Abstract
The ATP-binding cassette (ABC) transporter superfamily, one of the largest membrane protein families, plays a crucial role in multidrug resistance (MDR) in cancer by mediating the efflux of various chemotherapeutic agents, thereby lowering their intracellular concentrations and diminishing therapeutic effectiveness. Beyond drug efflux, these transporters are also involved in vital biological processes, such as signal transduction in cancer. Over the past few decades, extensive structural and functional research has provided valuable insights into ABC transporters’ broad substrate specificity and transport mechanisms, leading to promising strategies for overcoming MDR. This review will provide a structural understanding of the interactions between ABC transporters and inhibitors to develop novel cancer therapeutics. Additionally, we focus on methods such as irradiation-based immune therapies, thermal therapies, nanomedicine, CRISPR-Cas, and natural therapies that can genetically modify ABC transporters to reduce their expression or reverse the drug efflux ability. Knowledge gained from these approaches can then be translated into the development of new cancer therapeutics that can combat chemotherapy resistance.
1. Introduction
In the 21st century, cancer has become one of the leading causes of death globally, with the estimation that one in five men or women develop cancer in a lifetime [1,2]. According to the Global Cancer Observatory statistics, the total number of cancer cases (all types, across both sexes and all age groups) was recorded as 19, 976, 499, with 9, 743,832 deaths (48.7%) [3]. Over 90% of these cancer-related deaths are attributed to metastatic cancer, which has been increasingly linked to multidrug resistance (MDR) [4,5,6,7,8]. Chemotherapy, often combined with surgery, immunotherapy, or radiotherapy, remains one of the most effective cancer treatments [9]. However, several studies have shown that ATP-binding cassette (ABC) transporters play a significant role in the active efflux of drugs from the cell and tend to be associated with conferring MDR in cancer cells, resulting in ineffective chemotherapy [10,11]. Therefore, in recent years, there has been a surge in the development of strategies to target ABC transporters in the treatment and prevention of cancers [12,13,14].
2. The General Architecture of ABC Transporters
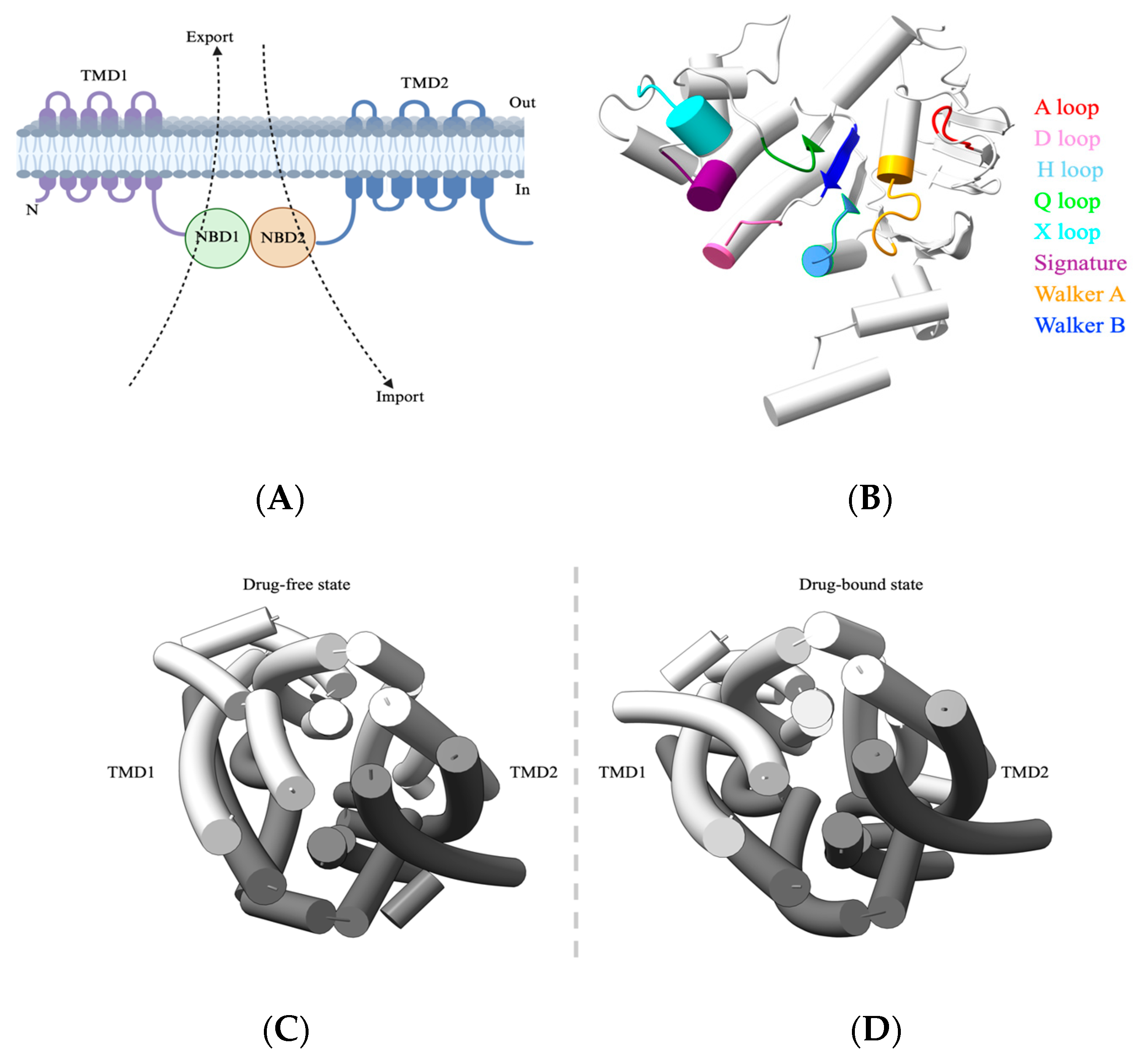
A typical ABC transporter comprises a nucleotide-binding domain (NBD) that is responsible for ATP binding and hydrolysis and a transmembrane domain (TMD) that facilitates substrate export (away from NBDs) or substrate import (toward NBDs) (Figure 1A). Although ABC transporters share a fundamental architecture, they show considerable diversity in substrate specificity, transport mechanisms, and regulation. NBDs are highly conserved hydrophilic domains and are located within the cytoplasm for ATP hydrolysis. NBDs comprise an alpha-helical domain, a RecA-like domain, and a beta domain, while the ATP binding occurs at the interface of two NBDs arranged in a head-to-tail orientation. These NBDs also include the Walker A motif (GXXGXGKS/T, X = any amino acid), which binds alpha and beta phosphates of ATP, while the glutamate and aspartate residues of Walker B (ΦΦΦΦD, Φ = hydrophobic) motifs coordinate with Mg2+ ions and water molecules to interact with the γ-phosphate group of ATP. The signature motif LSGGQ helps to orient the bound ATP molecule [15] (Figure 1B). In stark contrast to NBDs, TMDs are structurally diverse and recognize a wide range of substrates. They span the membrane and form channels to facilitate transport. Hence, TMDs dictate the characteristics of the substrates that are to be transported [16,17] (Figure 1C).
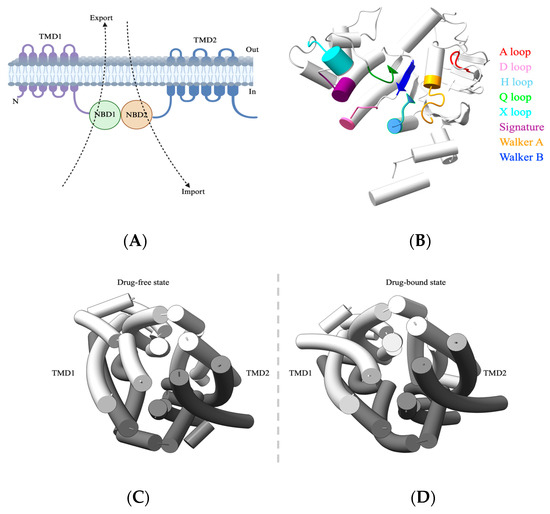
Figure 1.
General architecture of ABC transporter. (A) Schematic of ABC transporter domain architecture, with arrows indicating the direction of transport. (B) NBD of ABC transporters. Shown here is the NBD of ABCB1 (PDB-7A69) with the conserved ATPase domains separately labeled and colored. The NBD is oriented to show the NBD surface that constitutes the NBD–NBD interface. (C,D) Top view (over the membrane panel) of an arrangement of transmembrane helices in ABC transporters. Shown here are both drug-free (PDB-7A65) and drug-bound states (PDB-7A69) of ABCB1 respectively. Abbreviations: NBD, nucleotide-binding domain; TMD, transmembrane domain.
