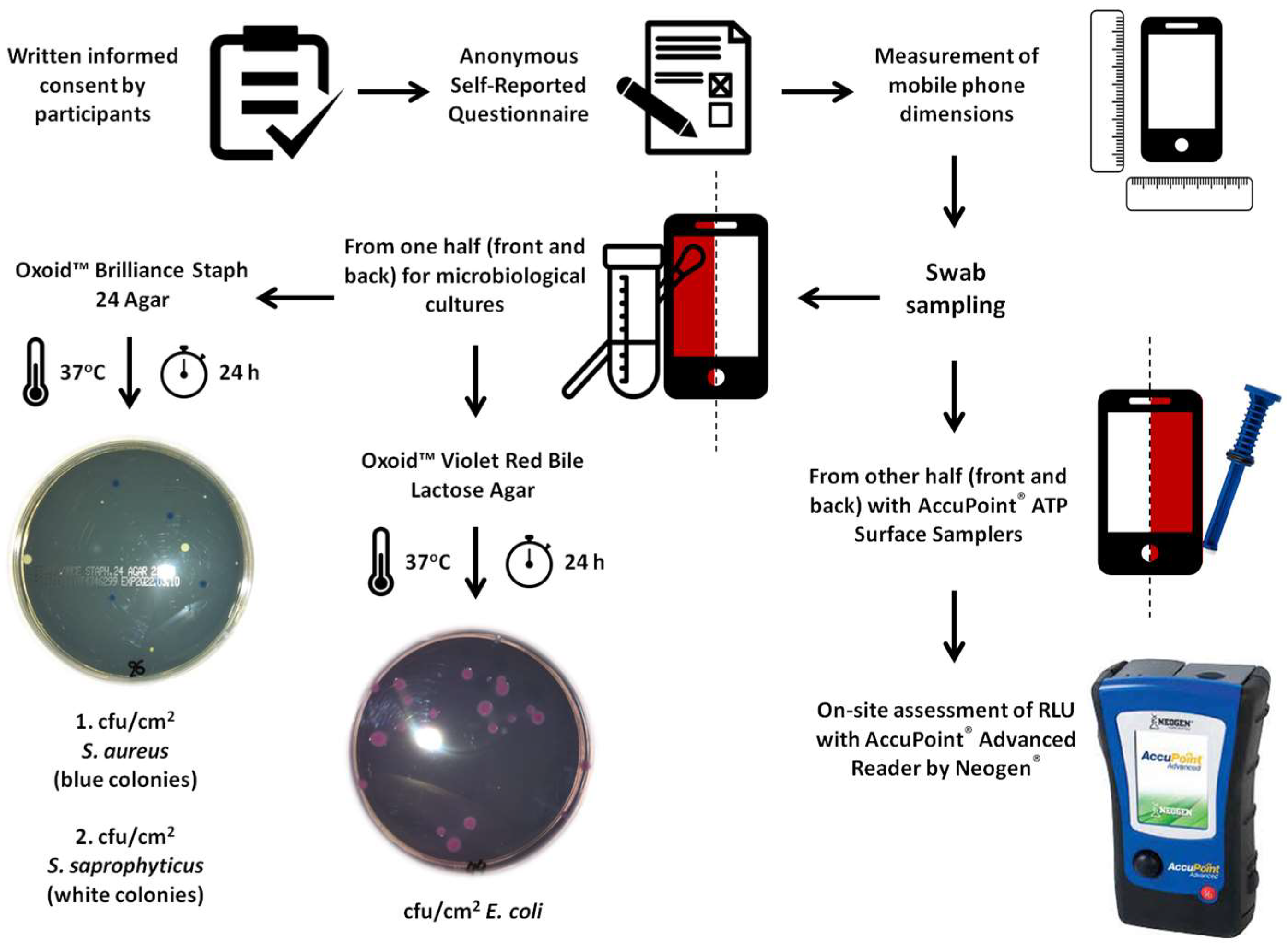
1. Introduction
In 1938, Price found that the microorganisms detected on the hands can be divided into two distinct categories, namely resident and transient microbial flora. The resident flora is mainly found under the superficial cells of the stratum corneum, as well as on the surface of the hands. On the contrary, transient flora colonizes the superficial layers of the skin and it is easier to remove with normal hand hygiene [
1]. Bacteria are the most dominant group of the human skin microbiome, including species of coagulase-negative staphylococci (CoNS), with
Staphylococcus epidermidis being the dominant resident species, anaerobic
Propionibacterium acnes,
Streptococcus,
Micrococcus,
Corynebacterium, and
Acinetobacter. Apart from the above bacterial species, the human skin is also colonized by various fungal species, such as
Candida,
Malassezia,
Cryptococcus, etc. [
2].
