Microbial Load of Hand Sanitizer Dispensers—A University Hospital Study
Abstract
1. Introduction
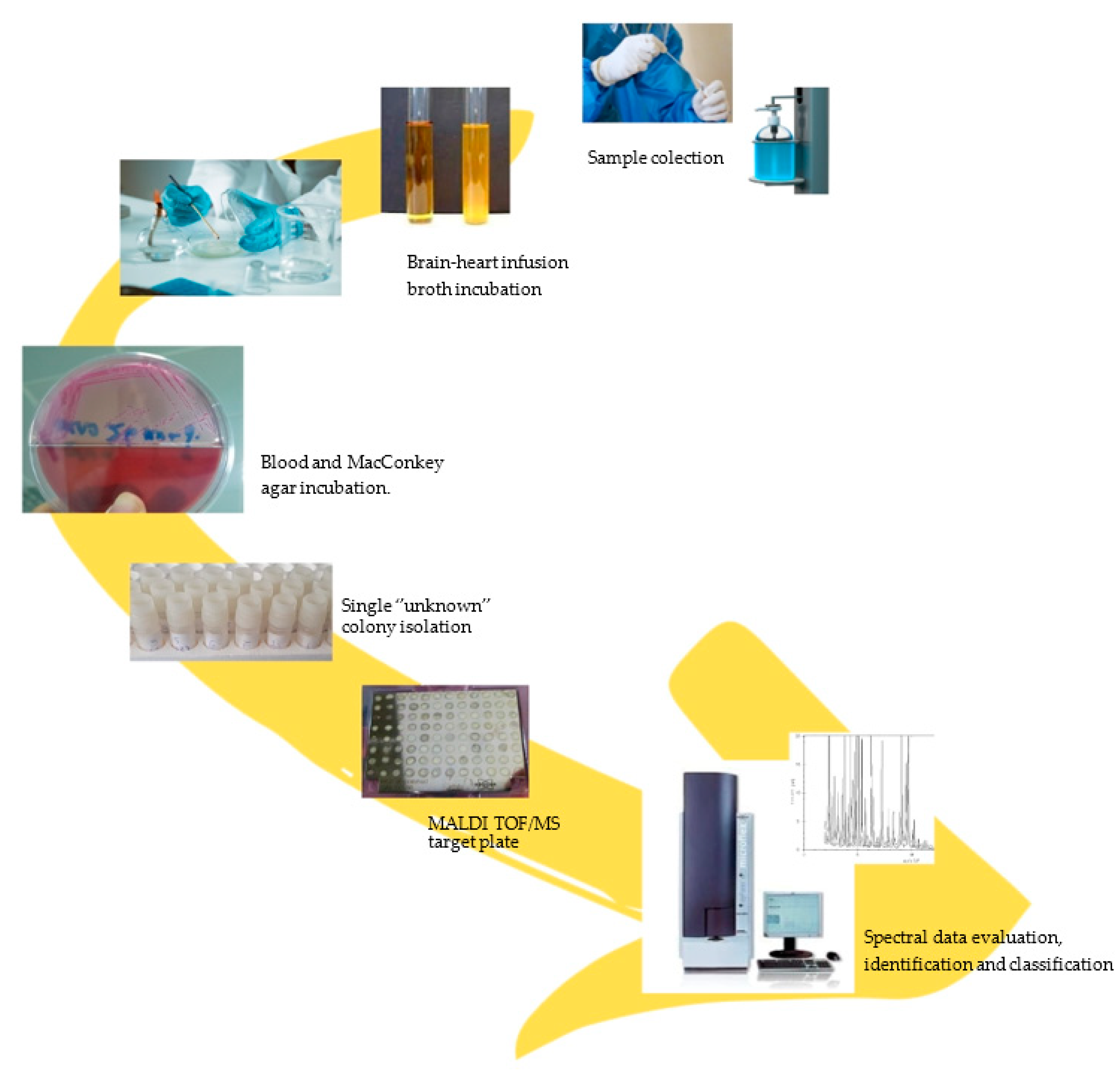
2. Materials and Methods
Sampling Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittet, D.; Allegranzi, B.; Sax, H.; Bertinato, L.; Concia, E.; Cookson, B.; Fabry, J.; Richet, H.; Philip, P.; Spencer, R.C.; et al. Considerations for a WHO European Strategy on Health-Care-Associated Infection, Surveillance, and Control. Lancet Infect. Dis. 2005, 5, 242–250. [Google Scholar] [CrossRef]
- Beyersmann, J.; Kneib, T.; Schumacher, M.; Gastmeier, P. Nosocomial Infection, Length of Stay, and Time-Dependent Bias. Infect. Control Hosp. Epidemiol. 2009, 30, 273–276. [Google Scholar] [CrossRef]
- Mielke, M. Prevention and Control of Nosocomial Infections and Resistance to Antibiotics in Europe–Primum Non-Nocere: Elements of Successful Prevention and Control of Healthcare-Associated Infections. Int. J. Med. Microbiol. 2010, 300, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Pittet, D.; Healthcare Infection Control Practices Advisory Committee. HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Prof. MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2002, 51, 1–45. [Google Scholar]
- World Health Organization (WHO). WHO Guidelines on Hand Hygiene in Health Care. Available online: http://whqlibdoc.who.int/publications/2009/9789241597906 (accessed on 21 July 2023).