3. Classification of ABC Transporters
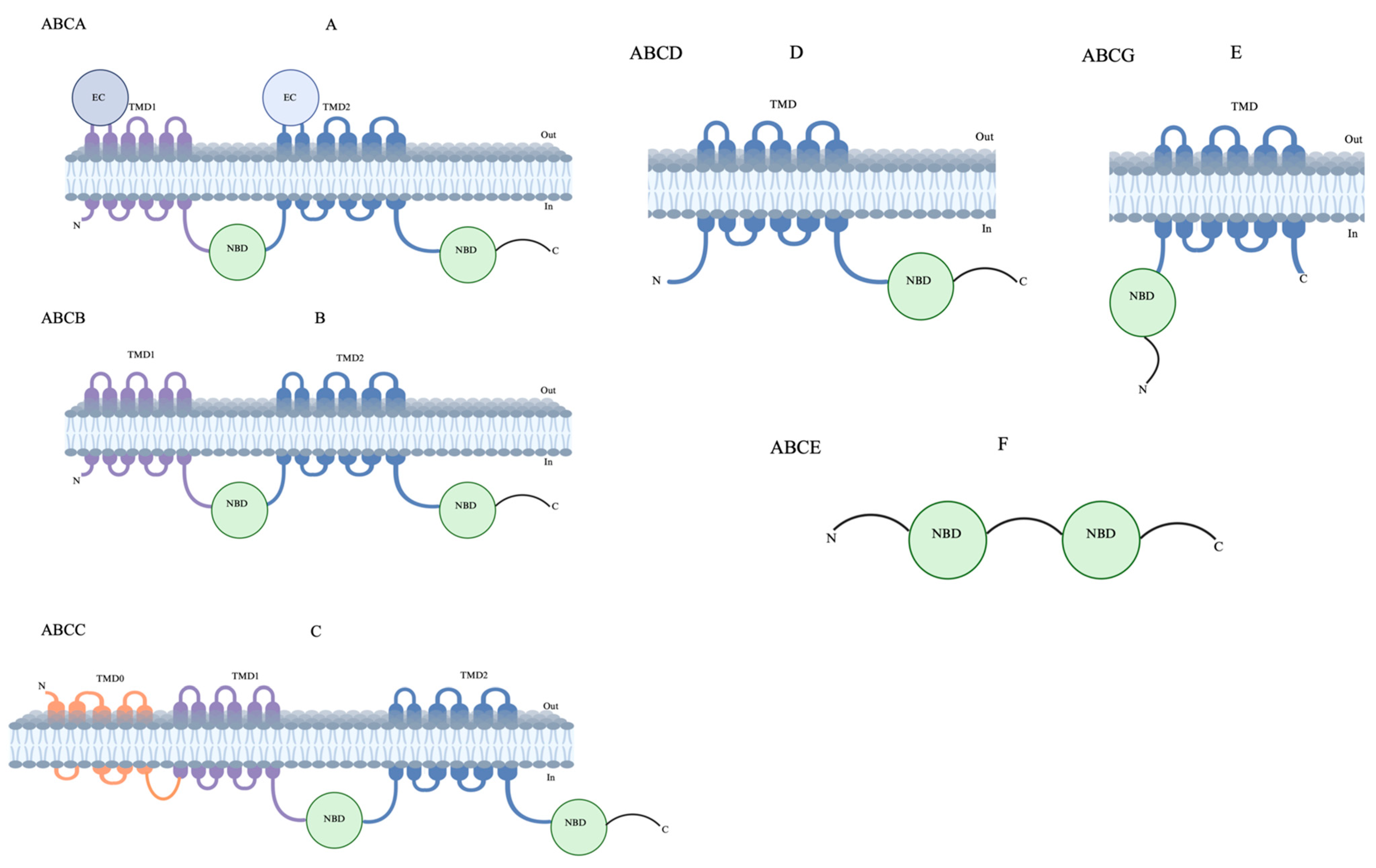
The human ABC transporters are classified into seven subfamilies, designated as ABCA to ABCG, according to the similarity of their amino acid sequences [18]. Of these, five (subfamilies ABCA–ABCD and ABCG) comprise transmembrane domains, while ABCE and ABCF lack transmembrane domains and are involved in cellular functions [19,20] (Figure 2). The human ABCA subfamily is the largest of all ABC transporters and encodes thirteen transporters. These transporters have remained comparatively understudied despite their role in lipid homeostasis and disease association [21,22]. The ABCB subfamily, arguably the most widely studied human ABC transporter, comprises ten transporters that can transport a wide range of substrates, including peptides, drugs, and ions. In this subfamily, ABCB1, also known as a multidrug resistance (MDR) protein, is the first identified member and plays a crucial role in the cellular detoxification of cancer cells. To date, a wide range of anticancer drugs have been identified as substrates for ABCB1, and this continues to be a key focus for therapeutic/diagnostic development [23,24,25]. The ABCC subfamily contains twelve transporters that feature a type I exporter fold, collectively classified as MDR-associated proteins. These transporters have diverse functions, including ion transport, toxin efflux, drug metabolism, as well as normal physiology [26,27,28]. Among these, ABCC1 is of considerable pharmacological and clinical importance as it is a major player in the MDR of cancer cells [29,30]. The ABCD subfamily contains four transporters, also known as adrenoleukodystrophy (ALD) transporters. These transporters are comparatively less studied and are mainly involved in fatty acid metabolism [31,32,33]. However, the role of ABCD transporters in cancer has remained inconclusive due to limited protein structural and topological information [34]. So far, five members have been found in the ABCG subfamily that assemble as homo/heterodimers adopting a type II exporter fold [35,36]. These transporters are found to be involved in either sterol/lipid transport or the mediation of multidrug transport. ABCG2, also known as breast cancer resistance protein (BCRP), is of extreme physiological importance and transports a wide variety of drugs and affects their pharmacokinetics [29]. Due to its critical role in toxin efflux and drug resistance in cancer cells, it remains an attractive target for therapeutic development [37,38]. Among these forty-eight human ABC transporters with distinct functions, approximately thirteen are directly relevant to MDR (Table 1) [39]. MDR was first described over 50 years ago in a subline of HeLa cells resistant to actinomycin D, with subsequent studies showing cross-resistance across different cell lines [40]. The discovery of a transporter responsible for actively expelling drugs from resistant cells, called P-glycoprotein (P-gp), altered the drug permeability in Chinese hamster ovary and fibroblast cells [41]. In this review, we focus on structural details and inhibitor binding of the most clinically relevant ABC transporters—ABCB1, ABCB4, ABCB6, ABCC1, and ABCG2—that cause MDR during cancer therapy. We emphasize the latest novel inhibitors that are currently in preclinical and clinical studies, followed by proposing emerging strategies for targeting ABC transporters to provide a broad perspective to understand MDR in cancers.
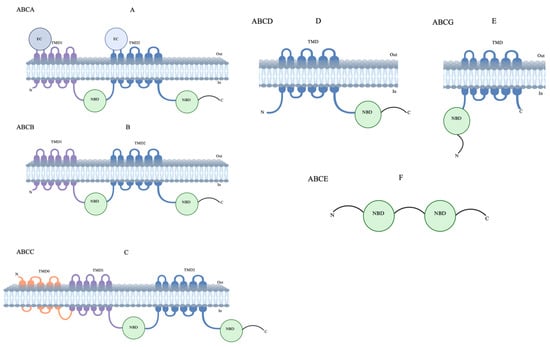
Figure 2.
Topological structures of ABC transporters. (A) ABCA, (B) ABCB, (C) ABCC, ABCD, and (D,E) ABCG have both nuclear-binding domains (NBDs) and transmembrane-binding domains (TMDs). (F) ABCE has only NMDs and does not have TMDs; also, their topological structures are unclear.

Table 1.
List of chemo resistant human ABC transporters. Data adapted from [42].
3.1. ABCB1
ABCB1 is expressed at several blood-organ barriers and is pharmacologically important because its overexpression in certain tumors reduces the uptake of specific orally administered drugs [42,43,44,45]. ABCB1 is the first identified and most characterized MDR transporter and has been long recognized as a viable target to overcome cancer [46]. Due to the clinical significance of ABCB1, its structure and mechanism have been extensively investigated [47,48,49,50,51]. Although high-resolution x-ray crystallography structures of ABCB1 were available earlier, it was possible to determine the structure of the human protein using only single-particle cryo-electron microscopy (cryo-EM) [50]. ABCB1 is a monomeric ABC transporter with pseudo-symmetric halves, each containing TMDs and NBDs (Figure 3A). The largely conserved NBDs dimerize to bind and hydrolyze ATP at their interface, while the TMDs create a large cavity exceeding 6000 Å3 in size through which substrates are transported [47]. ABCB1 has often been viewed as a “hydrophobic vacuum cleaner”, as most of the substrates that pass through this large cavity are hydrophobic in nature [52,53]. Numerous structural and functional studies have revealed the molecular basis of ABCB1 poly-specificity, allowing it to bind and efflux a diverse array of structurally dissimilar compounds [54,55]. Several studies have shown that hydrophobic residues that are located on the upper side of the pocket from TMDs (4 and 6, 10 and 12) play a critical role in substrate binding [47,53]. It is shown that different residues in the binding pocket present in TMDs may be involved in interactions with different substrates. For instance, the high-resolution structure of paclitaxel bound to ABCB1 depicts Q725, Q347, and Q990 as the interacting residues with paclitaxel, while zosuquidar bound to ABCB1 reveals M985 and F982 as the interacting residues [50,51,56] (Figure 3A). Additionally, recent high-resolution cryo-EM structures have shown that two inhibitor molecules can bind simultaneously within the cavity formed by the TMDs. One molecule occupies the central binding pocket, while the second binds to the access tunnel, which extends from the central binding pocket [53]. However, the exact purpose of ABCB1’s ability to bind two inhibitor molecules remains to be explored. Furthermore, the conserved glutamine Q475 in NBD1 coordinates with Mg2+ and gamma-phosphates of ATP; thus, they play an important role in ATP hydrolysis and drug transport [51]. Among the therapeutic compounds that are transported by ABCB1, vinblastine and vincristine, belonging to the vinca alkaloids family, have been extensively studied and used in antitumor therapies [57,58]. In addition to these, a plethora of anticancer drugs (Table 2) have been tested to overcome MDR in cancer. However, most of the inhibitory compounds that were developed showed promise in cellular assays but did not pass clinical trials due to selectivity, efficacy, and toxicity issues [59,60,61]. Nevertheless, these setbacks did not diminish interest in developing ABCB1 inhibitors, and research efforts are ongoing. This perseverance led to the discovery of the best-known small molecule inhibitor, MRK16, which was developed against leukemia resistant to adriamycin and the monoclonal antibody UIC2 [53,56,62]. While the developed monoclonal antibody was not successful in clinical applications, the small molecule MRK16 was found to interfere only with the transport of bulky substrates like vincristine and not with flat molecules such as doxorubicin [50,51,53]. Consequently, many drug developers are now focusing on discovering new compounds that can bypass the activity of ABCB1. The details of this approach will be discussed under the emerging strategies section later in the review.
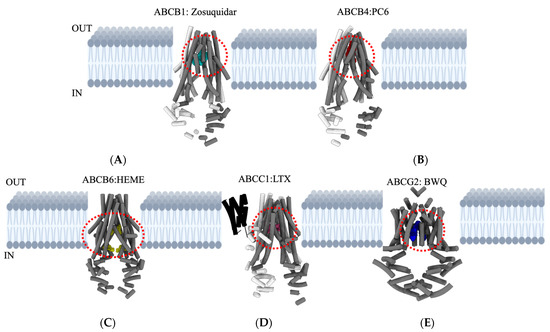
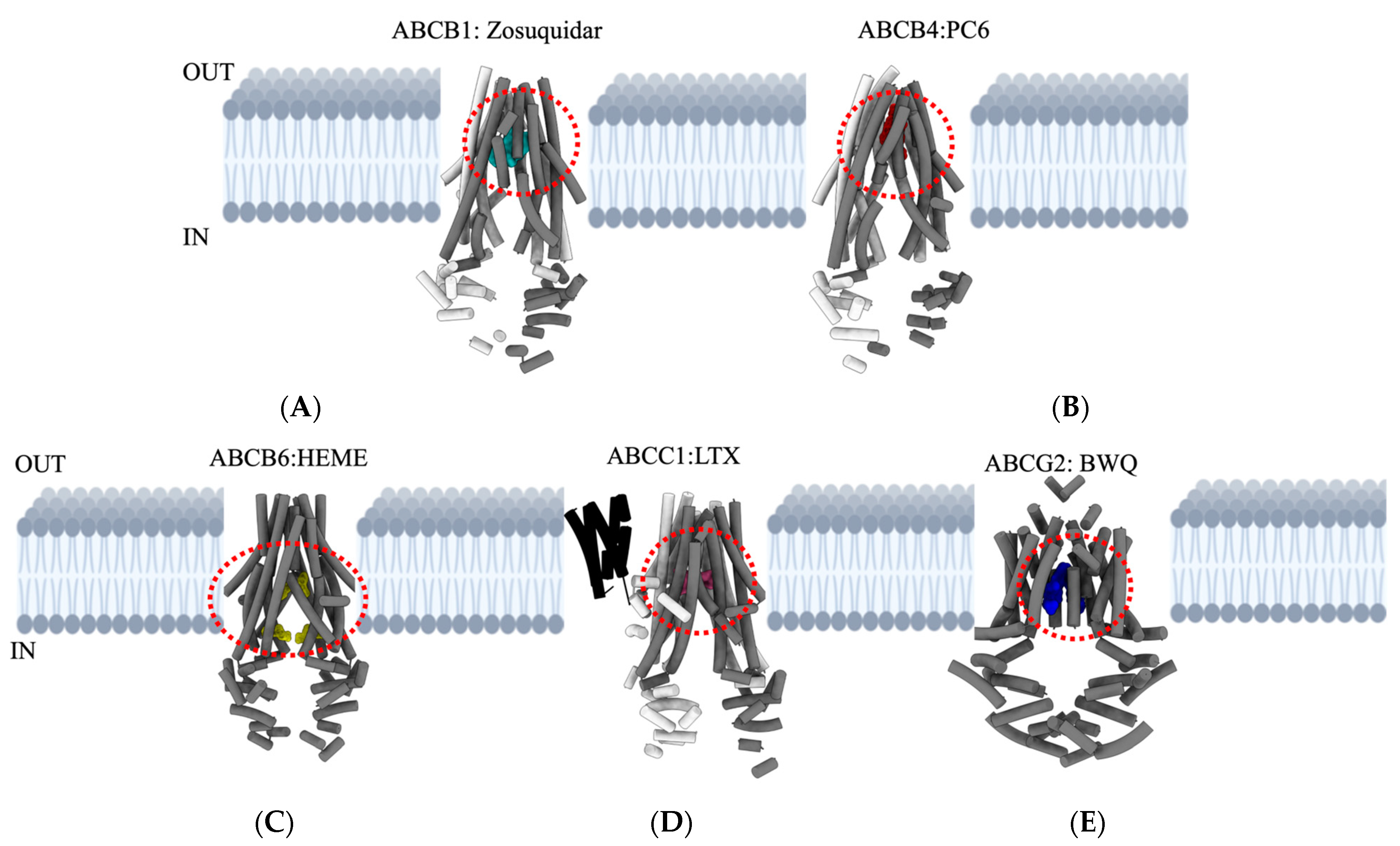
Figure 3.