Particularly, the hand microbiome appears to be more dynamic over time, demonstrating a greater bacterial diversity (>3 times more bacterial phylotypes per individual), than other skin sites. Hence, bacterial communities on hands are generally enriched with different species over time, compared to other human body areas. Additionally, the palms of women demonstrate greater bacterial diversity than those of men, with
Propionibacteria and
Corynebacterium being more abundant on male hands, while
Enterobacteriales,
Moraxellaceae,
Lactobacillaceae, and
Pseudomonaceae are more abundant on female hands. However, the diversity of fungal species on human hands is considered to be intermediate, compared to other skin sites of the human body [
3]. Moreover, the distribution and density of the hands’ microbiota depends on age and other environmental factors, such as the sebum secretion, pore blockage, as well as skin temperature and moisture. The existence of a healthy skin microbiota is based on the creation of a protective ecosystem against pathogens [
4].
Human hands are the main means of interpersonal, intrapersonal, and environmental transfer of microorganisms through touch [
3]. The transmissibility of transient flora depends on the type and number of microorganisms, as well as on the moisture of the skin [
1]. Therefore, the hands of some individuals may be more prone to being colonized with microorganisms, such as
Staphylococcus aureus and Gram negative bacteria such as
Escherichia coli, or yeasts [
1].
For instance, the hands of healthcare workers can be colonized with Gram positive microorganisms, such as
Staphylococcus aureus. These microorganisms are the main cause of hospital-acquired infections (HAIs), including bacteremia, pneumonia, and other skin and soft tissue infections [
5].
Staphylococcus aureus is considered to be a potentially pathogenic species, but it may be part of the normal skin and nasal mucosa flora in approximately 10–20% of healthy people [
2]. Although the normal skin flora is less likely to be associated with infections, there is the potential for it to be responsible for infections in sterile body cavities, such as the eyes, or on intact skin [
6]. Interestingly, the role that healthcare workers’ hands play in the spread of HAIs has been thoroughly investigated, but there are a limited number of studies investigating the hand hygiene of undergraduate students.
On the other hand, inanimate surfaces or so called “fomites” can act as reservoirs of pathogens, contributing to the spread of various infectious diseases [
7]. Mobile phones are a typical example of such fomite surfaces, since they are considered an essential “high-touch” handheld item in today’s society [
8]. They are part of the so-called “emotional technology” due to the personalized services provided through them, and are considered an integral accessory for both professional and private life [
6]. As this advanced technology becomes widespread, many people do not consciously realize how often they touch their phones or where they place them [
8].
Mobile phones can therefore act as a reservoir of microorganisms, especially when no cleaning or disinfection procedures are performed, increasing the risk of cross-contamination [
9] between the user’s skin and other surfaces or food [
10]. The generated heat during their operation, or when placed inside a pocket, creates the ideal conditions for the incubation of pathogenic microorganisms [
8]. Indicatively, several bacterial species have been identified on mobile phone surfaces, in healthcare, as well as in community settings. The most commonly isolated bacteria from mobile phone surfaces, which pose a potential health risk, are
Staphylococcus aureus, CoNS (with
Staphylococcus epidermidis and
Staphylococcus saprophyticus being the most dominant species), and
Escherichia coli [
7].
It is also worth mentioning that even the complacency that the use of protective cases, films, and screen protectors on mobile phones reduces the microbial load of mobile phones, seems to have been disproven. The application of such accessories does not affect the levels of microbial colonization on mobile phones, since their use remains unchanged. Only the use of alcohol-based disinfectants can substantially reduce the number of colonies on mobile phone surfaces, by 75% [
11].
As for the methods used, in order to assess the cleanliness of a surface, certain procedures such as visual inspections, microbial cultivations, as well as the application of bioluminescence tests are used [
12]. These tests are based on measuring the light produced by a bioluminescence enzyme reaction, between luciferin–luciferase and adenosine triphosphate (ATP). ATP is an energy molecule found in all living cells, the presence of which indicates improper cleaning and the contamination of a surface. The amount of light produced from this reaction is proportional to the amount of a sample’s ATP concentration. The light produced is measured by a luminometer, which displays the results in relative light units (RLUs) within a few seconds. Bioluminescence tests are widely used, due to their speed, ease of use, and cost-effectiveness [
13]. However, the measurement of ATP does not indicate the presence (or absence) of a particular microbial species, thus making it a quantitative indicator [
8]. As a result, it should not be considered a substitute for classic routine microbiological culture methods [
12].
