Non-Invasive In Vivo Bioimaging in Pigs
Abstract
:1. Introduction
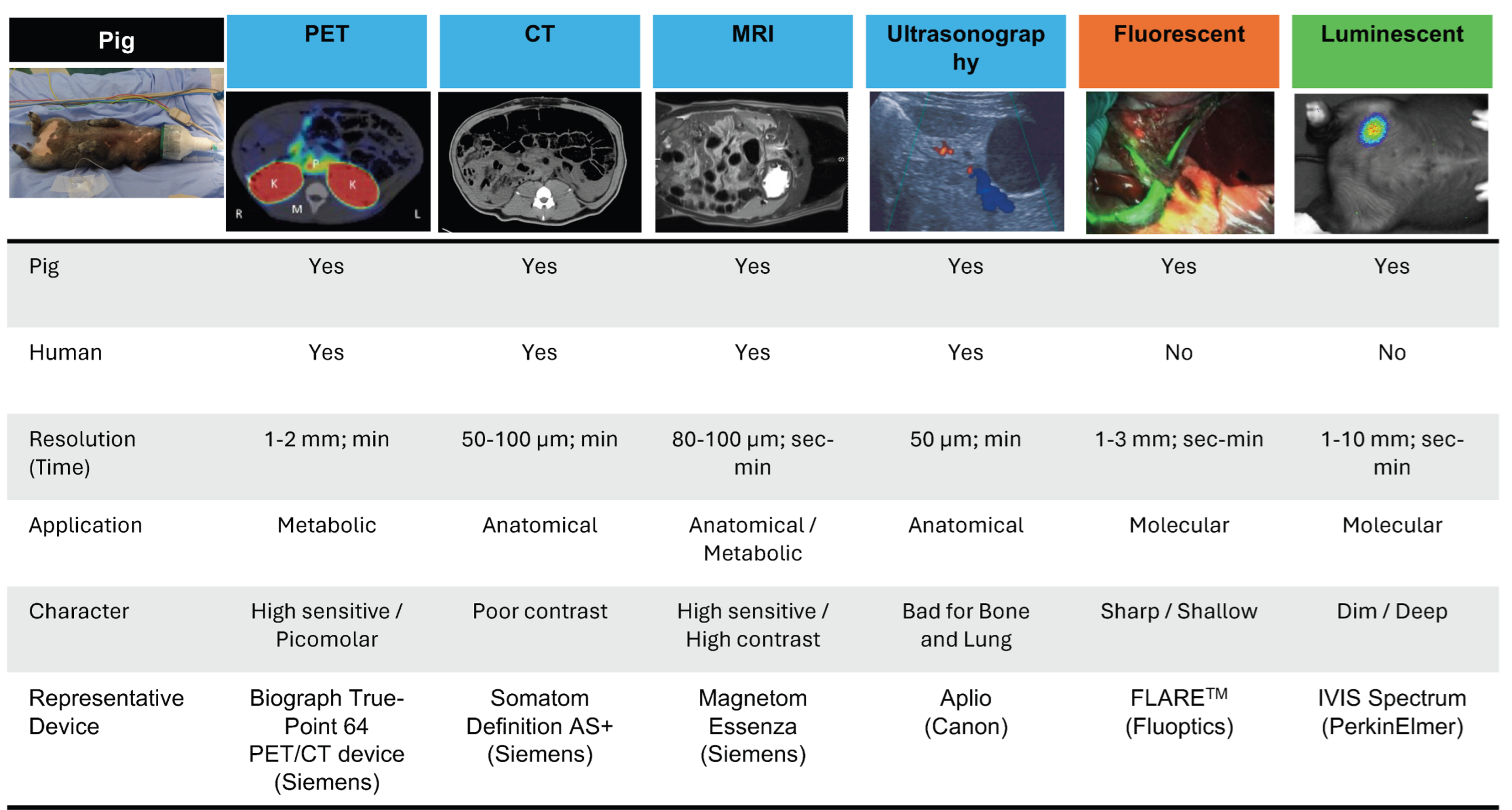
1.1. Common Imaging Techniques
1.2. Scope and Objectives
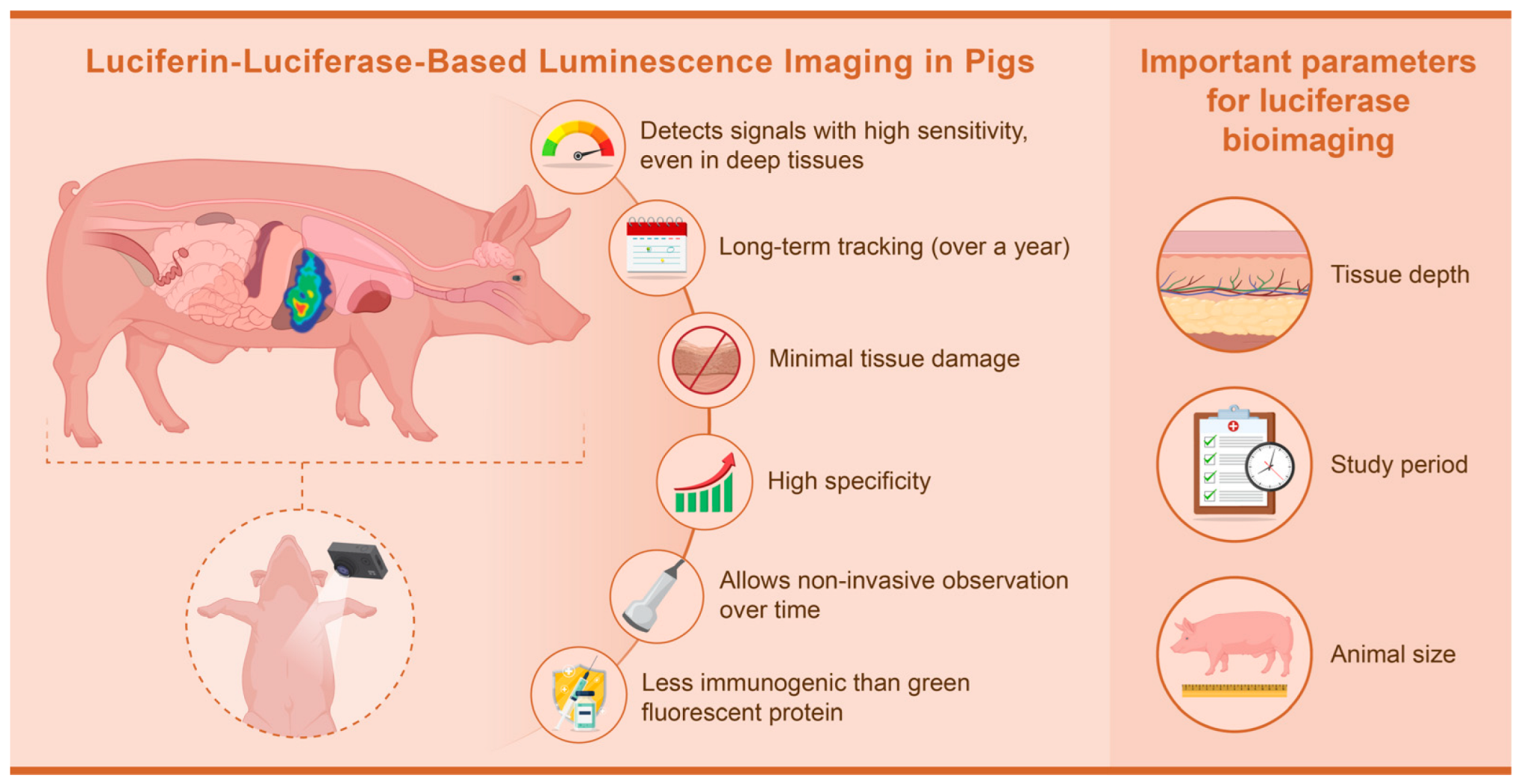
1.3. Reason for Selecting Luminescence Imaging
2. Conventional Non-Invasive In Vivo Imaging in Pigs
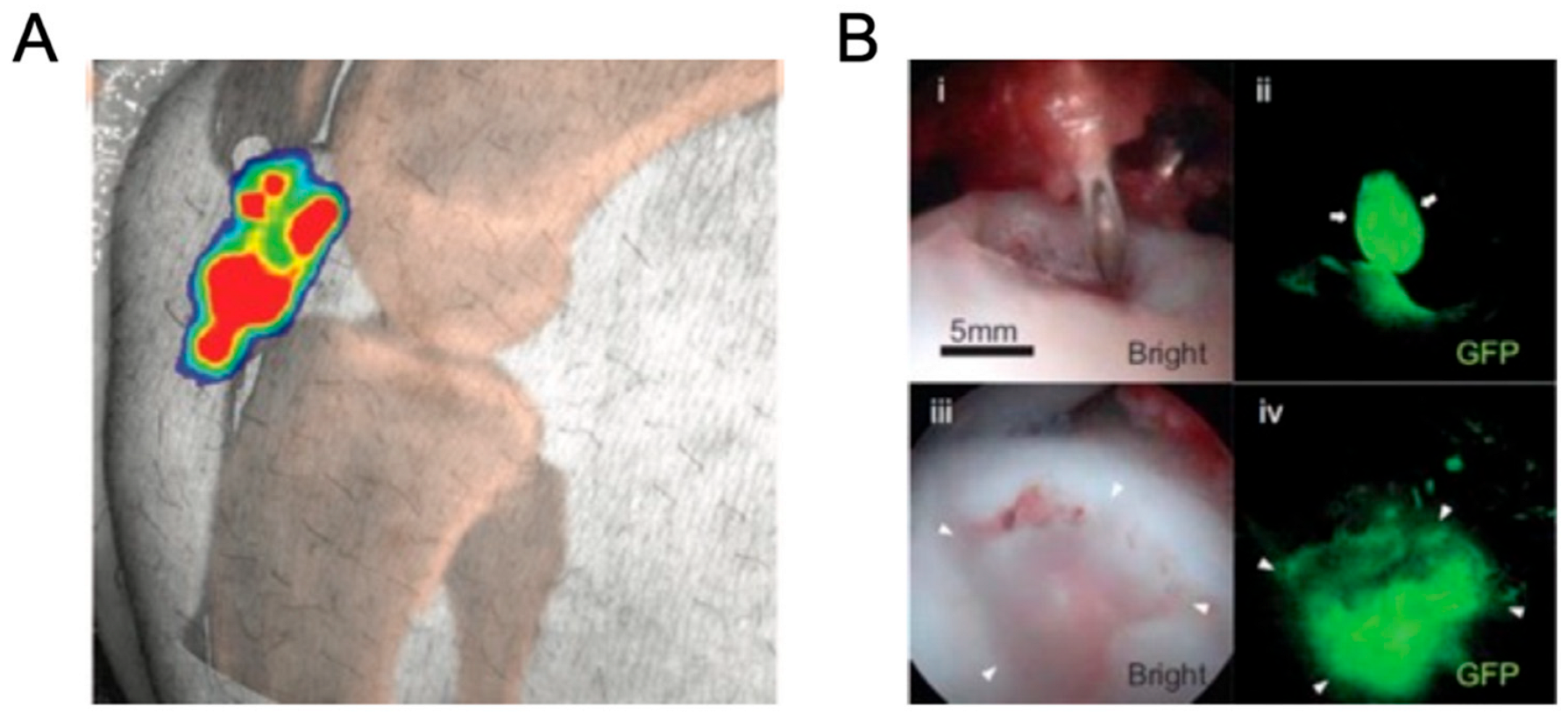
3. Luciferase Bioimaging in Pigs
4. Discussion
4.1. Important Parameters for Luciferase Bioimaging in Pigs
4.1.1. Depth
4.1.2. Study Period
4.1.3. Animal Size
4.1.4. Disadvantages of Luminescence Imaging for Research Using Pigs
4.2. Comparison of Fluorescent and Luminescent Labeling in Gene and Cell Therapy Research Using Pigs
4.2.1. Phototoxicity
4.2.2. Immunogenicity
4.3. Specific Points for Luminescence Bioimaging
4.3.1. Imaging Mechanisms
4.3.2. Efficacy and Sensitivity
4.3.3. Challenges and Limitations
4.3.4. Risk Assessment
4.3.5. Regulatory and Ethical Issues
4.3.6. Diagnostic Applications
5. Conclusions
6. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mettler, F.A. Essentials of Radiology, 2nd ed.; Elsevier Saunders: Philadelphia, PA, USA, 2005; ISBN 978-0-7216-0527-2. [Google Scholar]
- Jensen, J.A. Medical Ultrasound Imaging. Prog. Biophys. Mol. Biol. 2007, 93, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Biomed. Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Miglioretti, D.L.; Johnson, E.; Lee, C.; Feigelson, H.S.; Flynn, M.; Greenlee, R.T.; Kruger, R.L.; Hornbrook, M.C.; Roblin, D.; et al. Use of Diagnostic Imaging Studies and Associated Radiation Exposure for Patients Enrolled in Large Integrated Health Care Systems, 1996–2010. JAMA 2012, 307, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V.L. Technological Advancements in Cancer Diagnostics: Improvements and Limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, D.; Ahuja, K.; Grover, H.; Sharma, P.; Solanki, S.; Gupta, N.; Patel, L. Review of X-ray and Computed Tomography Scan Findings with a Promising Role of Point of Care Ultrasound in COVID-19 Pandemic. World J. Radiol. 2020, 12, 195–203. [Google Scholar] [CrossRef]
- Seeram, E. Computed Tomography: Physics and Technology: A Self Assessment Guide, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2022; ISBN 978-1-119-81932-5. [Google Scholar]
- Meulepas, J.M.; Ronckers, C.M.; Smets, A.M.J.B.; Nievelstein, R.A.J.; Jahnen, A.; Lee, C.; Kieft, M.; Laméris, J.S.; van Herk, M.; Greuter, M.J.W.; et al. Leukemia and Brain Tumors among Children after Radiation Exposure from CT Scans: Design and Methodological Opportunities of the Dutch Pediatric CT Study. Eur. J. Epidemiol. 2014, 29, 293–301. [Google Scholar] [CrossRef]
