Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm
Abstract
:1. Introduction
2. Materials and Methods
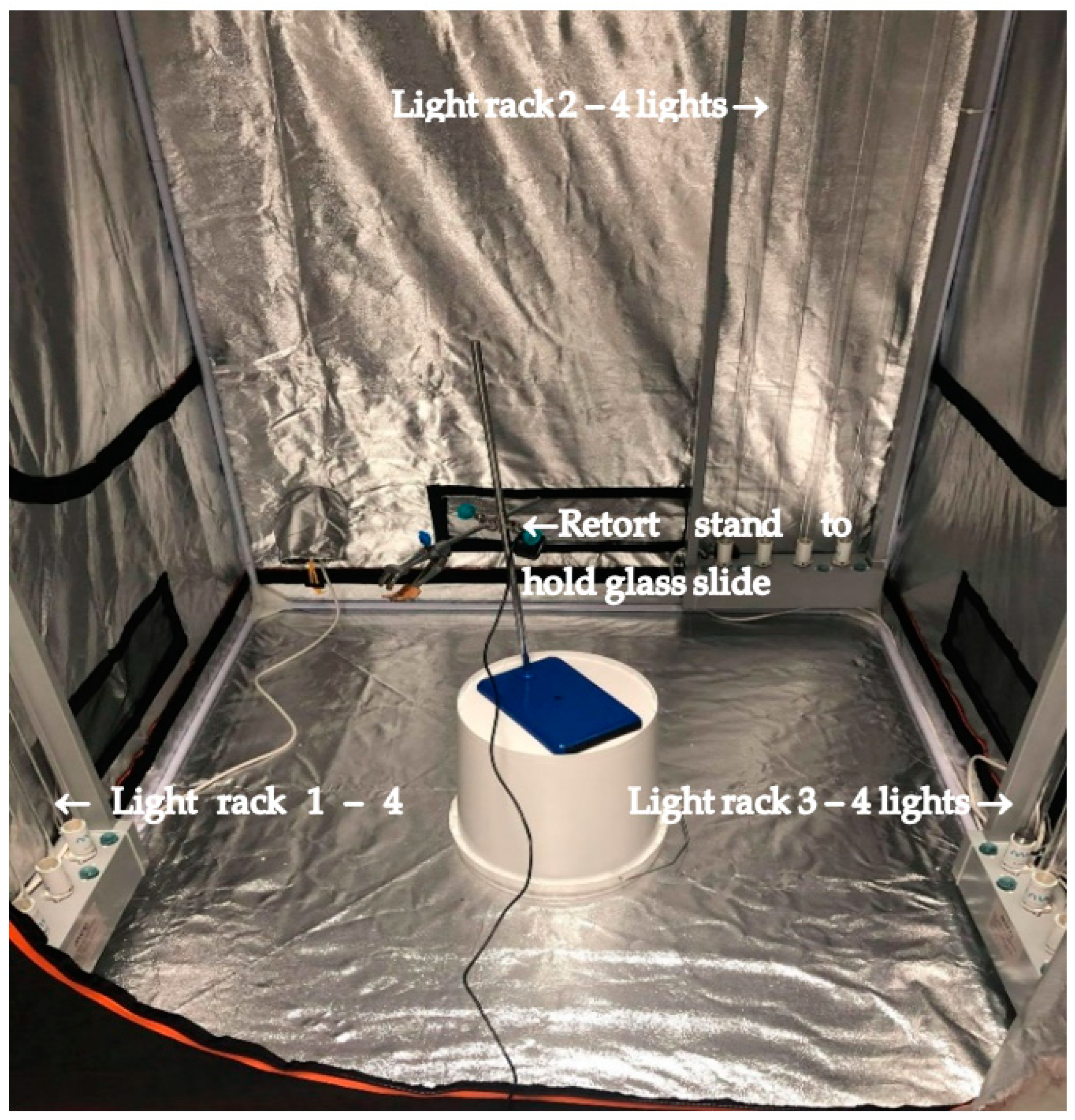
2.1. MUVI-UVC Disinfection System Setup
2.2. Microorganisms Studied
2.3. Microbial Growth, UV Exposure and Microbial Recovery
2.4. Biofilm Formation
2.5. UVC Exposure
2.6. Microbial Recovery
3. Results
3.1. Efficacy of UVC against Different Bacterial Species
3.2. Efficacy of UVC against Bacterial Biofilms
3.3. Efficacy of UVC against Coronavirus Fungus and Fungal Spores
3.4. Efficacy of UVC against S. aureus on Different Surfaces
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Rev. Anti-Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.; Hanslmeier, A. Ultraviolet Radiation in the Solar System; Springer: Berlin/Heidelberg, Germany, 2005; Volume 331. [Google Scholar]
- Gurzadyan, G.G.; Görner, H.; Schulte-Frohlinde, D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat. Res. 1995, 141, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Ossoff, S.F.; Lobe, D.C.; Dorfman, M.H.; Dumais, C.M.; Qualls, R.G.; Johnson, J.D. UV inactivation of pathogenic and indicator microorganisms. Appl. Environ. Microbiol. 1985, 49, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Nerandzic, M.M.; Cadnum, J.L.; Pultz, M.J.; Donskey, C.J. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect. Dis. 2010, 10, 197. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfectants used for environmental disinfection and new room decontamination technology. Am. J. Infect. Control 2013, 41, S36–S41. [Google Scholar] [CrossRef]
- Diffey, B. Physical barriers to protect humans from solar UV radiation exposure. In Sun Protection; IOP Publishing Ltd.: Bristol, UK, 2017; Chapter 6; pp. 6-1–6-24. [Google Scholar] [CrossRef]
- Kampf, G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect. Prev. Pract. 2020, 2, 100044. [Google Scholar] [CrossRef]
- Lei, H.; Li, Y.; Xiao, S.; Yang, X.; Lin, C.; Norris, S.L.; Wei, D.; Hu, Z.; Ji, S. Logistic growth of a surface contamination network and its role in disease spread. Sci. Rep. 2017, 7, 14826. [Google Scholar] [CrossRef]
- Carling, P.C.; Briggs, J.; Hylander, D.; Perkins, J. An evaluation of patient area cleaning in 3 hospitals using a novel targeting methodology. Am. J. Infect. Control 2006, 34, 513–519. [Google Scholar] [CrossRef]
- Carling, P.C.; Parry, M.M.; Rupp, M.E.; Po, J.L.; Dick, B.; Von Beheren, S.; Group, H.E.H.S. Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect. Control Hosp. Epidemiol. 2008, 29, 1035–1041. [Google Scholar] [CrossRef]
- Ghedini, E.; Pizzolato, M.; Longo, L.; Menegazzo, F.; Zanardo, D.; Signoretto, M. Which Are the Main Surface Disinfection Approaches at the Time of SARS-CoV-2? Front. Chem. Eng. 2021, 2, 589202. [Google Scholar] [CrossRef]
- Vickery, K.; Deva, A.; Jacombs, A.; Allan, J.; Valente, P.; Gosbell, I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 2012, 80, 52–55. [Google Scholar] [CrossRef]