- Picheansathian, W. A Systematic Review on the Effectiveness of Alcohol-Based Solutions for Hand Hygiene. Int. J. Nurs. Pract. 2004, 10, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Sax, H.; Pittet, D. Hand Hygiene and Healthcare System Change within Multi-Modal Promotion: A Narrative Review. J. Hosp. Infect. 2013, 83, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.W.S.; Ma, Y.; Liu, S.Y.; Pun, W.H.; Chua, S.L. Prevalence of Alcohol-Tolerant and Antibiotic-Resistant Bacterial Pathogens on Public Hand Sanitizer Dispensers. J. Hosp. Infect. 2022, 127, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M. The Pandemic of COVID-19 and Its Implications for the Purity and Authenticity of Alcohol-Based Hand Sanitizers: The Health Risks Associated with Falsified Sanitizers and Recommendations for Regulatory and Public Health Bodies. Res. Soc. Adm. Pharm. 2021, 17, 2050–2051. [Google Scholar] [CrossRef]
- Hansen, S.; Schwab, F.; Gastmeier, P.; Pittet, D.; Zingg, W.; Sax, H.; Gastmeier, P.; Hansen, S.; Grundmann, H.; van Benthem, B.; et al. Provision and Consumption of Alcohol-Based Hand Rubs in European Hospitals. Clin. Microbiol. Infect. 2015, 21, 1047–1051. [Google Scholar] [CrossRef]
- Toney-Butler, T.J.; Gasner, A.C.N. Hand Hygiene. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470254/ (accessed on 21 July 2023).
- Sax, H.; Allegranzi, B.; Uçkay, I.; Larson, E.; Boyce, J.; Pittet, D. ‘My Five Moments for Hand Hygiene’: A User-Centred Design Approach to Understand, Train, Monitor and Report Hand Hygiene. J. Hosp. Infect. 2007, 67, 9–21. [Google Scholar] [CrossRef]
- Suzuki, Y.; Morino, M.; Morita, I.; Yamamoto, S. The Effect of a 5-Year Hand Hygiene Initiative Based on the WHO Multimodal Hand Hygiene Improvement Strategy: An Interrupted Time-Series Study. Antimicrob. Resist. Infect. Control 2020, 9, 75. [Google Scholar] [CrossRef]
- Kendall, A.; Landers, T.; Kirk, J.; Young, E. Point-of-Care Hand Hygiene: Preventing Infection behind the Curtain. Am. J. Infect. Control 2012, 40, S3–S10. [Google Scholar] [CrossRef]
- Cure, L.; Van Enk, R.; Tiong, E. A Systematic Approach for the Location of Hand Sanitizer Dispensers in Hospitals. Health Care Manag. Sci. 2013, 17, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, S.; Lemmen, S.W. How Can Compliance with Hand Hygiene Be Improved in Specialized Areas of a University Hospital? J. Hosp. Infect. 2013, 83, S17–S22. [Google Scholar] [CrossRef]
- Cosgrove, S.E. The Relationship between Antimicrobial Resistance and Patient Outcomes: Mortality, Length of Hospital Stay, and Health Care Costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Kuster, S.; Roth, J.A.; Frei, R.; Meier, C.A.; Dangel, M.; Widmer, A.F. Handrub Dispensers per Acute Care Hospital Bed: A Study to Develop a New Minimum Standard. Antimicrob. Resist. Infect. Control 2021, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Weinhold, D.; Tong, E.; Birrell, F.; Doidge, S.; Ramritu, P.; Halton, K.; Lairson, D.; Whitby, M. Effect of Healthcare-Acquired Infection on Length of Hospital Stay and Cost. Infect. Control Hosp. Epidemiol. 2007, 28, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Lemmen, S.W.; Lewalter, K. Antibiotic Stewardship and Horizontal Infection Control Are More Effective than Screening, Isolation and Eradication. Infection 2018, 46, 581–590. [Google Scholar] [CrossRef]