- Beckett, K.R.; Moriarity, A.K.; Langer, J.M. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics 2015, 35, 1738–1750. [Google Scholar] [CrossRef]
- Yeh, B.M.; FitzGerald, P.F.; Edic, P.M.; Lambert, J.W.; Colborn, R.E.; Marino, M.E.; Evans, P.M.; Roberts, J.C.; Wang, Z.J.; Wong, M.J.; et al. Opportunities for New CT Contrast Agents to Maximize the Diagnostic Potential of Emerging Spectral CT Technologies. Adv. Drug Deliv. Rev. 2017, 113, 201–222. [Google Scholar] [CrossRef]
- Burstein, D. MRI for Development of Disease-Modifying Osteoarthritis Drugs. NMR Biomed. 2006, 19, 669–680. [Google Scholar] [CrossRef]
- Pando-Naude, V.; Toxto, S.; Fernandez-Lozano, S.; Parsons, C.E.; Alcauter, S.; Garza-Villarreal, E.A. Gray and White Matter Morphology in Substance Use Disorders: A Neuroimaging Systematic Review and Meta-Analysis. Transl. Psychiatry 2021, 11, 29. [Google Scholar] [CrossRef]
- Weiskopf, N.; Edwards, L.J.; Helms, G.; Mohammadi, S.; Kirilina, E. Quantitative Magnetic Resonance Imaging of Brain Anatomy and in Vivo Histology. Nat. Rev. Phys. 2021, 3, 570–588. [Google Scholar] [CrossRef]
- Zugni, F.; Mariani, L.; Lambregts, D.M.J.; Maggioni, R.; Summers, P.E.; Granata, V.; Pecchi, A.; Di Costanzo, G.; De Muzio, F.; Cardobi, N.; et al. Whole-Body MRI in Oncology: Acquisition Protocols, Current Guidelines, and Beyond. Radiol. Med. 2024, 129, 1352–1368. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, G.; Namavari, M.; Jouannot, E.B.; Hoehne, A.; Reeves, R.; Hardy, J.; Gambhir, S.S. Investigation of 6-[18F]-Fluoromaltose as a Novel PET Tracer for Imaging Bacterial Infection. PLoS ONE 2014, 9, e107951. [Google Scholar] [CrossRef]
- Saboury, B.; Morris, M.A.; Farhadi, F.; Nikpanah, M.; Werner, T.J.; Jones, E.C.; Alavi, A. Reinventing Molecular Imaging with Total-Body PET, Part I: Technical Revolution in Evolution. PET Clin. 2020, 15, 427–438. [Google Scholar] [CrossRef]
- Haroon, A.; Zumla, A.; Bomanji, J. Role of Fluorine 18 Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography in Focal and Generalized Infectious and Inflammatory Disorders. Clin. Infect. Dis. 2012, 54, 1333–1341. [Google Scholar] [CrossRef]
- Mohan, A.-M.; Beindorff, N.; Brenner, W. Nuclear Medicine Imaging Procedures in Oncology. In Metastasis; Stein, U.S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2294, pp. 297–323. ISBN 978-1-07-161349-8. [Google Scholar]