- Hu, H.; Johani, K.; Gosbell, I.B.; Jacombs, A.; Almatroudi, A.; Whiteley, G.S.; Deva, A.K.; Jensen, S.; Vickery, K. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: Combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J. Hosp. Infect. 2015, 91, 35–44. [Google Scholar] [CrossRef]
- Yezli, S.; Otter, J. Does the discovery of biofilms on dry hospital environmental surfaces change the way we think about hospital disinfection? J. Hosp. Infect. 2012, 81, 293–294. [Google Scholar] [CrossRef]
- Parvin, F.; Hu, H.; Whiteley, G.S.; Glasbey, T.; Vickery, K. Difficulty in removing biofilm from dry surfaces. J. Hosp. Infect. 2019, 103, 465–467. [Google Scholar] [CrossRef]
- Chowdhury, D.; Tahir, S.; Legge, M.; Hu, H.; Prvan, T.; Johani, K.; Whiteley, G.S.; Glasbey, T.O.; Deva, A.K.; Vickery, K. Transfer of dry surface biofilm in the healthcare environment: The role of healthcare workers’ hands as vehicles. J. Hosp. Infect. 2018, 100, e85–e90. [Google Scholar] [CrossRef]
- Ramos, C.C.R.; Roque, J.L.A.; Sarmiento, D.B.; Suarez, L.E.G.; Sunio, J.T.P.; Tabungar, K.I.B.; Tengco, G.S.C.; Rio, P.C.; Hilario, A.L. Use of ultraviolet-C in environmental sterilization in hospitals: A systematic review on efficacy and safety. Int. J. Health Sci. 2020, 14, 52–65. [Google Scholar]
- World Health Organization. WHO Guidelines on Tuberculosis Infection Prevention and Control: 2019 Update; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- McDevitt, J.J.; Rudnick, S.N.; Radonovich, L.J. Aerosol susceptibility of influenza virus to UV-C light. Appl. Environ. Microbiol. 2012, 78, 1666–1669. [Google Scholar] [CrossRef]
- Rogers, W. Steam and dry heat sterilization of biomaterials and medical devices. In Sterilisation of Biomaterials and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 20–55. [Google Scholar]
- Iwaguch, S.; Matsumura, K.; Tokuoka, Y.; Wakui, S.; Kawashima, N. Sterilization system using microwave and UV light. Colloids Surf. B Biointerfaces 2002, 25, 299–304. [Google Scholar] [CrossRef]
- Moore, G.; Ali, S.; Cloutman-Green, E.A.; Bradley, C.R.; Wilkinson, M.A.C.; Hartley, J.C.; Fraise, A.P.; Wilson, A.P.R. Use of UV-C radiation to disinfect non-critical patient care items: A laboratory assessment of the Nanoclave Cabinet. BMC Infect. Dis. 2012, 12, 174. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; Filipello, V.; Di Ciccio, P.A.; Pitozzi, A.; Ghidini, S.; Scali, F.; Ianieri, A.; Zanardi, E.; Losio, M.N.; Simon, A.C.; et al. Assessment of the Antibiotic Resistance Profile, Genetic Heterogeneity and Biofilm Production of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolated from The Italian Swine Production Chain. Foods 2020, 9, 1141. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Stapleton, F.; Summers, S.; Rice, S.A.; Willcox, M.D. Antibiotic Resistance Characteristics of Pseudomonas aeruginosa Isolated from Keratitis in Australia and India. Antibiotics 2020, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Enhancement of Antibiofilm Activity of Ciprofloxacin against Staphylococcus aureus by Administration of Antimicrobial Peptides. Antibiotics 2021, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cole, N.; Dutta, D.; Kumar, N.; Willcox, M.D.P. Antimicrobial activity of immobilized lactoferrin and lactoferricin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Gosbell, I.B.; Hu, H.; Jensen, S.O.; Espedido, B.A.; Tahir, S.; Glasbey, T.O.; Legge, P.; Whiteley, G.; Deva, A.; et al. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: Implications for infection control. J. Hosp. Infect. 2016, 93, 263–270. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.K.; Zhu, H.; Ozkan, J.; Wu, D.; Masoudi, S.; Bandara, R.; Borazjani, R.N.; Willcox, M.D. Bacterial adhesion to unworn and worn silicone hydrogel lenses. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2012, 89, 1095–1106. [Google Scholar] [CrossRef]
- Singh, D.; Joshi, K.; Samuel, A.; Patra, J.; Mahindroo, N. Alcohol-based hand sanitisers as first line of defence against SARS-CoV-2: A review of biology, chemistry and formulations. Epidemiol. Infect. 2020, 148, e229. [Google Scholar] [CrossRef]
- Hulkower, R.L.; Casanova, L.M.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. Am. J. Infect. Control 2011, 39, 401–407. [Google Scholar] [CrossRef]
- Welch, J.L.; Xiang, J.; Mackin, S.R.; Perlman, S.; Thorne, P.; O’Shaughnessy, P.; Strzelecki, B.; Aubin, P.; Ortiz-Hernandez, M.; Stapleton, J.T. Inactivation of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) and diverse RNA and DNA viruses on three-dimensionally printed surgical mask materials. Infect. Control Hosp. Epidemiol. 2021, 42, 253–260. [Google Scholar] [CrossRef]
- Rauth, A.M. The physical state of viral nucleic acid and the sensitivity of viruses to ultraviolet light. Biophys. J. 1965, 5, 257–273. [Google Scholar] [CrossRef]
- Budowsky, E.; Bresler, S.; Friedman, E.; Zheleznova, N. Principles of selective inactivation of viral genome. Arch. Virol. 1981, 68, 239–247. [Google Scholar] [CrossRef]
- Anderson, D.J.; Gergen, M.F.; Smathers, E.; Sexton, D.J.; Chen, L.F.; Weber, D.J.; Rutala, W.A. Decontamination of targeted pathogens from patient rooms using an automated ultraviolet-C-emitting device. Infect. Control Hosp. Epidemiol. 2013, 34, 466–471. [Google Scholar] [CrossRef]
- Andersen, B.; Bånrud, H.; Bøe, E.; Bjordal, O.; Drangsholt, F. Comparison of UV C light and chemicals for disinfection of surfaces in hospital isolation units. Infect. Control Hosp. Epidemiol. 2006, 27, 729–734. [Google Scholar] [CrossRef]
- Darnell, M.E.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef]
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Raeiszadeh, M.; Adeli, B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: Applicability, validation, and safety considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef]