Moreover, mobile phones are widely used by undergraduate students worldwide for social, as well as for academic purposes. Specifically, students who belong to faculties of health sciences tend to use their mobile phones during their clinical practice in hospitals, or in clinical laboratories. Similarly, students who attend curricula of other sciences also use their mobile phones during their classes or their internship in offices, where many people are usually present [
14]. This constant handling of mobile phones by undergraduate students, in all places and occasions, exposes their device to an array of microorganisms [
7]. Although the bacterial colonization of mobile phones in hospital settings has been thoroughly studied, there are only a few studies that investigated the contamination of mobile phones in the academic community [
15]. Most of these studies have been conducted in underdeveloped and developing Asian and African countries. In the literature, there are a limited number of recent studies on mobile phone hygiene among undergraduate students, concerning the European countries, as summarized in
Table 1.
Considering all the above issues, further investigation is imperative among the social group of undergraduate students. Therefore, the present study aims to fill the gap of the understudied field of hand hygiene and mobile phone hygiene among undergraduate students. Unlike the previous studies, the novelty of this research lies in the combined investigation of the knowledge, attitudes, and behaviors of Greek undergraduate students, concerning their hand hygiene, as well as their mobile phone hygiene habits, in relation to the microbial load on their mobile phone devices, which was also assessed. It is worth mentioning that no microorganisms were directly isolated from students’ hands, since that was not within the aims of this study. Instead, this study detected the most prevalent bacterial species on mobile phone surfaces, which are considered transient microbial flora on students’ hands. This indirect approach was selected, in order to demonstrate the degree of interaction between hands and mobile phones, with an emphasis on removing the hands’ transient flora through proper hand hygiene, as well as from the surfaces of students’ mobile phones through adequate cleaning/disinfection procedures. The results of this study are considered of great importance, since they provide a clear viewpoint of the students’ hygienic status and mindset, based on their sex, faculty, and year of study, where differences are expected, through the aspect of a European country like Greece. These findings are vital, especially in terms of public health policy making from the perspective of the current post-COVID circumstances.
4. Discussion
This study assessed the knowledge, attitudes, and behaviors of Greek undergraduate students on hand hygiene and mobile phone hygiene in a university setting, using an anonymous self-reported questionnaire. There was an equal share of male and female participants, as well as of students of health and other sciences, from all years of study. Most of the participants reached the university campus on all working days of each week via public transport. Τhis situation is a cause for concern, since there is an increased health risk faced by students, due to overcrowding and frequent contact with various surfaces, as indicated by a previous study [
37].
Taking into account the participants’ hand hygiene assessment, most students preferred hand washing, soap use, paper towels, and liquid soap, rather than gloves, alcohol disinfectants, hand dryer, and soap bar (refer to
Table 2). These findings are in agreement with the results of a previous study by Guzek et al., where the same questions were asked concerning the participants’ knowledge and beliefs on proper hand hygiene and personal protection [
23]. It seems by the declared answers that the participants of this study are well oriented, as far as the methods and means of hand hygiene. Speaking of hand hygiene, alcohol-based hand disinfectants, especially at concentrations between 60% and 80%, can significantly reduce the bacterial colonies on the skin, but they are incapable of eliminating most spores [
38], while soap washes away bacteria by dissolving the oily layer on the skin’s surface [
39]. There are still ongoing debates, however, regarding the effectiveness of alcohol-based hand disinfectants and soap, which are used to ward off pathogens [
38].
In addition, having studied the personal protective behaviors (refer to
Table 3), hand washing had the highest share among respondents, as also proved in the study by Guzek et al. [
23], followed by the use of face masks and alcohol disinfectants. Less popular personal protective behaviors were the avoidance of public places, medications or dietary supplements, and the use of disposable gloves. As for the participants’ hand washing frequency (refer to
Figure 2), although most students washed their hands frequently, there was still a considerable number of students who washed their hands inadequately. Previous studies demonstrated similar results, where the daily hand washing frequency of most students also ranged between 6–10 times [
40,
41]. Therefore, it is not enough just to prefer hand washing as a hand hygiene method, but it should also be performed with sufficient frequency as well.