- Gonzalez Rojas, N.; Cesarini, M.E.; Peker, G.; Da Prat, G.A.; Etcheverry, J.L.; Gatto, E.M. Review of Huntington’s Disease: From Basics to Advances in Diagnosis and Treatment. J. Neurol. Res. 2022, 12, 93–113. [Google Scholar] [CrossRef]
- Malaiya, A.; Singhai, M.; Singh, M.; Prajapati, S.K.; Choudhury, H.; Fatima, M.; Alexander, A.; Dubey, S.K.; Greish, K.; Kesharwani, P. Recent Update on the Alzheimer’s Disease Progression, Diagnosis and Treatment Approaches. Curr. Drug Targets 2022, 23, 978–1001. [Google Scholar] [CrossRef]
- Katal, S.; Eibschutz, L.S.; Saboury, B.; Gholamrezanezhad, A.; Alavi, A. Advantages and Applications of Total-Body PET Scanning. Diagnostics 2022, 12, 426. [Google Scholar] [CrossRef]
- Chelikam, N.; Vyas, A.; Desai, R.; Khan, N.; Raol, K.; Kavarthapu, A.; Kamani, P.; Ibrahim, G.; Madireddy, S.; Pothuru, S.; et al. Past and Present of Point-of-Care Ultrasound (PoCUS): A Narrative Review. Cureus 2023, 15, e50155. [Google Scholar] [CrossRef]
- Chan, V.; Perlas, A. Basics of Ultrasound Imaging. In Atlas of Ultrasound-Guided Procedures in Interventional Pain Management; Narouze, S.N., Ed.; Springer: New York, NY, USA, 2011; pp. 13–19. ISBN 978-1-4419-1679-2. [Google Scholar]
- Barik, S.; Spring, J.E.; Jones, M.H.; Luck, C.A. Routine Ultrasound Scanning in Pregnancy. The Benefits Are Clinical. BMJ 1993, 307, 559. [Google Scholar] [CrossRef]
- Bourgioti, C.; Konidari, M.; Gourtsoyianni, S.; Moulopoulos, L.A. Imaging during Pregnancy: What the Radiologist Needs to Know. Diagn. Interv. Imaging 2021, 102, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Albakri, A.A.; Alzahrani, M.M.; Alghamdi, S.H. Medical Imaging in Pregnancy: Safety, Appropriate Utilization, and Alternative Modalities for Imaging Pregnant Patients. Cureus 2024, 16, e54346. [Google Scholar] [CrossRef] [PubMed]
- Quarato, C.M.I.; Lacedonia, D.; Salvemini, M.; Tuccari, G.; Mastrodonato, G.; Villani, R.; Fiore, L.A.; Scioscia, G.; Mirijello, A.; Saponara, A.; et al. A Review on Biological Effects of Ultrasounds: Key Messages for Clinicians. Diagnostics 2023, 13, 855. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hanazono, Y.; Kunita, S. Swine used in the Medical University: Overview of 20 years of experience. Exp. Anim. 2018, 67, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Meomartino, L.; Gnudi, G.; Brunetti, A.; Di Giancamillo, M. Imaging Techniques in Veterinary Medicine. Part II: Computed Tomography, Magnetic Resonance Imaging, Nuclear Medicine. Eur. J. Radiol. Open 2023, 10, 100467. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Pane, S.; Iacovacci, V.; Koukourakis, N.; Czarske, J.; Menciassi, A.; Medina-Sánchez, M.; Schmidt, O.G. Medical Imaging of Microrobots: Toward In Vivo Applications. ACS Nano 2020, 14, 10865–10893. [Google Scholar] [CrossRef]