Overview of major ABC transporters that mediate cancer MDR. Structures and topologies are derived from a protein data bank (PDB) for the five ABC transporters bound to inhibitors/substrates. (A) ABCB1: Zosuquidar—PDB ID—7A6F. (B) ABCB4: PC6 (phosphatidyl choline)—PDB ID—7NIV. (C) ABCB6: HEME—PDB ID—7DNZ. (D) ABCC1: LTX (leukotriene A4 conjugated with glutathione)—PDB ID—5UJA. (E) ABCG2: BWQ (derivative of fumitremorgin C-related inhibitor Ko143)—PDB ID—6FFC. The area outlined with red dashed lines in each transporter indicates appropriate locations for drug-binding cavities. Figures are generated using UCSF Chimera X: Tools for structure building and analysis.

Table 2.
List of key residues within the binding pocket, substrates, and inhibitors of major ABC transporters that mediate cancer MDR. The residues that are involved in both substrate and inhibitor binding are shown in red.
3.2. ABCB4
ABCB4 was originally identified as a sister protein to ABCB1, and these two proteins share 85% sequence similarity [67]. Various studies in the literature have shown that ABCB4 is capable of recognizing and transporting some ABCB1 substrates with low capacity [68,69,70]; however, it is to be noted that this is likely not the physiologically relevant function of ABCB4. ABCB4 is expressed mainly in hepatocytes and is an active phospholipid translocator that is critical for bile generation [67,71,72,73]. The transport of phospholipids by ABCB4 is stimulated by bile salts exported by ABCB11, which, in turn, facilitates cholesterol export via ABCG5–ABCG8. Several mutations in ABCB4 are associated with progressive familial intrahepatic cholestasis, a severe liver disease [74,75]. In addition to this, dysfunction and epigenetic silencing of ABCB4 are directly evident in hepatobiliary malignancies [76,77,78]. Despite its importance in health and diseases, so far only two high-resolution structures of ABCB4 are available [63,79], partly due to difficulties in obtaining suitable amounts of functional and pure ABCB4. ABCB4 consists of six intracellular domains and six extracellular loops separated by twelve TMDs and contains two NBDs. TMD4 and TMD10 form a Y-shaped occluded cavity of ∼ 6500 Å3 at the center of the transporter, wherein the lipid phosphatidylcholine is bound [63], resembling the structure of the ABCB1 transporter [51,54] (Figure 3B). The phosphatidylcholine binding pocket is above residue W234, with its choline moiety stabilized by cation–π interactions with this residue. Azole antifungals, like posaconazole, inhibit ABCB4, reducing phosphatidylcholine secretion and increasing bile toxicity, which can lead to drug-induced liver injury (DILI) in susceptible patients [80,81].
3.3. ABCB6
ABCB6 is a homodimeric transporter comprising a single TMD and NBD with an N-terminal extension (TMD0), with TMDs responsible for substrate translocation, while the NBDs are involved in ATP binding and hydrolysis [17]. The functional role of TMD0 in ABCB6 is not fully understood, but earlier studies indicate that TMD0 may perform the regulatory, trafficking, localization, and stability roles of the transporter [82,83]. Since its first discovery in 1996 [84], ABCB6’s role has been shown in drug resistance [85,86] and toxic metal resistance [87] while primarily functioning as a porphyrin transporter [88,89,90]. Mutations in the ABCB6 gene are linked to tissue-specific disorders such as ocular coloboma [91], familial pseudohyperkalemia [92], porphyria, and dyschromatosis universalis hereditarian [93]. ABCB6 is demonstrated to transport a broad spectrum of substrates, including porphyrin metabolites [94], heavy metals [87], and organic compounds [95]. In addition to these, ABCB6 can interact with various anticancer drugs, including camptothecin, cisplatin, paclitaxel, doxorubicin, methotrexate, and vincristine [85,86,96,97,98,99]. The molecular mechanisms of transportation and substrate recognition for ABCB6 remained largely unclear until the first glimpse was provided by the cryo-EM structure very recently [64]. However, this study has a major limitation as it is mainly based on a 4.0 Å resolution model, which is borderline sufficient to identify side chains. This is followed by the determination of a few more cryo-EM structures of ABCB6 in the apo-state, nucleotide state, and cadmium ion-bound (Cd (II)) state to shed light on the mechanism that ABCB6 mediates to transport a broad range of substrates [64,100,101,102]. The overall structure of ABCB6 shows an elongated shape with 130 Å in height, and the two NBDs, like two “legs”, are separated by 35 Å (Figure 3C). In the apo-state, ABCB6 adopts an inward-facing conformation, and the TMD region contains a loop acting like a gate named a “plug”. The substrate-binding cavity within the TMD is divided into two parts: 1) closed cavity and 2) open-form cavity, which is different when compared to most substrate-binding pockets found in other ABC transporters. The “plug” of ABCB6 comprises flexible residues that provide flexibility for multi-substrate binding, and in the inward position, the “plug” acts as an obstructing substrate and could be opened during ATP-driven conformational changes in ABCB6. The “plug”, together with residue W546, provides a hydrophobic environment for substrate translocation, and the “plug” divides TMDs into two pockets, with pocket one playing a role in substrate recruitment on the other and pocket 2 for the transport of substrate. Moreover, it was found that ABCB6 displayed resistance to anticancer drugs such as 5-fluorouracil (5-FU), SN-38, and vincristine [85]. Therefore, obtaining more structural information about the specific regions within the transmembrane domains (TMDs) of ABCB6 is essential to gain mechanistic insights. This knowledge is crucial for designing targeted inhibitors or drugs that can effectively modulate ABCB6 activity.
3.4. ABCC1
ABCC1 is a single polypeptide chain consisting of TMDs and NBDs. It shares approximately 23% sequence identity with ABCB1 and exhibits a notable overlap in the range of substrates it transports [65]. Only one nucleotide-binding domain located on the NBD2 is responsible for hydrolyzing ATP and providing energy for translocation [103]. ABCC1 contains an N-terminal membrane-bound region (TMD0) domain that links to the transporter core through a Lo linker, and this forms the characteristic feature of the ABCC family proteins (Figure 3D). The loss or mutation of the Lo linker results in protein misfolding and defective function [104]. So far, ABCC1 seems to have the broadest substrate specificity of any ABCC subfamily member, at least in vitro [105]. A key question in understanding the multidrug resistance of ABCC1 is how a single transporter can recognize a wide range of structurally diverse molecules. This has led to significant interest in identifying the atomic determinants responsible for its ability to recognize such a wide range of substrates. The first structural insights into ABCC1 were provided by the bovine ABCC1, which shares 91% amino acid identity with human ABCC1. Later, the cryo-EM structure of the human counterpart was determined [65,103]. Structural studies of this transporter reveal that it forms a single, bipartite substrate-binding site, which enables the transporter to accommodate and recognize a broad spectrum of substrates with varying chemical structures. The binding pocket of the ABCC1 transporter is formed by two distinct bundles: TM1 and TM2. The residues on the inner-facing side of these bundles differ significantly in their properties [103]. Positively charged residues are in TM1, while hydrophobic residues are found in TM2. The positively charged region typically interacts with the glutathione (GSH) moiety, while the hydrophobic region binds to the substrate [65]. ABCB1 and ABCC1 are both ABC transporters that contribute to multidrug resistance but differ significantly in their functional properties [106]. ABCB1 primarily transports hydrophobic and weakly cationic compounds, while ABCC1 mainly transports organic acids. The translocation pathways of these transporters are chemically distinct, with ABCB1 having a hydrophobic pathway with acidic patches and ABCC1 having a basic pathway. ABCB1 uses a “hydrophobic vacuum” model, where substrates partition into the lipid bilayer before entering the transporter, while ABCC1 recruits substrates directly from the cytoplasm via its well-ordered transmembrane helices. ABCB1 substrate recognition is influenced by membrane partitioning, while ABCC1’s ability to recognize a wide range of substrates is due to the bipartite nature and flexibility of its substrate-binding site [51,54,65,103]. ABCB1 is shown to confer MDR to etoposide, daunorubicin, and vinblastine in ABCB1-transfected HEK293 cells [107]. Apart from a few reported inhibitors of ABCC1 (Table 2), there is a lack of potent inhibitors, highlighting the need for additional substrate-bound cryo-EM structures and biochemical validations. These are essential for achieving an atomic-level understanding of the poly-substrate specificity of ABCC1.
3.5. ABCG2
ABCG2 consists of six transmembrane helices and one ATP-binding site, with one nucleotide-binding domain (NBD) and one transmembrane domain (TMD) on each polypeptide chain. These chains dimerize to form a functional active transporter [66] (Figure 3E). This transporter recognizes and transports a broad spectrum of substrates, primarily hydrophobic, polycyclic, and relatively flat molecules. The transporter also recognizes endogenous substrates as well as exogenous cytotoxic compounds [108,109] (Table 2). In colon cancer, ABCG2 was observed to confer resistance against various chemotherapy drugs, including topotecan, SN-38, mitoxantrone, doxorubicin, and several tyrosine kinases [109,110,111]. Owing to its clinical relevance, ABCG2 is listed on the US Food and Drug Administration and the European Medicines Agency lists of transporters to be checked for transporter–drug interactions [112]. Over the years, there have been many in vivo studies on this transporter that provided information on localization, physiological functions, trafficking, and substrate specificity [113,114,115]. However, the mechanistic understanding of ABCG2 was limited due to a lack of high-resolution structure, which is partly because of the challenges associated with its overexpression and functional purification. While the crystal structure [116] and low-resolution electron microscopy [116,117] imaging provided the first structural glimpse of the transporter, it was only after the determination of the cryo-EM structure of human ABCC2 that the structural understanding became much clearer [66,118,119,120] (Figure 3E). These structures reveal an inward-open confirmation of ABCG2 with the binding pocket formed between TM2 and TM5a domains. The transmembrane helices and intracellular loops of ABCG2 are shorter than those of B-subfamily ABC transporters, resulting in a smaller distance between the NBDs and the membrane. Thus, ABCG2 is more compact [66]. Two standout features of the ABCG2 structures are the presence of 1) a deep hydrophobic cavity within two TMDs and 2) an external cavity located below the external loop separated by a “leucine plug” [66]. This structural arrangement suggests that ABCG2’s preference for flat substrates contrasts with ABCB1, which favors globular hydrophobic substrates [51]. As for its potential value in the treatment of cancer and pharmacotherapy, huge efforts have been invested over the past decades in the development of specific inhibitors against human ABCG2 [109,110,111,121]. The first and well-characterized ABCG2 inhibitor, fungal toxin fumitremorgin C (FTC), has been ruled out owing to its neurotoxicity [122], while its derivative Ko143 was less toxic but lacked specificity [123]. Attempts have also been made to develop ABCG2 inhibitors derived from tariquidar, a third-generation ABCB1 inhibitor [124,125,126]. In this context, the structures of ABCG2 bound to the Ko143-derived inhibitors and the tariquidar-derived inhibitor molecules have been determined [118]. It was shown that depending on their shape, the inhibitor molecules can symmetrically or asymmetrically occupy the binding pocket cavity within the TMDs. Additionally, febuxostat, an approved drug used in patients with hyperuricemia, was serendipitously identified as a strong ABCG2 inhibitor both in vitro and in vivo [127]. Furthermore, a recent study has demonstrated that two potential anticancer compounds are currently under clinical examination that could competitively inhibit both ABCG2 and ABCB1 [128]. Nonetheless, to the best of our knowledge, the clinical use of such drugs for ABCG2 inhibition has not yet been approved due to concerns about safety and specificity. This may have been a common reason responsible for the failure of the clinical development of ABCG2 inhibitors thus far. Therefore, much larger efforts are needed to understand the catalytic cycle of ABCG2 and, in general, other multidrug transporters in physiologically relevant conformational states, including their intermediates.