- Stilo, A.; Troiano, G.; Melcarne, L.; Gioffrè, M.E.; Nante, N.; Messina, G.; Laganà, P. Hand Washing in Operating Room: A Procedural Comparison. Epidemiol. Biostat. Public Health 2022, 13, 11734. [Google Scholar] [CrossRef]
- López-Gigosos, R.M.; Mariscal-López, E.; Gutierrez-Bedmar, M.; García-Rodriguez, A.; Mariscal, A. Evaluation of Antimicrobial Persistent Activity of Alcohol-Based Hand Antiseptics against Bacterial Contamination. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1197–1203. [Google Scholar] [CrossRef]
- Godoy, P.; Castilla, J.; Delgado-Rodríguez, M.; Martín, V.; Soldevila, N.; Alonso, J.; Astray, J.; Baricot, M.; Cantón, R.; Castro, A.; et al. Effectiveness of Hand Hygiene and Provision of Information in Preventing Influenza Cases Requiring Hospitalization. Prev. Med. 2012, 54, 434–439. [Google Scholar] [CrossRef]
- Park, G.W.; Barclay, L.; Macinga, D.; Charbonneau, D.; Pettigrew, C.A.; Vinje, J. Comparative Efficacy of Seven Hand Sanitizers against Murine Norovirus, Feline Calicivirus, and GII.4 Norovirus. J. Food Prot. 2010, 73, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Bolon, M.K. Hand Hygiene. Infect. Dis. Clin. N. Am. 2016, 30, 591–607. [Google Scholar] [CrossRef]
- 2023 Breakpoint Implementation Toolkit. Available online: https://clsi.org/meetings/ast/breakpoints-in-use-toolkit/ (accessed on 6 October 2023).
- Dalton, K.R.; Rock, C.; Carroll, K.C.; Davis, M.F. One Health in hospitals: How understanding the dynamics of people, animals, and the hospital built-environment can be used to better inform interventions for antimicrobial-resistant gram-positive infections. Antimicrob. Resist. Infect. Control 2020, 9, 78. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef]
- Nelli, A.; Voidarou, C.; Venardou, B.; Fotou, K.; Tsinas, A.; Bonos, E.; Fthenakis, G.C.; Skoufos, I.; Tzora, A. Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats. Biology 2022, 11, 1591. [Google Scholar] [CrossRef]
- Tzora, A.; Skoufos, S.; Bonos, E.; Fotou, K.; Karamoutsios, A.; Nelli, A.; Giannenas, I.; Tsinas, A.; Skoufos, I. Identification by MALDI-TOF MS and Antibiotic Resistance of Riemerella Anatipestifer, Isolated from a Clinical Case in Commercial Broiler Chickens. Vet. Sci. 2021, 8, 29. [Google Scholar] [CrossRef]
- Severn, M.M.; Williams, M.R.; Shahbandi, A.; Bunch, Z.L.; Lyon, L.M.; Nguyen, A.; Zaramela, L.S.; Todd, D.A.; Zengler, K.; Cech, N.B.; et al. The Ubiquitous Human Skin Commensal Staphylococcus hominis Protects against Opportunistic Pathogens. MBio 2022, 13, e00930-22. [Google Scholar] [CrossRef]
- Lunjani, N.; Hlela, C.; O’Mahony, L. Microbiome and Skin Biology. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 328–333. [Google Scholar] [CrossRef]
- Bieber, L.; Kahlmeter, G. Staphylococcus lugdunensis in Several Niches of the Normal Skin Flora. Clin. Microbiol. Infect. 2010, 16, 385–388. [Google Scholar] [CrossRef]
- Kocur, M.; Kloos, W.E.; Schleifer, K.-H. The Genus Micrococcus. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 961–971. [Google Scholar]
- Seng, P.; Barbe, M.; Pinelli, P.O.O.; Gouriet, F.; Drancourt, M.; Minebois, A.; Cellier, N.; Lechiche, C.; Asencio, G.; Lavigne, J.P.P.; et al. Staphylococcus caprae Bone and Joint Infections: A Re-Emerging Infection? Clin. Microbiol. Infect. 2014, 20, O1052–O1058. [Google Scholar] [CrossRef] [PubMed]
- Micropia Staphylococcus epidermidis Lives in Sweaty Places. Available online: https://www.micropia.nl/en/discover/microbiology/staphylococcus-epidermidis/ (accessed on 21 July 2023).