- Barbagianni, M.S.; Gouletsou, P.G. Modern Imaging Techniques in the Study and Disease Diagnosis of the Mammary Glands of Animals. Vet. Sci. 2023, 10, 83. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, Y.; Wang, X.; Li, H.; Qiu, J.; Lu, W. Application of photoacoustic computed tomography in biomedical imaging: A literature review. Bioeng. Transl. Med. 2023, 8, e10419. [Google Scholar] [CrossRef]
- Hirai, M.; Sakurada, T.; Ikeda, T.; Monden, Y.; Shimoizumi, H.; Yamagata, T. Developmental changes of the neural mechanisms underlying Level 2 visual perspective-taking: A functional near-infrared spectroscopy study. Dev. Psychobiol. 2022, 64, e22229. [Google Scholar] [CrossRef]
- Noel, S.J.; Jørgensen, H.J.H.; Knudsen, K.E.B. The use of near-infrared spectroscopy (NIRS) to determine the energy value of individual feedstuffs and mixed diets for pigs. Anim. Feed. Sci. Technol. 2022, 283, 115156. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Saito-Moriya, R.; Obata, R.; Maki, S.A. Near-Infrared luciferin Analogs for in vivo optical Imaging. In Bioluminescence—Technology and Biology; Suzuki, H., Ogoh, K., Eds.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83962-385-1. [Google Scholar]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.-Q. Large mammalian animal models of heart disease. J. Cardiovasc. Dev. Dis. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Higashi, A.; Izawa, Y.; Hishikawa, S. Organ perfusion during partial REBOA in haemorrhagic shock: Dynamic 4D-CT analyses in swine. Sci. Rep. 2022, 12, 18745. [Google Scholar] [CrossRef] [PubMed]
- Sasanuma, H.; Takahashi, T.; Kawai, S.; Saitsu, A.; Kurashina, W.; Iijima, Y.; Saito, T.; Takeshita, K. Morphological and histological evaluation of the tendon-bone junction in porcine shoulders to create a rotator cuff tear and repair model. J. Orthop. Sci. 2023. [Google Scholar] [CrossRef]
- Tomaru, Y.; Sugaya, H.; Yoshioka, T.; Arai, N.; Abe, T.; Tsukagoshi, Y.; Kamada, H.; Yamazaki, M.; Mishima, H. Effects of bone marrow-derived mesenchymal stem cell transplantation in piglet legg-calve-perthes disease models: A pilot study. J. Pediatr. Orthop. B 2023, 33, 358–362. [Google Scholar] [CrossRef]
