Li, W.; Osumi, D.; Miyoshi, E.; Taniguchi, N. N-glycan of ErbB family plays a crucial role in dimer formation and tumor promotion. Biochim. Biophys. Acta 2008, 1780, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Whitson, K.B.; Whitson, S.R.; Red-Brewer, M.L.; McCoy, A.J.; Vitali, A.A.; Walker, F.; Johns, T.G.; Beth, A.H.; Staros, J.V. Functional effects of glycosylation at Asn-579 of the epidermal growth factor receptor. Biochemistry 2005, 44, 14920–14931. [Google Scholar] [CrossRef] [PubMed]
- Freyer, J.P.; Sutherland, R.M. Selective dissociation and characterization of cells from different regions of multicell tumor spheroids. Cancer Res. 1980, 40, 3956–3965. [Google Scholar] [PubMed]
- Lottich, S.C.; Johnston, W.W.; Szpak, C.A.; Delong, E.R.; Thor, A.; Schlom, J. Tumor-associated antigen TAG-72: Correlation of expression in primary and metastatic breast carcinoma lesions. Breast Cancer Res. Treat. 1985, 6, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.R.; Zhong, X.; Rescorla, F.J.; Hickey, R.J.; Malkas, L.H.; Sandoval, J.A. Proteomic approaches in neuroblastoma: A complementary clinical platform for the future. Expert. Rev. Proteom. 2009, 6, 387–394. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay. Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Inamori, K.; Gu, J.; Ohira, M.; Kawasaki, A.; Nakamura, Y.; Nakagawa, T.; Kondo, A.; Miyoshi, E.; Nakagawara, A.; Taniguchi, N. High expression of N-acetylglucosaminyltransferase V in favorable neuroblastomas: Involvement of its effect on apoptosis. FEBS Lett. 2006, 580, 627–632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deng, W.; Gu, L.; Li, X.; Zheng, J.; Zhang, Y.; Duan, B.; Cui, J.; Dong, J.; Du, J. CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells. J. Transl. Med. 2016, 14, 32. [Google Scholar] [CrossRef]
- Mateus, A.R.; Seruca, R.; Machado, J.C.; Keller, G.; Oliveira, M.J.; Suriano, G.; Luber, B. EGFR regulates RhoA-GTP dependent cell motility in E-cadherin mutant cells. Hum. Mol. Genet. 2007, 16, 1639–1647. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Qu, R.; Zhang, L.; Huang, W. Rho A Regulates Epidermal Growth Factor-Induced Human Osteosarcoma MG63 Cell Migration. Int. J. Mol. Sci. 2018, 19, 1437. [Google Scholar] [CrossRef] [PubMed]
- Dyberg, C.; Fransson, S.; Andonova, T.; Sveinbjörnsson, B.; Lännerholm-Palm, J.; Olsen, T.K.; Forsberg, D.; Herlenius, E.; Martinsson, T.; Brodin, B.; et al. Rho-associated kinase is a therapeutic target in neuroblastoma. Proc. Natl. Acad. Sci. USA 2017, 114, E6603–E6612. [Google Scholar] [CrossRef] [PubMed]
- Lock, F.E.; Ryan, K.R.; Poulter, N.S.; Parsons, M.; Hotchin, N.A. Differential regulation of adhesion complex turnover by ROCK1 and ROCK2. PLoS ONE 2012, 7, e31423. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.; Liang, X.; Teo, J.L.; Duszyc, K.; Yap, A.S.; Gomez, G.A. ROCK1 but not ROCK2 contributes to RhoA signaling and NMIIA-mediated contractility at the epithelial zonula adherens. Mol. Biol. Cell 2017, 28, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Multhaupt, H.A.; Couchman, J.R. The Rho kinases I and II regulate different aspects of myosin II activity. J. Cell Biol. 2005, 170, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Abualsaud, N.; Caprio, L.; Galli, S.; Krawczyk, E.; Alamri, L.; Zhu, S.; Gallicano, G.I.; Kitlinska, J. Neuropeptide Y/Y5 Receptor Pathway Stimulates Neuroblastoma Cell Motility Through RhoA Activation. Front. Cell Dev. Biol. 2020, 8, 627090. [Google Scholar] [CrossRef]
- Fife, C.M.; Sagnella, S.M.; Teo, W.S.; Po’uha, S.T.; Byrne, F.L.; Yeap, Y.Y.; Ng, D.C.; Davis, T.P.; McCarroll, J.A.; Kavallaris, M. Stathmin mediates neuroblastoma metastasis in a tubulin-independent manner via RhoA/ROCK signaling and enhanced transendothelial migration. Oncogene 2017, 36, 501–511. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burch, A.P.; Hall, M.K.; Wease, D.; Schwalbe, R.A. Reduction of N-Acetylglucosaminyltransferase-I Activity Promotes Neuroblastoma Invasiveness and EGF-Stimulated Proliferation In Vitro. Int. J. Transl. Med. 2024, 4, 519-538. https://doi.org/10.3390/ijtm4030035
Burch AP, Hall MK, Wease D, Schwalbe RA. Reduction of N-Acetylglucosaminyltransferase-I Activity Promotes Neuroblastoma Invasiveness and EGF-Stimulated Proliferation In Vitro. International Journal of Translational Medicine. 2024; 4(3):519-538. https://doi.org/10.3390/ijtm4030035
Chicago/Turabian StyleBurch, Adam P., M. Kristen Hall, Debra Wease, and Ruth A. Schwalbe. 2024. "Reduction of N-Acetylglucosaminyltransferase-I Activity Promotes Neuroblastoma Invasiveness and EGF-Stimulated Proliferation In Vitro" International Journal of Translational Medicine 4, no. 3: 519-538. https://doi.org/10.3390/ijtm4030035
APA StyleBurch, A. P., Hall, M. K., Wease, D., & Schwalbe, R. A. (2024). Reduction of N-Acetylglucosaminyltransferase-I Activity Promotes Neuroblastoma Invasiveness and EGF-Stimulated Proliferation In Vitro. International Journal of Translational Medicine, 4(3), 519-538. https://doi.org/10.3390/ijtm4030035