- Schleifer, K.H.; Kloos, W.E. Isolation and Characterization of Staphylococci from Human Skin I. Amended Descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus and Descriptions of Three New Species: Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus. Int. J. Syst. Bacteriol. 1975, 25, 50–61. [Google Scholar] [CrossRef]
- Iwase, T.; Seki, K.; Shinji, H.; Mizunoe, Y.; Masuda, S. Development of a Real-Time PCR Assay for the Detection and Identification of Staphylococcus capitis, Staphylococcus haemolyticus and Staphylococcus warneri. J. Med. Microbiol. 2007, 56, 1346–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The Role of Environmental Contamination in the Transmission of Nosocomial Pathogens and Healthcare-Associated Infections. Curr. Infect. Dis. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Sharma, N.K.; Garg, R.; Baliga, S.; Bhat, K.G. Nosocomial Infections and Drug Susceptibility Patterns in Methicillin Sensitive and Methicillin Resistant Staphylococcus aureus. J. Clin. Diagn. Res. 2013, 7, 2178–2180. [Google Scholar] [CrossRef]
- Podkowik, M.; Park, J.Y.; Seo, K.S.; Bystroń, J.; Bania, J. Enterotoxigenic Potential of Coagulase-Negative Staphylococci. Int. J. Food Microbiol. 2013, 163, 34–40. [Google Scholar] [CrossRef]
- Gastmeier, P.; Stamm-Balderjahn, S.; Hansen, S.; Nitzschke-Tiemann, F.; Zuschneid, I.; Groneberg, K.; Rüden, H. How Outbreaks Can Contribute to Prevention of Nosocomial Infection: Analysis of 1,022 Outbreaks. Infect. Control Hosp. Epidemiol. 2005, 26, 357–361. [Google Scholar] [CrossRef]
- Rodríguez Fernández, L.; Martín Guerra, J.M.; Dueñas Gutiérrez, C.J. Role of Staphylococcus caprae in Nosocomial Infection. Enfermedades Infecc. Microbiol. Clin. 2020, 38, 455–456. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Widerström, M. Commentary: Significance of Staphylococcus epidermidis in health care-associated infections, from contaminant to clinically relevant pathogen: This is a wake-up call! J. Clin. Microbiol. 2016, 54, 1679–1681. [Google Scholar] [CrossRef]
- Marincola, G.; Liong, O.; Schoen, C.; Abouelfetouh, A.; Hamdy, A.; Wencker, F.D.R.; Marciniak, T.; Becker, K.; Koeck, R.; Ziebuhr, W. Antimicrobial resistance profiles of coagulase-negative staphylococci in community-based healthy individuals in Germany. Front. Public Health 2021, 9, 684456. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: Staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. Bioessays 2013, 35, 4–11. [Google Scholar] [CrossRef]
- John, J.; George, S.; Nori, S.R.C.; Nelson-Sathi, S. Phylogenomic analysis reveals the evolutionary route of resistant genes in Staphylococcus aureus. Genome Biol. Evol. 2019, 11, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Zell, C.; Resch, M.; Rosenstein, R.; Albrecht, T.; Hertel, C.; Götz, F. Characterization of Toxin Production of Coagulase-Negative Staphylococci Isolated from Food and Starter Cultures. Int. J. Food Microbiol. 2008, 127, 246–251. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The “accidental” Pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Szemraj, M.; Grazul, M.; Balcerczak, E.; Szewczyk, E.M. Staphylococcal Species Less Frequently Isolated from Human Clinical Specimens—Are They a Threat for Hospital Patients? BMC Infect. Dis. 2020, 20, 128. [Google Scholar] [CrossRef]