- Miura, M.; Miura, Y.; Iwazu, Y.; Mukai, H.; Sugiura, T.; Suzuki, Y.; Kato, M.; Kano, M.; Nagata, D.; Shiizaki, K.; et al. Removal of calciprotein particles from the blood using an adsorption column improves prognosis of hemodialysis miniature pigs. Sci. Rep. 2023, 13, 15026. [Google Scholar] [CrossRef]
- Fil, J.E.; Joung, S.; Zimmerman, B.J.; Sutton, B.P.; Dilger, R.N. High-Resolution Magnetic Resonance Imaging-Based Atlases for the Young and Adolescent Domesticated Pig (Sus Scrofa). J. Neurosci. Methods 2021, 354, 109107. [Google Scholar] [CrossRef]
- Simchick, G.; Shen, A.; Campbell, B.; Park, H.J.; West, F.D.; Zhao, Q. Pig brains have homologous resting-state networks with human brains. Brain Connect. 2019, 9, 566–579. [Google Scholar] [CrossRef]
- Norris, C.; Lisinski, J.; McNeil, E.; VanMeter, J.W.; VandeVord, P.; LaConte, S.M. MRI brain templates of the male Yucatan minipig. Neuroimage 2021, 235, 118015. [Google Scholar] [CrossRef]
- Hellman, A.; Maietta, T.; Clum, A.; Byraju, K.; Raviv, N.; Staudt, M.D.; Jeannotte, E.; Nalwalk, J.; Belin, S.; Poitelon, Y.; et al. Development of a common peroneal nerve injury model in domestic swine for the study of translational neuropathic pain Treatments. J. Neurosurg. 2021, 135, 1516–1523. [Google Scholar] [CrossRef]
- Alstrup, A.K.O.; Dollerup, M.R.; Simonsen, M.I.T.; Vendelbo, M.H. Preclinical imaging studies: Protocols, preparation, anesthesia, and animal care. Semin. Nucl. Med. 2023, 53, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Cumming, P.; Scheidegger, M.; Dornbierer, D.; Palner, M.; Quednow, B.B.; Martin-Soelch, C. Molecular and functional imaging studies of psychedelic drug action in animals and humans. Molecules 2021, 26, 2451. [Google Scholar] [CrossRef] [PubMed]
- Pilz, J.; Gloddek, N.; Lindheimer, F.; Lindner, M.J.; Puhr-Westerheide, D.; Ümütlü, M.; Cyran, C.; Seidensticker, M.; Lindner, R.; Kraetzl, M.; et al. Functional maturation and longitudinal imaging of intraportal neonatal porcine islet grafts in genetically diabetic pigs. Am. J. Transplant. 2024, 24, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Hyun, H.; Vargas, C.; Gravier, J.; Park, G.; Gioux, S.; Frangioni, J.V.; Henary, M.; Choi, H.S. Pancreas-targeted NIR fluorophores for dual-channel image-guided abdominal surgery. Theranostics 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Hemetsberger, R.; Wolbank, S.; Pichler, V.; Kaun, C.; Posa, A.; Petrasi, Z.; Petnehazy, Ö.; Hofer-Warbinek, R.; De Martin, R.; et al. Delayed recovery of myocardial blood flow after intracoronary stem cell administration. Stem Cell Rev. Rep. 2011, 7, 616–623. [Google Scholar] [CrossRef]