- Pirnie, M.; Linden, K.G.; Malley, J. Ultraviolet disinfection guidance manual for the final long term 2 enhanced surface water treatment rule. US Environ. Prot. Agency 2006, 2, 1–436. [Google Scholar]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Luppens, S.B.I.; Reij, M.W.; van der Heijden, R.W.L.; Rombouts, F.M.; Abee, T. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 2002, 68, 4194–4200. [Google Scholar] [CrossRef]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control. Hosp. Epidemiol. 2013, 34, 1288–1301. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Hota, S.; Hirji, Z.; Stockton, K.; Lemieux, C.; Dedier, H.; Wolfaardt, G.; Gardam, M.A. Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infect. Control Hosp. Epidemiol. 2009, 30, 25–33. [Google Scholar] [CrossRef]
- French, G.L. Clinical impact and relevance of antibiotic resistance. Adv. Drug Deliv. Rev. 2005, 57, 1514–1527. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Kadry, A.A.; Serry, F.M.; El-Ganiny, A.M.; El-Baz, A.M. Integron occurrence is linked to reduced biocide susceptibility in multidrug resistant Pseudomonas aeruginosa. Br. J. Biomed. Sci. 2017, 74, 78–84. [Google Scholar] [CrossRef]
- Chaoui, L.; Mhand, R.; Mellouki, F.; Rhallabi, N. Contamination of the surfaces of a health care environment by multidrug-resistant (MDR) bacteria. Int. J. Microbiol. 2019, 2019, 3236526. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Bilal, N.E.; Hamid, M.E. Increased multi-drug resistant Escherichia coli from hospitals in Khartoum state, Sudan. Afr. Health Sci. 2012, 12, 368–375. [Google Scholar] [CrossRef]
- Mutai, W.C.; Muigai, A.W.T.; Waiyaki, P.; Kariuki, S. Multi-drug resistant Salmonella enterica serovar Typhi isolates with reduced susceptibility to ciprofloxacin in Kenya. BMC Microbiol. 2018, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Katayama, Y.; Matsuo, M.; Sasaki, T.; Morimoto, Y.; Sekiguchi, A.; Baba, T. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2014, 20, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef]
- Awodele, O.; Emeka, P.; Agbamuche, H.; Akintonwa, A. The antimicrobial activities of some commonly used disinfectants on Bacillus subtilis, Pseudomonas aeruginosa and Candida albicans. Afr. J. Biotechnol. 2007, 6, 987–990. [Google Scholar]
- Akinbobola, A.; Sherry, L.; Mckay, W.G.; Ramage, G.; Williams, C. Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J. Hosp. Infect. 2017, 97, 162–168. [Google Scholar] [CrossRef]
- Wang, L.; Ye, C.; Guo, L.; Chen, C.; Kong, X.; Chen, Y.; Shu, L.; Wang, P.; Yu, X.; Fang, J. Assessment of the UV/Chlorine Process in the Disinfection of Pseudomonas aeruginosa: Efficiency and Mechanism. Environ. Sci. Technol. 2021, 55, 9221–9230. [Google Scholar] [CrossRef]
- Szeto, W.; Yam, W.C.; Huang, H.; Leung, D.Y.C. The efficacy of vacuum-ultraviolet light disinfection of some common environmental pathogens. BMC Infect. Dis. 2020, 20, 127. [Google Scholar] [CrossRef]
- Dosler, S.; Mataraci, E. In vitro pharmacokinetics of antimicrobial cationic peptides alone and in combination with antibiotics against methicillin resistant Staphylococcus aureus biofilms. Peptides 2013, 49, 53–58. [Google Scholar] [CrossRef]