- Mazzariol, A.; Lo Cascio, G.; Kocsis, E.; Maccacaro, L.; Fontana, R.; Cornaglia, G. Outbreak of Linezolid-Resistant Staphylococcus haemolyticus in an Italian Intensive Care Unit. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.M.; Tuncer, C.; Saruhan, I.; Erper, I.; Öztürk, M.; Akca, İ. Isolation and Characterization of Bacillus megaterium Isolates from Dead Pentatomids and Their Insecticidal Activity to Palomena Prasina Nymphs. Akad. Ziraat Derg. 2018, 7, 21–25. [Google Scholar] [CrossRef]
- Bocchi, M.B.; Cianni, L.; Perna, A.; Vitiello, R.; Greco, T.; Maccauro, G.; Perisano, C. A Rare Case of Bacillus megaterium Soft Tissues Infection. Acta Biomed. 2020, 91, e2020013. [Google Scholar] [CrossRef]
- Kuczewski, E.; Henaff, L.; Regard, A.; Argaud, L.; Lukaszewicz, A.-C.; Rimmelé, T.; Cassier, P.; Fredenucci, I.; Loeffert-Frémiot, S.; Khanafer, N.; et al. Bacterial Cross-Transmission between Inanimate Surfaces and Patients in Intensive Care Units under Real-World Conditions: A Repeated Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2022, 19, 9401. [Google Scholar] [CrossRef] [PubMed]
- Glasset, B.; Herbin, S.; Granier, S.A.; Cavalié, L.; Lafeuille, E.; Guérin, C.; Ruimy, R.; Casagrande-Magne, F.; Levast, M.; Chautemps, N.; et al. Bacillus cereus, a Serious Cause of Nosocomial Infections: Epidemiologic and Genetic Survey. PLoS ONE 2018, 13, e0194346. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Bacillus cereus, a Volatile Human Pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Delétoile, A.; Decré, D.; Courant, S.; Passet, V.; Audo, J.; Grimont, P.; Arlet, G.; Brisse, S. Phylogeny and Identification of Pantoea Species and Typing of Pantoea agglomerans Strains by Multilocus Gene Sequencing. J. Clin. Microbiol. 2009, 47, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Mackiewicz, B.; Kinga Lemieszek, M.; Golec, M.; Milanowski, J. Pantoea agglomerans: A Mysterious Bacterium of Evil and Good. Part III. Deleterious Effects: Infections of Humans, Animals and Plants. Ann. Agric. Environ. Med. 2016, 23, 197–205. [Google Scholar] [CrossRef]
- Palmer, M.; Steenkamp, E.T.; Coetzee, M.P.A.; Avontuur, J.R.; Chan, W.-Y.; van Zyl, E.; Blom, J.; Venter, S.N. Mixta Gen. Nov., a New Genus in the Erwiniaceae. Int. J. Syst. Evol. Microbiol. 2018, 68, 1396–1407. [Google Scholar] [CrossRef]
- Casalta, J.-P.; Fournier, P.-E.; Habib, G.; Riberi, A.; Raoult, D. Prosthetic Valve Endocarditis Caused by Pseudomonas luteola. BMC Infect. Dis. 2005, 5, 82. [Google Scholar] [CrossRef]
- Bayhan, G.I.; Senel, S.; Tanir, G.; Ozkan, S. Bacteremia Caused by Pseudomonas luteola in Pediatric Patients. Jpn. J. Infect. Dis. 2015, 68, 50–54. [Google Scholar] [CrossRef]
- Alhalimi, A.A.; AlShammari, L.T.; Al-Qurayn, A.K.; Al Rashed, A.S. Infective Endocarditis Caused by Pseudomonas luteola in a Pediatric Patient. A Case Report and Review of Literature. Am. J. Case Rep. 2022, 23, 935743. [Google Scholar] [CrossRef]
- Yousefi, F.; Shoja, S.; Honarvar, N. Empyema Caused by Pseudomonas luteola: A Case Report. Jundishapur J. Microbiol. 2014, 7, 10923. [Google Scholar] [CrossRef]