- Rodenberg, E.J.; Patel, D.S.; Shirley, B.; Young, B.W.; Taylor, A.F.; Steidinger, H.R.; Fisher, S.J.; Patel, A.N. Catheter-based retrograde coronary sinus infusion is a practical delivery technique for introducing biological molecules into the cardiac system. Catheter. Cardiovasc. Interv. 2019, 94, 669–676. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Jablonska, A.; Chu, C.; Gregg, L.; Bulte, J.W.M.; Koehler, R.C.; Walczak, P.; Janowski, M. Biodistribution of glial progenitors in a three dimensional-printed model of the piglet cerebral ventricular system. Stem Cells Dev. 2019, 28, 515–527. [Google Scholar] [CrossRef]
- Watano, R.; Ohmori, T.; Hishikawa, S.; Sakata, A.; Mizukami, H. Utility of microminipigs for evaluating liver-mediated gene expression in the presence of neutralizing antibody against vector capsid. Gene Ther. 2020, 27, 427–434. [Google Scholar] [CrossRef]
- Kremen, T.J.; Stefanovic, T.; Tawackoli, W.; Salehi, K.; Avalos, P.; Reichel, D.; Perez, M.J.; Glaeser, J.D.; Sheyn, D. A translational porcine model for human cell-based therapies in the treatment of posttraumatic osteoarthritis after anterior cruciate ligament injury. Am. J. Sports Med. 2020, 48, 3002–3012. [Google Scholar] [CrossRef]
- Nakamura, T.; Sekiya, I.; Muneta, T.; Hatsushika, D.; Horie, M.; Tsuji, K.; Kawarasaki, T.; Watanabe, A.; Hishikawa, S.; Fujimoto, Y.; et al. Arthroscopic, histological and mri analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 2012, 14, 327–338. [Google Scholar] [CrossRef]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically modified porcine-to-human cardiac xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Endo, K.; Hanazono, Y.; Kobayashi, E. In vivo luciferin–luciferase reaction in pigs using xenogeneic rat bone marrow transplantation: A case report and consideration of recent advance. Int. J. Mol. Sci. 2024, 25, 8609. [Google Scholar] [CrossRef] [PubMed]
- Hakamata, Y.; Murakami, T.; Kobayashi, E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation 2006, 81, 1179–1184. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hakamata, Y.; Enosawa, S.; Shang, K.-M.; Komatsu, H. Firefly rats: Illuminating the scientific community in transplantation research. Cell Transplant. 2024, 33, 9636897231224174. [Google Scholar] [CrossRef]