- Rodriguez Herrero, E.; Boon, N.; Pauwels, M.; Bernaerts, K.; Slomka, V.; Quirynen, M.; Teughels, W. Necrotrophic growth of periodontopathogens is a novel virulence factor in oral biofilms. Sci. Rep. 2017, 7, 1107. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, X.; Xie, J.; Xu, Z.; Liu, G.; Zhang, G. Investigation of Formation of Bacterial Biofilm upon Dead Siblings. Langmuir 2019, 35, 7405–7413. [Google Scholar] [CrossRef]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and Implications for Infection Control. Front. Microbiol. 2018, 9, 726. [Google Scholar] [CrossRef]
- Latgé, J.-P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef]
- und Anlagenbau, V.D.M. Hygienic Filling Machines of VDMA Class IV for Liquid and Viscous Foods Minimum Requirements and Basic Conditions for Operation in Accordance with Specification; Fachverband Nahrungsmittelmaschinen und Verpackungsmaschinen: Frankfurt, Germany, 2005. [Google Scholar]
- Racchi, I.; Scaramuzza, N.; Hidalgo, A.; Cigarini, M.; Berni, E. Sterilization of food packaging by UV-C irradiation: Is Aspergillus brasiliensis ATCC 16404 the best target microorganism for industrial bio-validations? Int. J. Food Microbiol. 2021, 357, 109383. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Nikaeen, M.; Stensvold, C.R.; Mirhendi, H. Ultraviolet irradiation: An effective inactivation method of Aspergillus spp. in water for the control of waterborne nosocomial aspergillosis. Water Res. 2012, 46, 5935–5940. [Google Scholar] [CrossRef]
- Narita, K.; Asano, K.; Naito, K.; Ohashi, H.; Sasaki, M.; Morimoto, Y.; Igarashi, T.; Nakane, A. Ultraviolet C light with wavelength of 222 nm inactivates a wide spectrum of microbial pathogens. J. Hosp. Infect. 2020, 105, 459–467. [Google Scholar] [CrossRef]
- Pechter, E.; Rosenman, K.D. Occupational health risks associated with use of environmental surface disinfectants in health care. Am. J. Infect. Control 2016, 44, 1755–1756. [Google Scholar] [CrossRef]
- Begum, M.; Hocking, A.D.; Miskelly, D. Inactivation of food spoilage fungi by ultra violet (UVC) irradiation. Int. J. Food Microbiol. 2009, 129, 74–77. [Google Scholar] [CrossRef]
- Hosein, I.; Madeloso, R.; Nagaratnam, W.; Villamaria, F.; Stock, E.; Jinadatha, C. Evaluation of a pulsed xenon ultraviolet light device for isolation room disinfection in a United Kingdom hospital. Am. J. Infect. Control 2016, 44, e157–e161. [Google Scholar] [CrossRef]

| Specification | Details |
|---|---|
| Dimensions (m) | 120 × 120 × 2000 |
| Voltage | 240 V ± 10% 50 Hz |
| UVC light frequency (Nm) | 253.7 |
| UV lamp | 40 W × 4 lights = 160 W per set × 3 |
| UV output (uW/cm2) | 1620 at 1 m (12 × 135 uW/cm2) |
| Microorganisms | Position of Sample | No. of Light Racks | Time of Exposure (min)/Killing % | |||
|---|---|---|---|---|---|---|
| 2 | 2.5 | 5 | 10 | |||
| S. aureus ATCC 6538 | Vertical | 1 light | 99.9 | 99.9 | 99.99 | 99.999 |
| 3 lights | 99.9 | 99.9 | 99.999 | 99.999 | ||
| Horizontal | 1 light | 99.9 | 99.9 | 99.99 | 99.999 | |