However, the duration of hand washing by the majority of undergraduate students is considered adequate (refer to
Table 2). Hands should be washed for at least 15 s, while making sure that the entire hands’ surface is properly cleaned [
42]. Additionally, while most participants always washed their hands no matter what, certain restrictions such as the absence of soap, or the proximity and accessibility of bathrooms, as well as the students’ forgetfulness, were the main declared reasons why they neglected washing their hands (refer to
Table 4). Other reasons (apart from the available choices) for neglecting hand washing, included participants’ boredom. Similar trends were observed in previous studies [
40], indicating forgetfulness [
41,
43,
44], as well as the above restrictions [
45], as the main reasons for neglecting hand washing. In order to solve this issue, students should have easy access to hand hygiene means and facilities, as well as find efficient ways to remind them and keep them motivated to wash their hands.
There is a strong association of colonization between the hands and surfaces. As demonstrated by the findings of a study that analyzed a total of 69 pairs of samples obtained from hands and surfaces, in all cases, the same bacterial species was recovered, both from the hand and the respective environmental surface from which the sample was taken. Among the recovered bacteria, 81% were Gram negative rods, while 19% were Gram positive cocci [
46]. This interconnectedness between the hands and surfaces is a cause for concern, especially when it comes to the spread of pathogens.
Concerning the students’ mobile phone hygiene assessment (refer to
Table 5), it is worth mentioning that all the participants in this study owned a touchscreen mobile phone device. Thus, the results of this study are not applicable to owners of keypad mobile phones. Moreover, protective back covers were applied to most participants’ mobile phones, along with the use of other accessories, such as headphones, handsfree, Bluetooth, etc. While most students used their mobile phones during classes, almost no one touched their devices while wearing disposable gloves. Finally, the majority of mobile phones were last cleaned by their owners approximately one week to one month prior to the time of participation. The above findings coincide with those of a previous study by Cicciarella Modica et al., where the same questions were assessed [
19]. However, other responses (apart from the available choices) indicated that a high share of mobile phones were never cleaned or last cleaned at their time of purchase. Similar response rates were also observed in the study by Ahmad et al., where 22.4% of participants cleaned their phones daily, 27% weekly, and 19.7% once a month [
47]. Hence, it is safe to say that students need to clean/disinfect their mobile phones more frequently, particularly after attending classes or visiting crowded places.
The microbial load on participants’ mobile phone devices was also assessed in this study, using conventional microbiological culture methods, as well as an on-site bioluminescence ATP measurement technique. In general, higher rates of
Staphylococcus spp. were expected in the mobile phone samples, since it is part of the normal flora of the human skin [
6]. Thus, the mobile phone devices of those who did not avoid touching their face as a personal protective behavior, as well as those who never washed their hands after blowing their nose, demonstrated a higher microbial load of total cfu/cm
2 in their Brilliance Staph 24 Agar culture results (refer to
Table 6). Similar culture results were observed among the mobile phone samples of participants who reached the university campus on foot or with a private vehicle, as well as surprisingly, among those who avoided touching sick people. It is worth mentioning that this study’s bacterial growth rate results match the results of the study by Cicciarella Modica et al., where 85% of the samples from 108 mobile phones of students of health sciences were also positive for staphylococci [
19].
In particular, the aforementioned variables concerning the participants’ means of transport, abstaining from touching their face, evading sick people, as well as hand washing after blowing their nose, demonstrated the same statistical significance tendency as above, in correlation with the cfu/cm
2 of
Staphylococcus saprophyticus (refer to
Table 7). Furthermore, a higher bacterial growth rate of
Staphylococcus aureus was observed among the mobile phone samples of students who reached the university campus 4 or 5 times a week, as well as among those who were unsure about their soap type preference, and surprisingly, among those who avoided touching sick people (refer to
Table 8). Accordingly, in a study sample of 27 mobile phones of secondary school students, the presence of potentially pathogenic microorganisms, including strains of
Staphylococcus aureus, were also found [
15]. It is therefore understood that certain everyday habits and behaviors, induce the colonization of students’ mobile phones with opportunistic pathogenic bacteria.