4. Mechanistic Overview of Drug Transport
The common structural features among the MDR-related ABC transporters feature a central substrate-binding pocket, which is remarkably flexible and can have the adaptability to accommodate a wide range of substrates [122]. The universal mechanism of drug translocation by MDR-related ABC transporters is commonly explained by the “alternating access” model. According to this model, the transporter undergoes significant conformational changes between two states: (1) inward-facing and (2) outward-facing. In the inward-facing conformation, the binding sites are accessible to substrates and ATP. Upon ATP binding, the transporter undergoes a conformational shift to the outward-facing state, which allows the substrates to be expelled. Following substrate release, ATP hydrolysis triggers the return of the transporter to its inward-facing conformation. These conformational changes, driven by ATP binding and hydrolysis, enable the transporter to efficiently transport substrates in/out of the membrane, thereby contributing to multidrug resistance [123]. Although the “alternating access” model is universal among MDR-related ABC transporters, there are minor distinctions among them. In the case of ABCB1, the bound drug is sealed by TM4 and TM10, which prevents the drug from returning to the cytosol. The two halves of the ABCB1 transporter move closer together, collapsing the substrate pocket and transitioning to an outward-facing conformation, facilitating substrate release before ATP hydrolysis [51]. In the case of ABCB4, which employs a similar mechanism as ABCB1, the central binding pocket harbors a phospholipid instead of a drug. This makes the proposed mechanism for ABCB4 unique, as it involves relatively subtle adaptations that fine-tune a general multidrug export mechanism specifically for phospholipid transport [63]. Compared to ABCB1, ABCB6 exhibits adaptations in both substrate binding and the ATP-triggered transport pathway. The long hydrophobic “plug” helps to form a flexible cavity that accommodates substrate binding. Following substrate binding, the surrounding helices adjust, and the “plug” reduces interactions, allowing ABCB6 to shift to an ATP-sensitive conformation [64]. In contrast, ABCC1 undergoes a rearrangement of pocket residues upon substrate binding rather than NBD movement [65]. In the case of ABCG2, the substrate recruitment occurs via the cytoplasm or directly through the membrane. The substrate first binds to cavity 1 of ABCG2, and upon ATP binding in NBDs, the substrate later moves to cavity 2 and is then expelled out of the cells [66].
5. Translational Role and Therapeutic Targeting Strategies for ABC Transporters
The highly conserved ABC transporters express in a tissue-specific manner, and their role in tumors is well documented [124,125]. According to the Gene Expression Atlas and the Human Protein Atlas, it is well known that ABC transporters overexpressed in a variety of cancers, for example, ABCB1, ABCC1, and ABCG2 overexpression, have been shown in acute myelocytic leukemia (AML), breast cancer, thyroid cancer, ovarian cancer, and multiple myeloma [126]. Overexpression of ABCB1 was also observed in melanoma and other multiple cancer cells. Research studies also confirmed that ABC transporters are one of the potential reasons for the development of MDR [129,130]. Despite their role in drug resistance, several ABC transporter inhibitors have been developed, particularly against ABCB1, ABCG2, and ABCC1. However, clinical studies failed with first- and second-generation ABC transporter inhibitors [131,132]. Recently Friedenberg et al. showed the beneficial effects of combination therapy using ABC transporter inhibitors. Studies revealed the use of valspodar (PSC-833) along with doxorubicin, vincristine, and dexamethasone versus valspodar alone in Phase III studies [133]. Third-generation inhibitors such as elacridar (GF120918), tariquidar (XR9576), zosuquidar (LY335979), and dofequidar were evaluated as improved selective inhibitors against ABC transporters [54]. Reports suggested that Phase III clinical studies were carried out using tariquidar in combination with paclitaxel along with either carboplatin or vincristine in patients with non-small-cell lung cancer [134,135]. However, this, along with other clinical studies performed at the National Cancer Institute (NIH, Bethesda, Rockville, MD, USA) with tariquidar, suggests that there is still optimism that therapeutic modulation of MDR mediated by ABC transporters may be beneficial to patients. Overall, these inhibitors showed limited clinical success; this topic has been extensively reviewed elsewhere [134,135]. Multiple reasons were recognized for the failure of these ABC inhibitors in clinical trials, such as side effects due to high drug toxicity, development of MDR due to multifactorial mechanisms, substrate overlap, and impaired drug clearance. For example, the inhibitor toxicity developed on ABC transporters could also cause the inhibition of metabolic cytochrome P450 enzyme function. Cytochrome P450 enzymes are becoming targets of ABC transporter inhibitors, particularly P-gp inhibitors tested in combination with anticancer drugs that were shown to be toxic at tolerable doses. The latest P-gp inhibitors have also been reported to exhibit toxicity by inhibiting metabolic cytochrome P450 enzymes [132,136,137,138,139,140]. Clinical trial investigations were terminated when using tariquidar, a P-gp inhibitor (third generation), combined with first-line chemotherapy in non-small-cell lung cancer patients [141,142]. To overcome these limitations, there is a need to develop more effective combinational therapies to overcome MDR resistance and chemotherapeutic failures for effective cancer treatments [53,142,143].
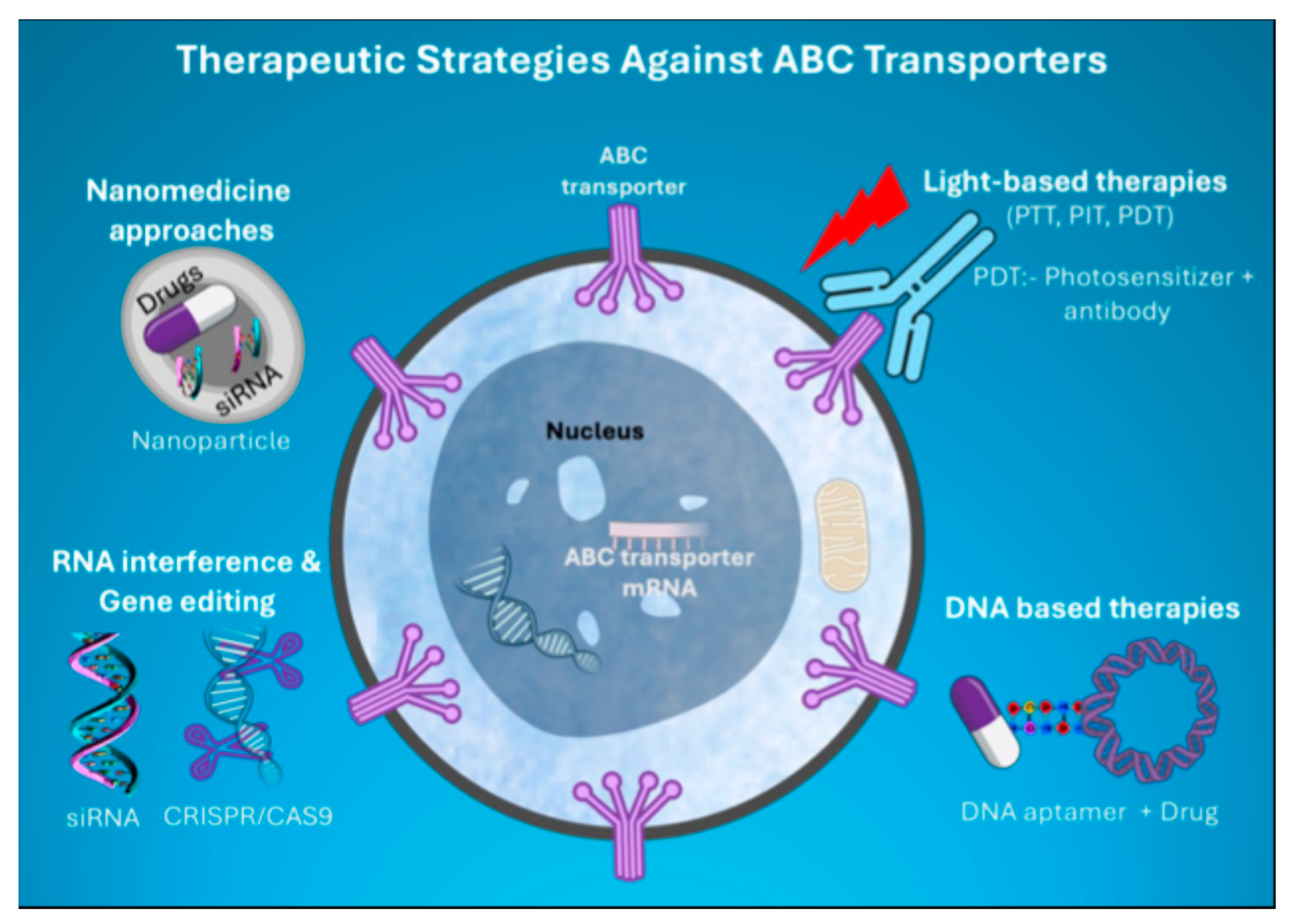
6. Novel Therapeutic Strategies Targeting ABC Transporters
Novel therapeutic strategies are developed to treat multidrug resistance (MDR) in diseases, especially cancer and autoimmune diseases. Widely used therapeutic approaches are light-based therapies coupled with inhibitors, nanomedicine-based therapies, RNA interference, and immunotherapy (Figure 4). Despite having advantages and disadvantages over different therapeutic approaches, we are highlighting some of them to discuss here. The current review addresses the advantages of applying novel therapeutic strategies coupled with ABC transporter inhibitors to overcome MDR.
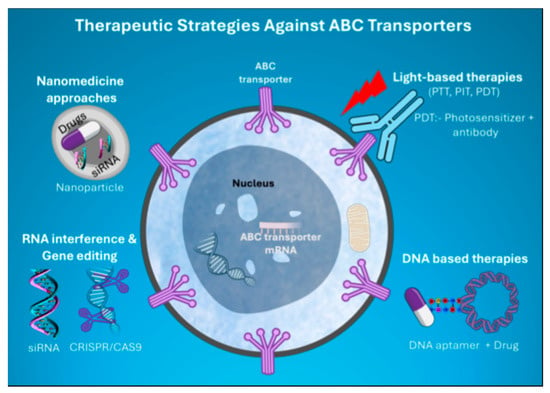
Figure 4.
Schematic illustrating the emerging therapies targeting major ABC transporters that mediate cancer MDR.
6.1. Light-Based Therapies Coupled with ABC Transporter Inhibitors
Light-based therapies have been widely used as approved therapies against cancers across the globe [144]. The synergistic role of light irradiation and drug release or activation in killing cancer cells has shown promising outcomes in preclinical studies. This approach is extensively used in treating cancers, such as bladder, head and neck, and skin cancers [144]. The light-based therapies include photothermal therapy, photoimmunotherapy, and photodynamic therapy (PDT). However, each method has its advantages and disadvantages [144,145,146,147,148]. In photothermal therapy, infrared radiation is used to generate heat on the targeted tissues to kill cancer cells. The limitation of this therapy is that the radiation can damage the targeted tissue and trigger further inflammation. With photoimmunotherapy, the radiation is combined with heat sensor-conjugated antibodies specific to target proteins expressed in cancer cells [145,146,147]. PDT utilizes light irradiation with a non-toxic photosensitizer agent that targets tumor cells and kills them by generating reactive oxygen species (ROS) [148]. These strategies are also being used for diagnostic and treatment purposes in the clinic. For example, benzoporphyrin derivative is an FDA-approved photosensitizer for age-related molecular degeneration and cancer, and it is also used as a conjugate with the ABCB1 monoclonal antibody UIC2 to target resistant cells [148,149]. PDT is the most advanced light-based treatment against cancers, and it is also very effective for all types of tumors associated with MDR. Coupling ABC transporter inhibitors with PDT may be a more effective approach to treating tumor-associated MDR. Recent studies identified new photosensitizer substrates: redaporfin for P-gp, BPD for ABCG2 and P-gp, and rose bengal for P-gp, ABCG2, and MRP1 [138,140,144]. It is shown that fumitremorgin [144,150], a P-gp inhibitor, significantly blocked the transport facilitated by ABCG2 and P-gp of rose bengal and BPD. MCF-7/VP cells were found to have reduced intracellular accumulation of rose bengal, which was restored with the MRP1 inhibitor (MK571) [151]. This study provides novel insights that the known temoporfin, talaporfin sodium, methylene blue, and indocyanine green are not the substrates of ABCG2, P-gp, or MRP1, indicating that identifying the specific ABC transporter substrate is important for the success of photodynamic therapy. Together, coupling ABC transporter inhibitors and light energy with specific photosensitizers may provide more effective treatment by limiting the exportation of drugs from the target cells.