- Lee, C.S.; Jung, Y.-T.; Park, S.; Oh, T.-K.; Yoon, J.-H. Lysinibacillus xylanilyticus Sp. Nov., a Xylan-Degrading Bacterium Isolated from Forest Humus. Int. J. Syst. Evol. Microbiol. 2010, 60, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.-D.; Seo, M.-J.; Lim, S.-I.; Lee, S.-Y. Genome Sequence of Lysinibacillus boronitolerans F1182, Isolated from a Traditional Korean Fermented Soybean Product. J. Bacteriol. 2012, 194, 5988. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Pal, A.; Bai, P.; Kour, A.; Sheeba, E.; Rajarajan, P.; Kausar, A.; Chatterjee, M.; Prasad, G.; Balayan, S.; et al. Co-Aggregation of Bacterial Flora Isolated from the Human Skin Surface. Microb. Pathog. 2019, 135, 103630. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, W.; Liu, Z.; Xue, X.; Gao, Q.; Wang, D.; Zhang, Y.; Zhang, J. Isolation and Identification of a Cytobacillus oceanisediminis Strain with Ochratoxin A Detoxification Ability. Food Control 2023, 151, 109797. [Google Scholar] [CrossRef]
- Mathews, S.L.; Pawlak, J.J.; Grunden, A.M. Isolation of Paenibacillus glucanolyticus from Pulp Mill Sources with Potential to Deconstruct Pulping Waste. Bioresour. Technol. 2014, 164, 100–105. [Google Scholar] [CrossRef]
- Eiref, S.D.; Leitman, I.M.; Riley, W. Hand Sanitizer Dispensers and Associated Hospital-Acquired Infections: Friend or Fomite? Surg. Infect. 2012, 13, 137–140. [Google Scholar] [CrossRef] [PubMed]

| Sample Origin | Bacterial Species Best Match (MALDI-TOF) | Score Value 1 |
|---|---|---|
| Neurosurgery ward (corridor/entrance) | Staphylococcus cohnii | 2.32 |
| Maternity ward Ι (corridor/entrance) | Staphylococcus warneri | 2.04 |
| Maternity ward ΙΙ (corridor/entrance) | Staphylococcus epidermidis, Bacillus megaterium, Staphylococcus epidermidis | 2.19 2.20 2.07 |
| Maternity ward ΙΙΙ (corridor/entrance) | Staphylococcus hominis | 2.36 |
| Maternity ward ΙV (corridor/entrance) | Staphylococcus hominis | 2.37 |
| Main hospital entrance I | Solibacillus silvestris | 1.73 |
| Main hospital entrance II | Staphylococcus caprae | 2.05 |
| Main hospital entrance III | Staphylococcus epidermidis, Micrococcus luteus | 2.13 2.44 |
| Main hospital entrance IV | Pseudomonas luteola | 2.33 |
| Chemotherapy ward (waiting room) | Staphylococcus aureus, Pantoea agglomerans | 2.31 2.32 |
| Outpatient clinics (entrance) | Staphylococcus caprae | 2.21 |
| Outpatient clinics (surgeries) | Staphylococcus lugdunensis Staphylococcus epidermidis | 2.22 2.14 |
| Outpatient clinics (waiting room) | Mixta calida | 1.80 |
| Outpatient clinics (renal clinic waiting room) | Staphylococcus haemolyticus | 2.03 |
| Outpatient clinics (gynecology clinic waiting room) | Staphylococcus saprophyticus | 2.10 |
| CAT scan (waiting room) | Lysinibacillus xylanilyticus | 2.02 |
| Postoperative care (corridor/entrance) | Bacillus cereus | 2.03 |
| Postoperative care (info desk) | No organism identification possible | 1.33 |
| Neurological clinic (waiting room) | No organism identification possible | 1.36 |
| Cafeteria (University) | Bacillus megaterium | 2.21 |
| Dept. of Histology (University) | Cytobacillus oceanisediminis | 2.00 |
| Dept. of Hygiene (University) | Paenibacillus amyloliquefaciens, Staphylococcus xylosus | 2.00 1.82 |
| Antimicrobial Agents | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class of Antibiotics | Amphenicols | Fluoroquinolones | Aminoglycosides | Macrolide | Sulfonamides | Tetracyclines | β-Lactams | Glycopeptide | Rifampicin | |||||
| Isolates | CHL (30 mcg) | CIP (5 mcg) | GEN (10 mcg) | ERY (15 mcg) | TRS (1.25 + 23.75 mcg) | TET (30 mcg) | AMC (20/10 mcg) | CXI (30 mcg) | OXA (1 mcg) | AMP (10 mcg) | PIP (10 mcg) | VAN (30 mcg) | RIF (5 mcg) | |
| 1 | Staphylococcus cohnii | S | S | S | S | S | S | S | S | S | S | S | S | - |
| 2 | Staphylococcus warneri | S | S | S | S | S | S | S | S | S | S | S | S | - |