In this study,
Staphylococcus spp. (namely those of
Staphylococcus saprophyticus and
Staphylococcus aureus) were the most prevalent bacteria, followed by
Escherichia coli, as also observed by over a third of the previous studies conducted both in healthcare and community settings [
48,
49,
50,
51]. For instance, in a study conducted in a Turkish hospital, higher levels of
Staphylococcus aureus and
Escherichia coli were observed on the hands of 60 intensive care unit nurses after ending their shift, compared to the beginning of their shift [
52]. Another study also showed that the hands of nursing staff with skin disease are twice as likely to be colonized with Gram positive bacteria, such as
Staphylococcus aureus, and Gram negative bacteria that are resistant to third-generation cephalosporins, such as
Escherichia coli [
53]. However, in a study among 66 students of health professions aged 19–22 years old, 9.1% of the participants’ hands tested positive for
Escherichia coli colonization before their clinical practice, while after their clinical practice, this percentage decreased to 6.1% of the participants [
5].
The mobile phone devices of those who did not select hand washing as a personal protective behavior demonstrated a higher microbial load of
Escherichia coli in cfu/cm
2. Similar results were observed among those who preferred the use of liquid soap, instead of soap bar, and those who did not know which of these was more effective (refer to
Table 9). Since
Escherichia coli is part of the normal intestinal flora, its presence on hands and consequently on mobile phones indicates fecal contamination due to poor personal and hand hygiene [
54], especially after visiting the restroom. Accordingly, although
Staphylococcus saprophyticus is part of the normal human flora of the perineum, rectum, urethra, cervix, and gastrointestinal tract, it is associated with lower urinary tract infections (UTIs) in young and middle-aged women [
19]. Thus, its presence on human hands can only be considered transient flora, indicating poor personal and hand hygiene as well.
The bacterial survival on surfaces ranges from hours to months, depending on a variety of factors, such as the environmental conditions, the bacterial strain properties, as well as the characteristics of the surface itself. In the study by Simmonds–Cavanagh, the viability of a variety of pathogenic bacteria on mobile phones in healthcare was assessed.
Bacillus cereus, followed by CoNS,
Acinetobacter baumannii,
Enterococcus faecalis, and
Staphylococcus aureus were found to be the most successful pathogenic bacteria to remain viable on mobile phones at the 28-day cut-off. On the other hand,
Escherichia coli and
Pseudomonas aeruginosa remained viable on mobile phones and were last detected at 6 h, while
Pseudomonas strutzeri was last detected at 7 days. In general, the Gram positive bacteria remained viable longer than the Gram negative bacteria, perhaps due to the thinner protective peptidoglycan layers of Gram negative bacteria. Additionally, all bacteria remained viable on mobile phones for at least 6 h, which is long enough to be transmitted to a clinical setting and out to the community. It is therefore obvious that mobile phone cleaning/disinfection should occur in combination with proper hand hygiene [
55].
In contrast, in terms of the ATP measurement results (refer to
Table 10), participants who equally preferred the use of liquid soap and soap bar, followed by those who did not know which of these was more effective, demonstrated higher RLU values compared to the rest of the participants. This finding is an indication that liquid soap could potentially be more efficient in removing organic matter from the hands compared to soap bars, but further research is required for safer conclusions. Similarly, hand washing reluctance (“I do not feel like doing it”) as a declared reason for neglecting hand washing also led to higher RLU values on students’ mobile phones as expected. Interestingly, a declared hand washing frequency between 6–30 times led to lower RLU values compared to the extremes. Hence, it is safe to say that a moderate daily hand washing frequency is ideally required in order to maintain a low microbial load level on our hands while keeping a healthy and balanced microbiome. However, the fact that most mobile phone samples exceeded the “fail” threshold of 300 RLU remains a cause for concern. Indicatively, in a previous study where the microbial load on mobile devices of healthcare students and nursing staff was assessed, the defined cut-off value was exceeded 10 times by the RLU values of nurses’ mobile phone samples, and up to 20 times by the RLU values of the students’ mobile phone samples [
9].
It is worth mentioning that no correlation between the cfu/cm
2 and RLU values was observed in this study. This probably happened because the participants’ mobile phones were colonized with plenty of other bacterial and fungal (e.g.,
Candida) species, as well as with ATP-containing organic matter, apart from those isolated by the selective culture media used in this study, which affected the RLU values. Correlations between cfu/cm
2 and RLU have also been previously investigated, leading to mixed results as well. While some reports indicated that high cfu/cm
2 values are usually correlated with high RLU values [
8], other studies found no correlation between cfu/cm
2 and RLU values [
13]. Likewise in the present study (Refer to
Table 11), while high RLU values were correlated with high cfu/cm
2 of
Staphylococcus aureus, this did not apply in the cases of
Staphylococcus saprophyticus and
Escherichia coli.