- Takaku, Y.; Murai, K.; Ukai, T.; Ito, S.; Kokubo, M.; Satoh, M.; Kobayashi, E.; Yamato, M.; Okano, T.; Takeuchi, M.; et al. In Vivo Cell Tracking by Bioluminescence Imaging after Transplantation of Bioengineered Cell Sheets to the Knee Joint. Biomaterials 2014, 35, 2199–2206. [Google Scholar] [CrossRef]
- Ashmore-Harris, C.; Iafrate, M.; Saleem, A.; Fruhwirth, G.O. Non-Invasive Reporter Gene Imaging of Cell Therapies, Including T Cells and Stem Cells. Mol. Ther. 2020, 28, 1392–1416. [Google Scholar] [CrossRef]
- Salinas-Jazmín, N.; Rosas-Cruz, A.; Velasco-Velázquez, M. Reporter Gene Systems for the Identification and Characterization of Cancer Stem Cells. World J. Stem Cells 2021, 13, 861–876. [Google Scholar] [CrossRef]
- Yoon, S.; Cheon, S.Y.; Park, S.; Lee, D.; Lee, Y.; Han, S.; Kim, M.; Koo, H. Recent Advances in Optical Imaging through Deep Tissue: Imaging Probes and Techniques. Biomater. Res. 2022, 26, 57. [Google Scholar] [CrossRef]
- Refaat, A.; Yap, M.L.; Pietersz, G.; Walsh, A.P.G.; Zeller, J.; Del Rosal, B.; Wang, X.; Peter, K. In Vivo Fluorescence Imaging: Success in Preclinical Imaging Paves the Way for Clinical Applications. J. Nanobiotechnol. 2022, 20, 450. [Google Scholar] [CrossRef]
- Wu, D.; Lombaert, I.M.A.; DeLeon, M.; Pradhan-Bhatt, S.; Witt, R.L.; Harrington, D.A.; Trombetta, M.G.; Passineau, M.J.; Farach-Carson, M.C. Immunosuppressed Miniswine as a Model for Testing Cell Therapy Success: Experience with Implants of Human Salivary Stem/Progenitor Cell Constructs. Front. Mol. Biosci. 2021, 8, 711602. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chang, S.; Guo, W.; Zhang, S.; Chen, Z.K. Progress in Liver Transplant Tolerance and Tolerance-Inducing Cellular Therapies. Front. Immunol. 2020, 11, 1326. [Google Scholar] [CrossRef] [PubMed]
- Magidson, V.; Khodjakov, A. Circumventing Photodamage in Live-Cell Microscopy. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 114, pp. 545–560. ISBN 978-0-12-407761-4. [Google Scholar]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Allport, J.R.; Weissleder, R. In Vivo Imaging of Gene and Cell Therapies. Exp. Hematol. 2001, 29, 1237–1246. [Google Scholar] [CrossRef]
- Saito-Moriya, R.; Nakayama, J.; Kamiya, G.; Kitada, N.; Obata, R.; Maki, S.A.; Aoyama, H. How to select firefly luciferin analogues for in vivo imaging. Int. J. Mol. Sci. 2021, 22, 1848. [Google Scholar] [CrossRef]
- Srinivas, M.; Aarntzen, E.H.J.G.; Bulte, J.W.M.; Oyen, W.J.; Heerschap, A.; de Vries, I.J.M.; Figdor, C.G. Imaging of cellular therapies. Adv. Drug Deliv. Rev. 2010, 62, 1080–1093. [Google Scholar] [CrossRef]