6.2. DNA-Based Therapeutics
Single-stranded DNA aptamers are highly selective toward specific proteins and are used to conjugate drugs [152]. A DNA aptamer conjugated with doxorubicin was developed against ABCG2 proteins and assessed for its role in resistant breast cancer cells and found to potentiate the uptake and cytotoxicity of doxorubicin [153]. Further, DNA aptamers were designed against ABCG2-glycosylated extracellular regions using molecular engineering methods. The combination of the glycosylated DNA aptamer with a monoclonal anti-ABCG2 complex was shown to block ABCG2-mediated doxorubicin transport and, thus, reverse MDR in resistant HepG2 liver cancer cells [154]. Besides single-stranded DNA aptamers, DNA-capped quantum dots encapsulated in gold nanoparticles carrying doxorubicin were used to target ABCC1 mRNA expression, which remarkably increased the efficacy of the drug in resistant cancer cells [155]. Another study designed a catalytic DNA or DNAzyme to target ABCB1 mRNA, which further significantly decreased drug resistance in MCF breast cancer cells [156]. However, for all these studies, further clinical trials are needed to prove their in vivo efficacy.
6.3. Nanomedicine Approaches
The nanoparticle (NP)-based medicinal approach is becoming popular in target cell drug delivery due to the unique characteristics of nanoparticles, such as increased drug stability, sustained drug release, and extended life toward target drug delivery. Nanomedicine is a great choice for combating MDR in chemotherapy. NPs ranging as low as 10 nm to 200 nm in size, coupled with chemotherapeutic agents, were delivered selectively to target cancer cells. NPs coupled with ABC transporter inhibitors have shown progressive results toward drug stability and delivery approaches. Several drugs already in the clinic with nanoformulations (such as Lipusu, Doxil, Abraxane, and Genoxil-PM) are encouraging to fight against MDR in cancers [148,157,158]. Recent studies evaluated testing nanomedicine platforms like mesoporous silica (MSN), poly-lactic nanoparticles (PLAs), and graphene quantum dots (GQDs), along with selected drugs to inhibit MDR1 (P-glycoprotein) and MRP1 [157,159,160]. PLA, MSN, and GQDs were reported to be non-toxic in MDA-MB-231 cancer cells. It has been reported that MRP1 overexpression in MDR and targeting MRP1 via nanomedicine might be a useful way to overcome MDR [129]. These studies revealed that PLA exhibits moderate inhibition of doxorubicin efflux by MRP1, and MSN is reported to be a strong inhibitor of doxorubicin efflux by MRP1. Doxorubicin is a chemotherapeutic drug used in multiple cancer treatments. NPs coated with chemotherapeutic agents alone could not overcome MDR in the tumor environment, whereas combinational therapy that includes ABC transporters and chemotherapeutic agents might be effective in preventing long-term MDR in chemoresistance diseases.
6.4. RNA Interference and Gene Editing
RNA interference (RNAi) is a biological process that can be used to regulate target gene expression. RNAi regulates gene expression by interfering with messenger RNA (mRNA). Research studies suggested that RNAi has become a promising tool for gene therapy to reverse MDR mediated by ABC transporters [129]. Currently, RNA interference to ABC transporters will be carried out in multiple ways, such as small interfering RNA (siRNA), short hairpin RNA (shRNA), and microRNA (miRNA) [161,162,163]. However, each of them has advantages and disadvantages in transiently regulating gene expression. Importantly, clustered regularly interspaced short palindromic repeats/Cas9 (CRISPR/Cas9) show permanent regulation of gene expression in a specific target-oriented way. Here, we discuss the application of miRNAs and CRISPR/Cas9 regulation of ABC transporter gene expression.
6.5. miRNA Targeting ABC Transporters
Small noncoding RNA molecules of 20–30 nucleotides, referred to as microRNAs, exhibit hairpin structures. miRNAs are important modulators contributing to the adaptive regulation of ABC transporters. The ABC transporter family member P-gp, encoded by the ABCB1 gene, became the most targeted gene using miRNA [163,164]. Since ABCB1 is also known as the MDR1 gene, most researchers focused on regulating ABCB1 gene expression, and here, we are emphasizing research work carried out targeting the ABCB1 gene using miRNA [163,164] (Table 3).

Table 3.
List of miRNAs targeting major ABC transporters that mediate cancer MDR. The miRNA targets are referenced from [165,166].
6.6. CRISPR/Cas9 Targeting ABC Transporter
Researchers identified that the clustered regulatory interspaced short palindromic repeats/(CRISPR)-associated protein 9 (CRISPR/Cas9) system provides more precise gene editing technology at an affordable cost. The mechanism behind this gene editing can be simply explained as the CRISPR/Cas9 system specifically targets and cleaves a specific DNA sequence, which is performed by Cas9 nuclease and interlinked with single-guide RNA (sgRNA) [166,167,168]. By using CRISPR/Cas9 technology, the ABC transporter gene family is targeted for gene regulation. The principal ideology of using a CRISPR/Cas9-mediated target of the ABC transporter family was to reduce drug efflux as well as to sensitize cancer cells to chemotherapeutic drugs. Here, providing several research studies showed effective gene regulation of ABC transporters using CRISPR/Cas9 technology. Research studies showed that CRISPR/Cas9 was used to reverse the resistance of ovarian cancer cells to doxorubicin. Research studies also reported that CRISPR/Cas9-derived knockout of ABCB1 can improve the response to temozolomide, carmustine, and lomustine in a glioblastoma multiforme cell model [169,170]. It is reported that targeting the knockdown of the ABCB1 gene using CRISPR/Cas9 technology significantly increased the accumulation of doxorubicin inside the cells and enhanced chemosensitivity in HCT-8/v cancer cells [170,171], showing that doxorubicin cell death increased upon CRISPR/Cas9 knockout of MDR1 in doxorubicin-resistant breast cancer cells [172,173]. Targeting the ABCB1 gene using CRISPR/Cas9 technology in ovarian cancer cells leads to increased sensitivity for chemotherapeutic drugs [169,173]. Given the many studies that are currently progressing, it is hoped that CRISPR/Cas9 technology will find solutions to overcome ABC transporter-mediated MDR and chemotherapeutic resistance. Also, the way will be opened for the clinical use of this technology to overcome drug resistance in cancers.
Overall, these multifaceted approaches targeting ABC transporters provide significant potential to overcome MDR in cancer, making treatment more effective and adaptable (Figure 4).
7. Conclusions, Open Questions, and Future Perspectives
With significant advancements in structural biology techniques, our understanding of ABC transporter topology, ligand binding, and the substrate transport cycle has greatly improved. This review thoroughly discusses these topics and highlights their role in cancer MDR. However, several mechanistic insights into these transporters remain elusive. For example, the role of the flexible linker region (50–100 amino acids), which typically connects the first NBD to the second TMD, remains enigmatic for most ABC transporters [174,175]. In the case of ABCB1, it is shown that shortening the linker region reduces the conformational flexibility, affecting the substrate transport [176]. Nevertheless, the precise role of the central linker region warrants further investigation in future research. Similarly, the role of TMD0, which is exclusively found in the ABCB family and typically links TMD1 by a flexible linker, remains largely unknown [65,177,178,179,180]. Although it appears that TMD0 does not influence substrate binding and transport, it may have a potential contribution to the occurrence of MDR. Hence, dedicated research is needed to understand the comprehensive role of TMD0 in ABC transporters. Another important area for investigation is specific lipid interactions of ABC transporters, particularly regarding both lipid transport and structural insights, as these remain challenging. The advent of AlphaFold [181] has been valuable in making the structures of human ABC transporters available for three-dimensional analysis and structure-based in silico drug design. These predicted models, which currently represent single states, offer limited insight into small-molecule, lipid, and protein interactions. However, the latest version of AlphaFold3, which can predict biomolecular interactions, needs to be thoroughly evaluated as part of future projects focused on ABC transporters [182]. Taken together, all these highlight the need for more exploratory studies that elucidate the mechanisms of ABC transporters, particularly the MDR-related ones.
Author Contributions
Conceptualization: R.S.K.N. and E.K.N.; writing—original draft preparation: R.S.K.N., G.P.V. and E.K.N.; writing—review and editing: R.S.K.N. and E.K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jokhadze, N.; Das, A.; Dizon, D.S. Global cancer statistics: A healthy population relies on population health. CA A Cancer J. Clin. 2024, 74, 224–226. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Akkol, E.K.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Cancer multidrug resistance. Nat. Biotechnol. 2000, 18, IT18–IT20. [CrossRef]
- Bin Emran, T.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Cui, Q.; Yang, Y.; Ji, N.; Wang, J.-Q.; Ren, L.; Yang, D.-H.; Chen, Z.-S. Gaseous Signaling Molecules and Their Application in Resistant Cancer Treatment: From Invisible to Visible. Futur. Med. Chem. 2019, 11, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed]
- Allikmets, R.; Schriml, L.M.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar]
- Giacomini, K.M.; Yee, S.W.; Koleske, M.L.; Zou, L.; Matsson, P.; Chen, E.C.; Kroetz, D.L.; Miller, M.A.; Gozalpour, E.; Chu, X. New and Emerging Research on Solute Carrier and ATP Binding Cassette Transporters in Drug Discovery and Development: Outlook From the International Transporter Consortium. Clin. Pharmacol. Ther. 2022, 112, 540–561. [Google Scholar] [CrossRef] [PubMed]
- Stockner, T.; Gradisch, R.; Schmitt, L. The role of the degenerate nucleotide binding site in type I ABC exporters. FEBS Lett. 2020, 594, 3815–3838. [Google Scholar] [CrossRef]
- Rosenberg, M.F.; Kamis, A.B.; Callaghan, R.; Higgins, C.F.; Ford, R.C. Three-dimensional Structures of the Mammalian Multidrug Resistance P-glycoprotein Demonstrate Major Conformational Changes in the Transmembrane Domains upon Nucleotide Binding. J. Biol. Chem. 2003, 278, 8294–8299. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Aller, S.G.; Beis, K.; Carpenter, E.P.; Chang, G.; Chen, L.; Dassa, E.; Dean, M.; Van Hoa, F.D.; Ekiert, D.; et al. Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett. 2020, 594, 3767–3775. [Google Scholar] [CrossRef]
- Borst, P.; Elferink, R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef] [PubMed]
- Murina, V.; Kasari, M.; Takada, H.; Hinnu, M.; Saha, C.K.; Grimshaw, J.W.; Seki, T.; Reith, M.; Putrinš, M.; Tenson, T.; et al. ABCF ATPases Involved in Protein Synthesis, Ribosome Assembly and Antibiotic Resistance: Structural and Functional Diversification across the Tree of Life. J. Mol. Biol. 2018, 431, 3568–3590. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Quiles, C.; Mateo-Bonmatí, E.; Micol, J.L. ABCE Proteins: From Molecules to Development. Front. Plant Sci. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Mujawar, Z.; Tamehiro, N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis 2010, 211, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Zhang, Z.; Yue, J.; Fang, Q.; Gong, X. Cryo-EM structures of the human surfactant lipid transporter ABCA3. Sci. Adv. 2022, 8, eabn3727. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Geier, A.; Elferink, R.P.J.O. ABC of oral bioavailability: Transporters as gatekeepers in the gut. Gut 2003, 52, 1788–1795. [Google Scholar] [CrossRef]
- Durmus, S.; Hendrikx, J.J.; Schinkel, A.H. Apical ABC transporters andcancer chemotherapeutic drugdisposition. Adv. Cancer Res. 2015, 125, 1–41. [Google Scholar]
- U.S. Food & Drug Administration. Invitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions: Guidance for Industry. Guidance Doc., US FDA. 2020. Available online: https://www.fda.gov/media/135587/download (accessed on 2 January 2025).