Participants’ field of study had greatly influenced the microbial load on their mobile phones. Undergraduate students of health sciences demonstrated lower RLU values on their mobile phone samples compared to those of students of other sciences (refer to
Table 12). In contrast, a higher microbial load of
Escherichia coli was observed on the mobile phone surfaces of students of health sciences, obviously due to their job’s nature, compared to those of students of other sciences. Previous studies also indicated a statistically significant association between the participants’ faculty and their mobile phones’ microbial load [
43], where the isolation of
Escherichia coli was higher from the touchscreen mobile phones of healthcare workers (
p < 0.05) [
56], due to their job’s nature as well. In this study, faculty was a determinant for other declared personal protective behaviors as well, where most students of health sciences considered staying at home and the use of face masks as equally effective, while most students of other sciences did not know which of these was more effective. However, most undergraduate students of other sciences considered liquid soap and soap bars as equally effective, while most students of health sciences preferred the use of liquid soap.
Soap preference was also determined by the participants’ year of study (refer to
Table 13). The majority of first-year, second-year, and third-year undergraduate students preferred the use of liquid soap, while an equal share of second-year and final-year students considered the liquid soap and soap bar as equally effective. Furthermore, there was an equal share of protective back covers applied to the majority of the mobile phones of students from all years of study. In addition, a higher microbial load of
Escherichia coli was observed on the mobile phone surfaces of first-year and third-year students compared to those of the second-year and final-year students. This finding indicates that healthcare students are probably becoming more aware of the health hazards and prevention methods at work as they proceed through their curricula. Additionally, it is no coincidence that participants who preferred the use of liquid soap, either based on their faculty or their year of study, demonstrated a higher microbial load of
Escherichia coli cfu/cm
2. However, the mobile phones of those who preferred the use of soap bars demonstrated higher cfu/cm
2 of
Staphylococcus aureus. These contradictory results concerning the efficacy of each soap type against certain bacteria require further investigation by future studies.
Moreover, similar trends were observed between the majority of male and female participants concerning their preference for paper towels, their hand washing duration, as well as the abstaining from touching their face, as a declared personal protective behavior (refer to
Table 14). However, male participants provided higher response rates of forgetfulness compared to female participants as a reason for neglecting hand washing, probably due to temperament factors and characteristics of the participants’ personality. In contrast, the mobile phone samples of female participants, which tested positive for
Staphylococcus aureus, were three times more than those of male participants. Finally, it is worth noting that apart from an indication of higher
Staphylococcus aureus contamination of females’ mobile phones, which requires further investigation by future studies, no further statistically significant differences were observed between the microbial load of the participants’ mobile phones and their sex. Previous studies also indicated no differences in the cfu/cm
2 and RLU values on mobile phones of male and female undergraduate students [
8,
36].
Generally, the identification of differences in knowledge, attitudes, and behaviors of young adults aged between 18–29 years old on hand hygiene and mobile phone hygiene could be useful in public health policy making and health education planning, since they are considered a heterogeneous social group [
43]. To the best of the authors’ knowledge, this is the first study that investigates the knowledge, attitudes, and behaviors of Greek undergraduate students on hand hygiene and mobile phone hygiene, combined with an assessment of the microbial load on their mobile phone devices, based on their sex, faculty, and year of study. According to this study’s results, emphasis should be placed on the education of undergraduate students, especially those in their junior years of non-healthcare and healthcare curricula, on hand hygiene and mobile phone hygiene topics. As proposed by other studies [
57,
58], the inclusion of such courses in their main curriculum enhances the knowledge, attitudes, and behaviors of future healthcare and non-healthcare professionals.
Finally, results should be interpreted with some caution, since the limitations of the present study include the following: the self-reporting of information regarding the participants’ hand hygiene and mobile phone hygiene habits [
59]; the cross-sectional study design, which does not allow for causality conclusions [
60,
61]; the convenience sampling method used, which affects the generalizability of these results [
62]; the rather small study sample given the number of variables that were analyzed; as well as the exclusive study of specific bacterial species and not any other additional bacterial or fungal species (e.g.,
Candida). Nevertheless, this study provides a clear view and additional information on the students’ knowledge, attitudes, and behaviors on hand hygiene and mobile phone hygiene associated with the microbial contamination of their mobile phone devices. Longitudinal data are necessary to further unravel the hand hygiene and mobile phone hygiene habits of undergraduate students, as well as their mobile phones’ degree of contamination. Future research should focus on investigating the transmissibility patterns of pathogenic bacteria due to mobile phone use among the social group of undergraduate students, as well as their viability on students’ mobile phone surfaces.