- Sharifian, S.; Homaei, A.; Hemmati, R.; Luwor, R.B.; Khajeh, K. The Emerging Use of Bioluminescence in Medical Research. Biomed. Pharmacother. 2018, 101, 74–86. [Google Scholar] [CrossRef]
- Kiyama, M.; Saito, R.; Iwano, S.; Obata, R.; Niwa, H.; Maki, S.A. Multicolor Bioluminescence Obtained Using Firefly Luciferin. Curr. Top. Med. Chem. 2016, 16, 2648–2655. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A Luciferin Analogue Generating Near-Infrared Bioluminescence Achieves Highly Sensitive Deep-Tissue Imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef]
- Kim, S.-B.; Paulmurugan, R. Bioluminescent imaging systems for assay developments. Anal. Sci. 2021, 37, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Maki, S.A. Precise In Vivo Optical Imaging of the Human Body Based on Artificial Firefly Bioluminescence System:To Challenge Single-Cell Imaging of the Human Body. Kagaku Seibutsu 2022, 60, 72–78. [Google Scholar] [CrossRef]
- Uchibori, R.; Teruya, T.; Ido, H.; Ohmine, K.; Sehara, Y.; Urabe, M.; Mizukami, H.; Mineno, J.; Ozawa, K. Functional Analysis of an Inducible Promoter Driven by Activation Signals from a Chimeric Antigen Receptor. Mol. Ther. Oncolytics 2019, 12, 16–25. [Google Scholar] [CrossRef]
- Kashiwakura, Y.; Endo, K.; Ugajin, A.; Kikuchi, T.; Hishikawa, S.; Nakamura, H.; Katakai, Y.; Baatartsogt, N.; Hiramoto, T.; Hayakawa, M.; et al. Efficient Gene Transduction in Pigs and Macaques with the Engineered AAV Vector AAV.GT5 for Hemophilia B Gene Therapy. Mol. Ther. Methods Clin. Dev. 2023, 30, 502–514. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, T.; Endo, K.; Hanazono, Y.; Kobayashi, E. Non-Invasive In Vivo Bioimaging in Pigs. Int. J. Transl. Med. 2024, 4, 570-583. https://doi.org/10.3390/ijtm4030039
Abe T, Endo K, Hanazono Y, Kobayashi E. Non-Invasive In Vivo Bioimaging in Pigs. International Journal of Translational Medicine. 2024; 4(3):570-583. https://doi.org/10.3390/ijtm4030039
Chicago/Turabian StyleAbe, Tomoyuki, Kazuhiro Endo, Yutaka Hanazono, and Eiji Kobayashi. 2024. "Non-Invasive In Vivo Bioimaging in Pigs" International Journal of Translational Medicine 4, no. 3: 570-583. https://doi.org/10.3390/ijtm4030039
APA StyleAbe, T., Endo, K., Hanazono, Y., & Kobayashi, E. (2024). Non-Invasive In Vivo Bioimaging in Pigs. International Journal of Translational Medicine, 4(3), 570-583. https://doi.org/10.3390/ijtm4030039