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updat. 2021, 54, 100743. [Google Scholar] [CrossRef]
- Chen, Z.-S.; Hopper-Borge, E.; Belinsky, M.G.; Shchaveleva, I.; Kotova, E.; Kruh, G.D. Characterization of the Transport Properties of Human Multidrug Resistance Protein 7 (MRP7, ABCC10). Mol. Pharmacol. 2003, 63, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hopper-Borge, E.; Xu, X.; Shen, T.; Shi, Z.; Chen, Z.-S.; Kruh, G.D. Human Multidrug Resistance Protein 7 (ABCC10) Is a Resistance Factor for Nucleoside Analogues and Epothilone B. Cancer Res. 2008, 69, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2004, 204, 216–237. [Google Scholar] [CrossRef]
- Wang, L.; Johnson, Z.L.; Wasserman, M.R.; Levring, J.; Chen, J.; Liu, S. Characterization of the kinetic cycle of an ABC transporter by single-molecule and cryo-EM analyses. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Quazi, F.; Lenevich, S.; Molday, R.S. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat. Commun. 2012, 3, 925. [Google Scholar] [CrossRef] [PubMed]
- Roermund, C.W.T.; Visser, W.F.; Ijlst, L.; Cruchten, A.; Boek, M.; Kulik, W.; Waterham, H.R.; Wanders, R.J.A. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl–CoA esters. FASEB J. 2008, 22, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- van Roermund, C.W.; Visser, W.F.; Ijlst, L.; Waterham, H.R.; Wanders, R.J. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid β-oxidation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2011, 1811, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Feng, Z.; Hou, W.-T.; Jiang, Y.-L.; Wang, L.; Sun, L.; Zhou, C.-Z.; Chen, Y. Cryo-EM structure of human lysosomal cobalamin exporter ABCD4. Cell Res. 2019, 29, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, C.; Sun, Y.; Song, W.; Lin, J.; Li, J.; Guan, X. p62/mTOR/LXRα pathway inhibits cholesterol efflux mediated by ABCA1 and ABCG1 during autophagy blockage. Biochem. Biophys. Res. Commun. 2019, 514, 1093–1100. [Google Scholar] [CrossRef]
- Hegyi, Z.; Homolya, L. Functional Cooperativity between ABCG4 and ABCG1 Isoforms. PLoS ONE 2016, 11, e0156516. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, J.T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1–27. [Google Scholar]
- Szakács, G.; Váradi, A.; Özvegy-Laczka, C.; Sarkadi, B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox). Drug Discov. Today 2008, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Saeed, M.E.M.; Munder, M.; Spuller, A.; Greten, H.J.; Efferth, T. Effect of ABC transporter expression and mutational status on survival rates of cancer patients. Biomed. Pharmacother. 2020, 131, 110718. [Google Scholar] [CrossRef]
- Goldstein, M.N.; Slotnick, I.J.; Journey, L.J. In Vitro Studies with Hela Cell Lines Sensitive and Resistant to Actinomycin D. Ann. N. Y. Acad. Sci. 1960, 89, 474–483. [Google Scholar] [CrossRef]
- Juliano, R.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 455, 152–162. [Google Scholar] [CrossRef]
- Alam, A.; Locher, K.P. Structure and Mechanism of Human ABC Transporters. Annu. Rev. Biophys. 2023, 52, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.F. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol. Sci. 2004, 25, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- The International Transporter Consortium; International Transporter Consortium; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Ling, V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2005, 580, 998–1009. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Oldham, M.L.; Zhang, Q.; Chen, J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 2012, 490, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Kodan, A.; Yamaguchi, T.; Nakatsu, T.; Sakiyama, K.; Hipolito, C.J.; Fujioka, A.; Hirokane, R.; Ikeguchi, K.; Watanabe, B.; Hiratake, J.; et al. Structural basis for gating mechanisms of a eukaryotic P-glycoprotein homolog. Proc. Natl. Acad. Sci. USA 2014, 111, 4049–4054. [Google Scholar] [CrossRef]
- Alam, A.; Kowal, J.; Broude, E.; Roninson, I.; Locher, K.P. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 2019, 363, 753–756. [Google Scholar] [CrossRef]
- Kim, Y.; Chen, J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science 2018, 359, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2017, 61, 5108–5121. [Google Scholar] [CrossRef] [PubMed]
- Nosol, K.; Romane, K.; Irobalieva, R.N.; Alam, A.; Kowal, J.; Fujita, N.; Locher, K.P. Cryo-EM structures reveal distinct mechanisms of inhibition of the human multidrug transporter ABCB1. Proc. Natl. Acad. Sci. USA 2020, 117, 26245–26253. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Therapeutic Potential and Utility of Elacridar with Respect to P-glycoprotein Inhibition: An Insight from the Published In Vitro, Preclinical and Clinical Studies. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 915–933. [Google Scholar] [CrossRef]
- Srinivas, N.R. Understanding the role of tariquidar, a potent Pgp inhibitor, in combination trials with cytotoxic drugs: What is missing? Cancer Chemother. Pharmacol. 2016, 78, 1097–1098. [Google Scholar] [CrossRef]
- Alam, A.; Küng, R.; Kowal, J.; McLeod, R.A.; Tremp, N.; Broude, E.V.; Roninson, I.B.; Stahlberg, H.; Locher, K.P. Structure of a zosuquidar and UIC2-bound human-mouse chimeric ABCB1. Proc. Natl. Acad. Sci. USA 2018, 115, E1973–E1982. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorganic Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Z.; Li, A.; Zhang, Z.; Xu, S. Double-sides sticking mechanism of vinblastine interacting with α,β-tubulin to get activity against cancer cells. J. Biomol. Struct. Dyn. 2018, 37, 4080–4091. [Google Scholar] [CrossRef]
- Crowley, E.; McDevitt, C.A.; Callaghan, R. Generating inhibitors of P-glycoprotein: Where to, now? Methods Mol. Biol. 2010, 596, 405–432. [Google Scholar] [PubMed]
- Hyafil, F.; Vergely, C.; Du Vignaud, P.; Grand-Perret, T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993, 53, 4595–4602. [Google Scholar] [PubMed]
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: A randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef]
- Olsen, J.A.; Alam, A.; Kowal, J.; Stieger, B.; Locher, K.P. Structure of the human lipid exporter ABCB4 in a lipid environment. Nat. Struct. Mol. Biol. 2019, 27, 62–70. [Google Scholar] [CrossRef]
- Wang, C.; Cao, C.; Wang, N.; Wang, X.; Wang, X.; Zhang, X.C. Cryo-electron microscopy structure of human ABCB6 transporter. Protein Sci. 2020, 29, 2363–2374. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.S.; Lee, H.Y.; Kim, S.; Kim, J.W.; Jin, M.S. Cryo-EM structure of cadmium-bound human ABCB6. Commun. Biol. 2024, 7, 1–13. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Huang, Q.; Yang, Y.; Zhang, J.-T.; Zhong, M.-Z.; Zhou, H.-H.; Liu, Z.-Q. Characterization and analyses of multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphisms in Chinese population. Pharmacogenetics Genom. 2009, 19, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Mechetner, E.B.; Roninson, I.B. Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc. Natl. Acad. Sci. USA 1992, 89, 5824–5828. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.-Y.; Terada, T. Molecular Mechanisms for Biliary Phospholipid and Drug Efflux Mediated by ABCB4 and Bile Salts. BioMed Res. Int. 2014, 2014, 954781. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; van Helvoort, A.; van Meer, G.; Szabó, K.; Welker, E.; Szakács, G.; Váradi, A.; Sarkadi, B.; Borst, P. MDR3 P-glycoprotein, a Phosphatidylcholine Translocase, Transports Several Cytotoxic Drugs and Directly Interacts with Drugs as Judged by Interference with Nucleotide Trapping. J. Biol. Chem. 2000, 275, 23530–23539. [Google Scholar] [CrossRef] [PubMed]
- Sticova, E.; Jirsa, M. ABCB4 disease: Many faces of one gene deficiency. Ann. Hepatol. 2020, 19, 126–133. [Google Scholar] [CrossRef]
- Morita, S.-Y.; Tsuda, T.; Horikami, M.; Teraoka, R.; Kitagawa, S.; Terada, T. Bile salt-stimulated phospholipid efflux mediated by ABCB4 localized in nonraft membranes. J. Lipid Res. 2013, 54, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.; Schinkel, A.; Elferink, R.; Groen, A.; Wagenaar, E.; van Deemter, L.; Mol, C.; Ottenhoff, R.; van der Lugt, N.; van Roon, M.; et al. Homozygous disruption of the murine MDR2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993, 75, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.L. Bile Formation and Secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- de Vree, J.M.L.; Jacquemin, E.; Sturm, E.; Cresteil, D.; Bosma, P.J.; Aten, J.; Deleuze, J.-F.; Desrochers, M.; Burdelski, M.; Bernard, O.; et al. Mutations in the MDR 3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl. Acad. Sci. USA 1998, 95, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Elferink, R.P.J.O.; Paulusma, C.C. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflügers Arch. Eur. J. Physiol. 2006, 453, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, D.; Fotsing, G.; Barbu, V.; Beauchant, M. ABCB4/MDR3 gene mutations and cholangiocarcinomas. J. Hepatol. 2012, 57, 467–468. [Google Scholar] [CrossRef]
- Mhatre, S.; Wang, Z.; Nagrani, R.; Badwe, R.; Chiplunkar, S.; Mittal, B.; Yadav, S.; Zhang, H.; Chung, C.C.; Patil, P.; et al. Common genetic variation and risk of gallbladder cancer in India: A case-control genome-wide association study. Lancet Oncol. 2017, 18, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, S.; Herkt, S.C.; Richter, A.M.; Fuhrmann, L.; El-Nikhely, N.; Seeger, W.; Savai, R.; Dammann, R.H. ABCB4 is frequently epigenetically silenced in human cancers and inhibits tumor growth. Sci. Rep. 2014, 4, 6899. [Google Scholar] [CrossRef]
- Ishigami, M.; Tominaga, Y.; Nagao, K.; Kimura, Y.; Matsuo, M.; Kioka, N.; Ueda, K. ATPase activity of nucleotide binding domains of human MDR3 in the context of MDR1. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, Z.M.; Synal-Hermanns, U.; Yoker, A.; Locher, K.P.; Stieger, B. Role of Multidrug Resistance Protein 3 in Antifungal-Induced Cholestasis. Mol. Pharmacol. 2016, 90, 23–34. [Google Scholar] [CrossRef] [PubMed]
- E Frampton, J.; Scott, L.J. Posaconazole. Drugs 2008, 68, 993–1016. [Google Scholar] [CrossRef] [PubMed]