| 3 lights | 99.9 | 99.9 | 99.999 | 99.999 | ||
| S. aureus SA31 * | Vertical | 1 light | 99.9 | 99.9 | 99.9 | 99.999 |
| 3 lights | 99.9 | 99.9 | 99.999 | 99.999 | ||
| Horizontal | 1 light | 99.9 | 99.9 | 99.99 | 99.999 | |
| 3 lights | 99.9 | 99.9 | 99.999 | 99.999 | ||
| P. aeruginosa 6294 | Vertical | 1 light | ND | 85 | 98 | 99.999 |
| 3 lights | ND | 95 | 99.999 | 99.999 | ||
| Horizontal | 1 light | ND | 99 | 99.99 | 99.999 | |
| 3 lights | ND | 99.9 | 99.999 | 99.999 | ||
| P. aeruginosa PA219 # | Horizontal | 1 light | ND | ND | 99.99 | 99.999 |
| 3 lights | ND | ND | 99.999 | 99.999 | ||
| Escherichia coli K12 (ATCC 10798) | Vertical | 1 light | ND | 99 | 99.99 | 99.999 |
| 3 lights | ND | 99.9 | 99.999 | 99.999 | ||
| Horizontal | 1 light | ND | 90 | 99.99 | 99.999 | |
| 3 lights | ND | 99 | 99.999 | 99.999 | ||
| Salmonella typhi ATCC 700730 | Vertical | 1 light | ND | 95 | 99.99 | 99.999 |
| 3 lights | ND | 99.9 | 99.999 | 99.999 | ||
| Horizontal | 1 light | ND | 95 | 99.99 | 99.999 | |
| 3 lights | ND | 99.9 | 99.999 | 99.999 | ||
| Microorganisms | Materials | No. of Light Racks | Time of Exposure (min)/Killing % | Viable Bacteria Recovered from the Untreated Biofilm | ||
|---|---|---|---|---|---|---|
| 2 | 5 | 10 | ||||
| Wet surface biofilm | Glass | 2 | 92 | 99.99 | 99.99 | 46.6 × 106 |
| Dry surface biofilm | ND | 99.99 | 99.99 | 43.1 × 106 | ||
| Microorganisms | Position of Sample | No. of Light Racks | Time of Exposure (min)/Killing % | ||||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 20 | 30 | |||
| Candida auris CBS 12373 | Horizontal | 1 light | ND | 99.9 | 99.99 | ND | ND |
| 3 lights | ND | 99.999 | 99.999 | ND | ND | ||
| Aspergillus niger (spores) ATCC 16404 | 1 light | ND | ≤1 | 88 | 99.9 | 99.99 | |
| 3 lights | ND | ≤1 | 90 | 99.9 | 99.99 | ||
| Coronavirus (SARS-CoV-2 surrogate; MHV-I; ATCC VR-261) | 1 light | 99.9 | 99.999 | ND | ND | ND | |
| 3 lights | 99.9 | 99.999 | ND | ND | ND | ||
| Microorganisms | Testing Surface | Position | No. of Lights | Time of Exposure (min)/Killing % | |
|---|---|---|---|---|---|
| 5 | 10 | ||||
| S. aureus ATCC 6538 | Vinyl | Horizontal | 1 | 89 | 99.999 |
| 3 | 99.999 | 99.999 | |||
| Ceramic | 1 | 99.99 | 99.999 | ||
| 3 | 99.999 | 99.999 | |||
| Formica | 1 | 99.99 | 99.999 | ||
| 3 | 99.999 | 99.999 | |||
| Steel | 1 | 83 | 99.999 | ||
| 3 | 96 | 99.999 | |||
| Plastic | 1 | 99.99 | 99.999 | ||
| 3 | 99.999 | 99.999 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; McDonald, M.; Mundada, K.; Willcox, M. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene 2022, 2, 120-131. https://doi.org/10.3390/hygiene2030010
Khan M, McDonald M, Mundada K, Willcox M. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene. 2022; 2(3):120-131. https://doi.org/10.3390/hygiene2030010
Chicago/Turabian StyleKhan, Mahjabeen, Murray McDonald, Kaustubh Mundada, and Mark Willcox. 2022. "Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm" Hygiene 2, no. 3: 120-131. https://doi.org/10.3390/hygiene2030010