| 3 | Staphylococcus epidermidis | R | I | S | R | S | R | S | S | S | S | R | S | - |
| 4 | Bacillus megaterium | - | S | S | S | S | S | S | - | - | S | S | S | S |
| 5 | Staphylococcus epidermidis | S | S | S | S | S | S | S | S | S | S | R | S | - |
| 6 | Staphylococcus hominis | S | S | S | R | S | S | S | S | S | S | R | S | - |
| 7 | Staphylococcus hominis | S | S | S | R | S | S | S | S | S | S | R | S | - |
| 8 | Staphylococcus caprae | R | S | S | R | S | S | S | S | S | R | R | S | - |
| 9 | Staphylococcus epidermidis | S | S | S | R | S | R | S | S | S | I | R | S | - |
| 10 | Micrococcus luteus | S | S | S | S | S | S | S | - | - | S | S | S | - |
| 11 | Staphylococcus aureus | - | - | S | R | S | S | S | S | S | I | R | - | - |
| 12 | Staphylococcus caprae | S | S | S | R | S | S | S | S | S | S | R | S | - |
| 13 | Staphylococcus lugdunensis | S | S | S | R | S | S | S | S | S | S | S | S | - |
| 14 | Staphylococcus epidermidis | S | S | R | S | S | S | S | S | S | S | S | S | - |
| 15 | Staphylococcus haemolyticus | S | S | S | I | S | S | S | S | S | S | S | S | - |
| 16 | Staphylococcus saprophyticus | S | S | S | R | S | I | S | S | S | S | S | S | - |
| 17 | Lysinibacillus xylanilyticus | S | S | S | S | S | S | S | - | - | S | S | S | R |
| 18 | Bacillus cereus | S | S | S | S | I | S | R | - | - | R | R | S | R |
| 19 | Bacillus megaterium | S | S | S | S | S | S | S | - | - | S | S | S | R |
| 20 | Cytobacillus oceanisediminis | S | S | S | S | S | S | S | - | - | S | S | S | R |
| 21 | Paenibacillus amyloliquefaciens | S | S | S | S | S | S | S | - | - | S | S | S | R |
| Antimicrobial Agents | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class of Antibiotics | Amphenicols | Polymyxin | Fluroquinolones | Aminoglycosides | Sulfonamides | Tetracyclines | β-Lactams | Carbapenem | ||||||
| Isolates | CHL (30 mcg) | COL (10 mcg) | CIP (5 mcg) | NAL (30 mcg) | GEN (10 mcg) | TOB (10 mcg) | SXT (1.25 + 23.75 mcg) | DOX (80 mcg) | TET (30 mcg) | AMC (20/10 mcg) | AMP (10 mcg) | IMI (10 mcg) | MER (10 mcg) | |
| 22 | Pseudomonas luteola | S | S | S | S | S | S | S | - | - | I | I | S | S |
| 23 | Pantoea agglomerans | S | S | S | S | S | S | S | - | - | R | S | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanis, C.; Giorgi, E.; Stavropoulou, E.; Voidarou, C.; Skoufou, M.; Nelli, A.; Tzora, A.; Tsigalou, C.; Bezirtzoglou, E. Microbial Load of Hand Sanitizer Dispensers—A University Hospital Study. Hygiene 2023, 3, 450-464. https://doi.org/10.3390/hygiene3040034
Stefanis C, Giorgi E, Stavropoulou E, Voidarou C, Skoufou M, Nelli A, Tzora A, Tsigalou C, Bezirtzoglou E. Microbial Load of Hand Sanitizer Dispensers—A University Hospital Study. Hygiene. 2023; 3(4):450-464. https://doi.org/10.3390/hygiene3040034
Chicago/Turabian StyleStefanis, Christos, Elpida Giorgi, Elisavet Stavropoulou, Chrysoula (Chrysa) Voidarou, Maria Skoufou, Aikaterini Nelli, Athina Tzora, Christina Tsigalou, and Eugenia Bezirtzoglou. 2023. "Microbial Load of Hand Sanitizer Dispensers—A University Hospital Study" Hygiene 3, no. 4: 450-464. https://doi.org/10.3390/hygiene3040034
APA StyleStefanis, C., Giorgi, E., Stavropoulou, E., Voidarou, C., Skoufou, M., Nelli, A., Tzora, A., Tsigalou, C., & Bezirtzoglou, E. (2023). Microbial Load of Hand Sanitizer Dispensers—A University Hospital Study. Hygiene, 3(4), 450-464. https://doi.org/10.3390/hygiene3040034