- Kiss, K.; Kucsma, N.; Brozik, A.; Tusnady, G.E.; Bergam, P.; van Niel, G.; Szakacs, G. Role of the N-terminal transmembrane domain in the endo-lysosomal targeting and function of the human ABCB6 protein. Biochem. J. 2015, 467, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Aguilar-Bryan, L.; Vaxillaire, M.; Dechaume, A.; Wang, Y.; Dean, M.; Moitra, K.; Bryan, J.; Schuetz, J.D. Conserved Intramolecular Disulfide Bond Is Critical to Trafficking and Fate of ATP-binding Cassette (ABC) Transporters ABCB6 and Sulfonylurea Receptor 1 (SUR1)/ABCC8. J. Biol. Chem. 2011, 286, 8481–8492. [Google Scholar] [CrossRef]
- Boswell-Casteel, R.C.; Fukuda, Y.; Schuetz, J.D. ABCB6, an ABC Transporter Impacting Drug Response and Disease. AAPS J. 2017, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Kamijo, Y.; Nishizawa, Y.; Tabata, S.; Horikuchi, F.; Yamamoto, M.; Kawahara, K.; Shinsato, Y.; Tachiwada, T.; Chen, Z.-S.; et al. Expression of ABCB6 is related to resistance to 5-FU, SN-38 and vincristine. Anticancer Res. 2014, 34, 4767–4773. [Google Scholar] [PubMed]
- Yasui, K.; Mihara, S.; Zhao, C.; Okamoto, H.; Saito-Ohara, F.; Tomida, A.; Funato, T.; Yokomizo, A.; Naito, S.; Imoto, I.; et al. Alteration in Copy Numbers of Genes as a Mechanism for Acquired Drug Resistance. Cancer Res. 2004, 64, 1403–1410. [Google Scholar] [CrossRef]
- Rakvacs, Z.; Kucsma, N.; Gera, M.; Igriczi, B.; Kiss, K.; Barna, J.; Kovacs, D.; Vellai, T.; Bencs, L.; Reisecker, J.M.; et al. The human ABCB6 protein is the functional homologue of HMT-1 proteins mediating cadmium detoxification. Cell. Mol. Life Sci. 2019, 76, 4131–4144. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.A.; Ardehali, H. Mitochondrial ATP–binding cassette proteins. Transl. Res. 2007, 150, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Schuetz, J.D. The Role of ABCG2 and ABCB6 in Porphyrin Metabolism and Cell Survival. Curr. Pharm. Biotechnol. 2011, 12, 647–655. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Xie, T.; Schuetz, J.D. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol. Ther. 2007, 114, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, F.; Bu, J.; Liu, X.; Du, W.; Dong, J.; Cooney, J.D.; Dubey, S.K.; Shi, Y.; Gong, B.; et al. ABCB6 Mutations Cause Ocular Coloboma. Am. J. Hum. Genet. 2012, 90, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, I.; Alper, S.L.; Delaunay, J.; Auriemma, C.; Russo, R.; Asci, R.; Esposito, M.R.; Sharma, A.K.; Shmukler, B.E.; Brugnara, C.; et al. Missense mutations in the ABCB6 transporter cause dominant familialpseudohyperkalemia. Am. J. Hematol. 2012, 88, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, D.; Zhang, J.; Chen, X.; Huang, M.; Archacki, S.; Tian, Y.; Ren, W.; Mei, A.; Zhang, Q.; et al. Mutations in ABCB6 Cause Dyschromatosis Universalis Hereditaria. J. Investig. Dermatol. 2013, 133, 2221–2228. [Google Scholar] [CrossRef]
- Krishnamurthy, P.C.; Du, G.; Fukuda, Y.; Sun, D.; Sampath, J.; Mercer, K.E.; Wang, J.; Sosa-Pineda, B.; Murti, K.G.; Schuetz, J.D. Identification of a mammalian mitochondrial porphyrin transporter. Nature 2006, 443, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Polireddy, K.; Khan, M.M.T.; Chavan, H.; Young, S.; Ma, X.; Waller, A.; Garcia, M.; Perez, D.; Chavez, S.; Strouse, J.J.; et al. A Novel Flow Cytometric HTS Assay Reveals Functional Modulators of ATP Binding Cassette Transporter ABCB6. PLoS ONE 2012, 7, e40005. [Google Scholar] [CrossRef] [PubMed]
- Januchowski, R.; Zawierucha, P.; Andrzejewska, M.; Ruciński, M.; Zabel, M. Microarray-based detection and expression analysis of ABC and SLC transporters in drug-resistant ovarian cancer cell lines. Biomed. Pharmacother. 2013, 67, 240–245. [Google Scholar] [CrossRef]
- Park, S.; Shimizu, C.; Shimoyama, T.; Takeda, M.; Ando, M.; Kohno, T.; Katsumata, N.; Kang, Y.-K.; Nishio, K.; Fujiwara, Y. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. Treat. 2006, 99, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Varatharajan, S.; Abraham, A.; Karathedath, S.; Ganesan, S.; Lakshmi, K.M.; Arthur, N.; Srivastava, V.M.; George, B.; Srivastava, A.; Mathews, V.; et al. Atp-Binding Casette Transporter Expression in Acute Myeloid Leukemia: Association with in Vitro Cytotoxicity and Prognostic Markers. Pharmacogenomics 2017, 18, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, S.; Tian, M.; Zhang, L.; Guo, R.; Zhuo, W.; Yang, M. Molecular insights into the human ABCB6 transporter. Cell Discov. 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.S.; Park, J.G.; Kim, J.W.; Ju, S.; Choi, S.H.; Kim, S.; Kim, N.J.; Hong, S.; Kang, J.Y.; et al. Structural Insights into Porphyrin Recognition by the Human ATP-Binding Cassette Transporter ABCB6. Mol. Cells 2022, 45, 575–587. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, J.G.; Jang, E.; Choi, S.H.; Kim, S.; Kim, J.W.; Jin, M.S. W546 stacking disruption traps the human porphyrin transporter ABCB6 in an outward-facing transient state. Commun. Biol. 2023, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.L.; Chen, J. Structural Basis of Substrate Recognition by the Multidrug Resistance Protein MRP1. Cell 2017, 168, 1075–1085.e9. [Google Scholar] [CrossRef] [PubMed]
- Conseil, G.; Arama-Chayoth, M.; Tsfadia, Y.; Cole, S.P.C. Structure-guided probing of the leukotriene C4 binding site in human multidrug resistance protein 1 (MRP1; ABCC1). FASEB J. 2019, 33, 10692–10704. [Google Scholar] [CrossRef] [PubMed]
- Bakos, É.; Evers, R.; Calenda, G.; Tusnády, G.E.; Szakács, G.; Váradi, A.; Sarkadi, B. Characterization of the amino-terminal regions in the human multidrug resistance protein (MRP1). J. Cell Sci. 2000, 113, 4451–4461. [Google Scholar] [CrossRef]
- Slot, A.J.; Molinski, S.V.; Cole, S.P. Mammalian multidrug-resistance proteins (MRPs). Essays Biochem. 2011, 50, 179–207. [Google Scholar] [CrossRef]
- Seelig, A. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 1998, 251, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Kowal, J.; Ni, D.; Jackson, S.M.; Manolaridis, I.; Stahlberg, H.; Locher, K.P. Structural Basis of Drug Recognition by the Multidrug Transporter ABCG2. J. Mol. Biol. 2021, 433, 166980. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of Human ABCG2: From Technical Background to Recent Updates With Clinical Implications. Front. Pharmacol. 2019, 10, 208. [Google Scholar] [CrossRef]
- Houghton, P.J.; Germain, G.S.; Harwood, F.C.; Schuetz, J.D.; Stewart, C.F.; Buchdunger, E.; Traxler, P. Imatinib Mesylate Is a Potent Inhibitor of the ABCG2 (BCRP) Transporter and Reverses Resistance to Topotecan and SN-38 in Vitro. Cancer Res. 2004, 64, 2333–2337. [Google Scholar] [CrossRef]
- Candeil, L.; Gourdier, I.; Peyron, D.; Vezzio, N.; Copois, V.; Bibeau, F.; Orsetti, B.; Scheffer, G.L.; Ychou, M.; Khan, Q.A.; et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int. J. Cancer 2004, 109, 848–854. [Google Scholar] [CrossRef]
- Hillgren, K.M.; Keppler, D.; A Zur, A.; Giacomini, K.M.; Stieger, B.; E Cass, C.; Zhang, L. Emerging Transporters of Clinical Importance: An Update From the International Transporter Consortium. Clin. Pharmacol. Ther. 2013, 94, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, A.; Ierano, C.; Szakacs, G.; Robey, R.W.; Bates, S.E. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011, 50, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Massey, P.R.; Amiri-Kordestani, L.; Bates, S.E. ABC Transporters: Unvalidated Therapeutic Targets in Cancer and the CNS. Anti-Cancer Agents Med. Chem. 2010, 10, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kinch, L.N.; Borek, D.M.; Wang, J.; Wang, J.; Urbatsch, I.L.; Xie, X.-S.; Grishin, N.V.; Cohen, J.C.; Otwinowski, Z.; et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 2016, 533, 561–564. [Google Scholar] [CrossRef]
- Rosenberg, M.F.; Bikadi, Z.; Hazai, E.; Starborg, T.; Kelley, L.; Chayen, N.E.; Ford, R.C.; Mao, Q. Three-dimensional structure of the human breast cancer resistance protein (BCRP/ABCG2) in an inward-facing conformation. Acta Crystallogr. Sect. D Struct. Biol. 2015, 71, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Manolaridis, I.; Jackson, S.M.; Taylor, N.M.I.; Kowal, J.; Stahlberg, H.; Locher, K.P. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 2018, 563, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ni, D.; Kowal, J.; Manolaridis, I.; Jackson, S.M.; Stahlberg, H.; Locher, K.P. Structures of ABCG2 under turnover conditions reveal a key step in the drug transport mechanism. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Yang, Y.; Cai, C.-Y.; Lei, Z.-N.; Wang, J.-Q.; Gupta, P.; Teng, Q.-X.; Chen, Z.-S.; Kong, D.; Yang, D.-H. VS-4718 Antagonizes Multidrug Resistance in ABCB1- and ABCG2-Overexpressing Cancer Cells by Inhibiting the Efflux Function of ABC Transporters. Front. Pharmacol. 2018, 9, 1236. [Google Scholar] [CrossRef]
- Jones, P.M.; George, A.M. The Switch and Reciprocating Models for the Function of ABC Multidrug Exporters: Perspectives on Recent Research. Int. J. Mol. Sci. 2023, 24, 2624. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Hu, J.; Zhu, X.; Yin, H.; Yin, J. Oxidative stress-mediated up-regulation of ABC transporters in lung cancer cells. J. Biochem. Mol. Toxicol. 2022, 36, e23095. [Google Scholar] [CrossRef]
- Sajid, A.; Rahman, H.; Ambudkar, S.V. Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters. Nat. Rev. Cancer 2023, 23, 762–779. [Google Scholar] [CrossRef]
- Lainey, E.; Sébert, M.; Thépot, S.; Scoazec, M.; Bouteloup, C.; Leroy, C.; De Botton, S.; Galluzzi, L.; Fenaux, P.; Kroemer, G. Erlotinib antagonizes ABC transporters in acute myeloid leukemia. Cell Cycle 2012, 11, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.C.; Wiese, M. HM30181 Derivatives as Novel Potent and Selective Inhibitors of the Breast Cancer Resistance Protein (BCRP/ABCG2). J. Med. Chem. 2015, 58, 3910–3921. [Google Scholar] [CrossRef]
- Miyata, H.; Takada, T.; Toyoda, Y.; Matsuo, H.; Ichida, K.; Suzuki, H. Identification of Febuxostat as a New Strong ABCG2 Inhibitor: Potential Applications and Risks in Clinical Situations. Front. Pharmacol. 2016, 7, 518. [Google Scholar] [CrossRef]
- Mohammad, I.S.; He, W.; Yin, L. Insight on Multidrug Resistance and Nanomedicine Approaches to Overcome MDR. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 473–509. [Google Scholar] [CrossRef] [PubMed]
- Pote, M.S.; Gacche, R.N. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, A.; Chen, M.; Liu, J. ABC Transporter Inhibitors in Reversing Multidrug Resistance to Chemotherapy. Curr. Drug Targets 2015, 16, 1356–1371. [Google Scholar] [CrossRef]
- Falasca, M.; Linton, K.J. Investigational ABC transporter inhibitors. Expert Opin. Investig. Drugs 2012, 21, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Friedenberg, W.R.; Rue, M.; Blood, E.A.; Dalton, W.S.; Shustik, C.; Larson, R.A.; Sonneveld, P.; Greipp, P.R. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95). Cancer 2006, 106, 830–838. [Google Scholar] [CrossRef]
- Kelly, R.J.; Draper, D.; Chen, C.C.; Robey, R.W.; Figg, W.D.; Piekarz, R.L.; Chen, X.; Gardner, E.R.; Balis, F.M.; Venkatesan, A.M.; et al. A Pharmacodynamic Study of Docetaxel in Combination with the P-glycoprotein Antagonist Tariquidar (XR9576) in Patients with Lung, Ovarian, and Cervical Cancer. Clin. Cancer Res. 2011, 17, 569–580. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Chen, J.-J.; Kumar, P.; Chen, K.; Sodani, K.; Patel, A.; Chen, Y.-L.; Chen, S.-D.; Jiang, W.-Q.; Chen, Z.-S. Reversal of MRP7 (ABCC10)-Mediated Multidrug Resistance by Tariquidar. PLoS ONE 2013, 8, e55576. [Google Scholar] [CrossRef]
- Krapf, M.K.; Gallus, J.; Spindler, A.; Wiese, M. Synthesis and biological evaluation of quinazoline derivatives–A SAR study of novel inhibitors of ABCG2. Eur. J. Med. Chem. 2018, 161, 506–525. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Sahebkar, A. Protective effects of curcumin against doxorubicin-induced toxicity and resistance: A review. Crit. Rev. Oncol. 2018, 122, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Noro, R.; Seike, M.; Matsuda, K.; Hirao, M.; Fukuizumi, A.; Takano, N.; Miyanaga, A.; Gemma, A. Inhibitors of ABCB1 and ABCG2 overcame resistance to topoisomerase inhibitors in small cell lung cancer. Thorac. Cancer 2022, 13, 2142–2151. [Google Scholar] [CrossRef]
- Yarla, N.S. Bioactive Flavonoids as ABC Transporters Inhibitors for Reversion of Multidrug Resistance in Cancer. J. Mar. Sci. Res. Dev. 2013, 4, 1000e123. [Google Scholar]
- van der Noord, V.E.; van der Stel, W.; Louwerens, G.; Verhoeven, D.; Kuiken, H.J.; Lieftink, C.; Grandits, M.; Ecker, G.F.; Beijersbergen, R.L.; Bouwman, P.; et al. Systematic screening identifies ABCG2 as critical factor underlying synergy of kinase inhibitors with transcriptional CDK inhibitors. Breast Cancer Res. 2023, 25, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef]
- Yu, M.; Ocana, A.; Tannock, I.F. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: Why has it failed to provide clinical benefit? Cancer Metastasis Rev. 2012, 32, 211–227. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Hu, Z.; Mao, X.; Zi, X.; Xia, K.; Tang, B.; Zhang, R. IGHMBP2 -related clinical and genetic features in a cohort of Chinese Charcot–Marie–Tooth disease type 2 patients. Neuromuscul. Disord. 2016, 27, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef]
- Bui, K.C.; Ho, V.H.; Nguyen, H.H.; Dang, T.C.; Ngo, T.H.; Nguyen, T.M.L.; Nguyen, L.T.; Dang, T.L.; Tran, T.T.; Le, Q.H.; et al. X-ray-irradiated K562 feeder cells for expansion of functional CAR-T cells. Biochem. Biophys. Rep. 2022, 33, 101399. [Google Scholar] [CrossRef]
- Du, J.; Kageyama, S.-I.; Hirata, H.; Motegi, A.; Nakamura, M.; Hirano, Y.; Okumura, M.; Yamashita, R.; Tsuchihara, K.; Hojo, H.; et al. Comparative analysis of the immune responses in cancer cells irradiated with X-ray, proton and carbon-ion beams. Biochem. Biophys. Res. Commun. 2021, 585, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, M.; Fujita, M.; Karasawa, K. Combining Carbon-Ion Irradiation and PARP Inhibitor, Olaparib Efficiently Kills BRCA1-Mutated Triple-Negative Breast Cancer Cells. Breast Cancer Basic Clin. Res. 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, W.; Yu, S.; Xia, S.-L.; Liu, Y.-N.; Yang, G.-J. Application of MOF-based nanotherapeutics in light-mediated cancer diagnosis and therapy. J. Nanobiotechnol. 2022, 20, 1–28. [Google Scholar] [CrossRef]
- De Vera, A.A.; Gupta, P.; Lei, Z.; Liao, D.; Narayanan, S.; Teng, Q.; Reznik, S.E.; Chen, Z.-S. Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo. Cancer Lett. 2018, 442, 91–103. [Google Scholar] [CrossRef]
- Bihorel, S.; Camenisch, G.; Lemaire, M.; Scherrmann, J.-M. Modulation of the Brain Distribution of Imatinib and its Metabolites in Mice by Valspodar, Zosuquidar and Elacridar. Pharm. Res. 2007, 24, 1720–1728. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Tavakkol-Afshari, J.; Brook, A.; Jafari-Anarkooli, I. Direct toxicity of Rose Bengal in MCF-7 cell line: Role of apoptosis. Food Chem. Toxicol. 2009, 47, 855–859. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Duan, Q.; Ran, C.; Fu, T.; Liu, Y.; Tan, W. Recent progress of aptamer–drug conjugates in cancer therapy. Acta Pharm. Sin. B 2023, 13, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, Y.; Liu, Z.; Zhao, R.; Wan, Q.; Chen, X.; Tang, B.; Zhou, Y.; Lin, Y. Self-Assembled Multivalent Aptamer Drug Conjugates: Enhanced Targeting and Cytotoxicity for HER2-Positive Gastric Cancer. ACS Appl. Mater. Interfaces 2023, 15, 43359–43373. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Wang, Y.; Zalath, M.; Liu, D.; Cardillo, T.M.; Goldenberg, D.M. Combining ABCG2 Inhibitors with IMMU-132, an Anti–Trop-2 Antibody Conjugate of SN-38, Overcomes Resistance to SN-38 in Breast and Gastric Cancers. Mol. Cancer Ther. 2016, 15, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Pieper, S.; Onafuye, H.; Mulac, D.; Cinatl, J.; Wass, M.N.; Michaelis, M.; Langer, K. Incorporation of doxorubicin in different polymer nanoparticles and their anticancer activity. Beilstein J. Nanotechnol. 2019, 10, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Lage, H. Gene Therapeutic Approaches to Overcome ABCB1-Mediated Drug Resistance. Recent Results Cancer Res. 2016, 209, 87–94. [Google Scholar]
- Varlas, S.; Neal, T.J.; Armes, S.P. Polymerization-induced self-assembly and disassembly during the synthesis of thermoresponsive ABC triblock copolymer nano-objects in aqueous solution. Chem. Sci. 2022, 13, 7295–7303. [Google Scholar] [CrossRef]
- Yadav, P.; Ambudkar, S.V.; Prasad, N.R. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnol. 2022, 20, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Rychlik, B.; Storm, G.; Kiessling, F.; Lammers, T. Multidrug resistance: Physiological principles and nanomedical solutions. Adv. Drug Deliv. Rev. 2013, 65, 1852–1865. [Google Scholar] [CrossRef]
- de la Puente, P.; Azab, A.K. Nanoparticle delivery systems, general approaches, and their implementation in multiple myeloma. Eur. J. Haematol. 2017, 98, 529–541. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, S.; Zhu, X.; Xia, J.; Wu, Q.; Wang, S.; Xie, W.; Zhang, Y. The novel ABC transporter ABCH1 is a potential target for RNAi-based insect pest control and resistance management. Sci. Rep. 2015, 5, srep13728. [Google Scholar] [CrossRef]
- Hokaiwado, N.; Takeshita, F.; Banas, A.; Ochiya, T. RNAi-based drug discovery and its application to therapeutics. IDrugs 2008, 11, 274–278. [Google Scholar]
- Uchino, K.; Ochiya, T.; Takeshita, F. RNAi Therapeutics and Applications of MicroRNAs in Cancer Treatment. Ultrasound Med. Biol. 2013, 43, 596–607. [Google Scholar] [CrossRef]
- Tong, A.W. Small RNAs and Non-Small Cell Lung Cancer. Curr. Mol. Med. 2006, 6, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, S.; Werk, A.N.; Cascorbi, I. MicroRNAs and their relevance to ABC transporters. Br. J. Clin. Pharmacol. 2013, 77, 587–596. [Google Scholar] [CrossRef]
- Szczepanek, J.; Skorupa, M.; Tretyn, A. MicroRNA as a Potential Therapeutic Molecule in Cancer. Cells 2022, 11, 1008. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Wegler, C.; Gazit, M.; Issa, K.; Subramaniam, S.; Artursson, P.; Karlgren, M. Expanding the Efflux In Vitro Assay Toolbox: A CRISPR-Cas9 Edited MDCK Cell Line with Human BCRP and Completely Lacking Canine MDR1. J. Pharm. Sci. 2021, 110, 388–396. [Google Scholar] [CrossRef]
- Radtke, L.; Majchrzak-Celińska, A.; Awortwe, C.; Vater, I.; Nagel, I.; Sebens, S.; Cascorbi, I.; Kaehler, M. CRISPR/Cas9-induced knockout reveals the role of ABCB1 in the response to temozolomide, carmustine and lomustine in glioblastoma multiforme. Pharmacol. Res. 2022, 185, 106510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, S.; Liu, H.; Li, L.; Song, J.; Wang, G.; Wang, H.; Wu, Y.; Shao, Z.; Fu, R. Infection risk in autoimmune hematological disorders with low-dose rituximab treatment. J. Clin. Lab. Anal. 2020, 34. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M. Protein Kinases and Cross-talk between Post-translational Modifications in the Regulation of Drug Transporters. Mol. Pharmacol. 2022, 103, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Sun, A.; Cao, W.; Eliason, A.; Mendez, K.M.; Getzler, A.J.; Tsuda, S.; Diao, H.; Mukori, C.; Bruno, N.E.; et al. Physiological expression and function of the MDR1 transporter in cytotoxic T lymphocytes. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Norouzi-Barough, L.; Sarookhani, M.R.; Salehi, R.; Sharifi, M.; Moghbelinejad, S. CRISPR/Cas9, a new approach to successful knockdown of ABCB1/P-glycoprotein and reversal of chemosensitivity in human epithelial ovarian cancer cell line. Iran. J. Basic Med. Sci. 2018, 21, 181–187. [Google Scholar] [CrossRef]
- Hrycyna, C.A.; Airan, L.E.; Germann, U.A.; Ambudkar, S.V.; Pastan, I.; Gottesman, M.M. Structural Flexibility of the Linker Region of Human P-Glycoprotein Permits ATP Hydrolysis and Drug Transport. Biochemistry 1998, 37, 13660–13673. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Esser, L.; Zhou, F.; Pluchino, K.M.; Shiloach, J.; Ma, J.; Tang, W.-K.; Gutierrez, C.; Zhang, A.; Shukla, S.; Madigan, J.P.; et al. Structures of the Multidrug Transporter P-glycoprotein Reveal Asymmetric ATP Binding and the Mechanism of Polyspecificity. J. Biol. Chem. 2017, 292, 446–461. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wang, F.; Cheng, M.; Mao, Y.; Fang, S.; Wang, L.; Zhou, C.; Hou, W.; Chen, Y. Placing steroid hormones within the human ABCC3 transporter reveals a compatible amphiphilic substrate-binding pocket. EMBO J. 2023, 42, e113415. [Google Scholar] [CrossRef]
- Johnson, Z.L.; Chen, J. ATP Binding Enables Substrate Release from Multidrug Resistance Protein 1. Cell 2018, 172, 81–89.e10. [Google Scholar] [CrossRef]
- Kroll, T.; Prescher, M.; Smits, S.H.J.; Schmitt, L. Structure and Function of Hepatobiliary ATP Binding Cassette Transporters. Chem. Rev. 2020, 121, 5240–5288. [Google Scholar] [CrossRef] [PubMed]
- Bakos, E.; Evers, R.; Szakács, G.; Tusnády, G.E.; Welker, E.; Szabó, K.; de Haas, M.; van Deemter, L.; Borst, P.; Váradi, A.; et al. Functional Multidrug Resistance Protein (MRP1) Lacking the N-terminal Transmembrane Domain. J. Biol. Chem. 1998, 273, 32167–32175. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).