Healthy Aging in Menopause: Prevention of Cognitive Decline, Depression and Dementia through Physical Exercise
Abstract
:1. Introduction
2. Methodology
3. Aging
4. Menopause
5. Neuroinflammation
6. Cognitive Impairment
7. Depression
8. Cognitive Decline and Alzheimer’s Disease
9. Physical Exercise
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rowe, J.W. Editorial: Successful Aging: Evolution of a Concept. J. Nutr. Health Aging 2023, 27, 194–195. [Google Scholar] [CrossRef]
- Seifert, C.; Storch, S.; Bahring, R. Modulation of Kv4.2/KChIP3 interaction by the ceroid lipofuscinosis neuronal 3 protein CLN3. J. Biol. Chem. 2020, 295, 12099–12110. [Google Scholar] [CrossRef]
- Ungvari, Z.; Csiszar, A. The emerging role of IGF-1 deficiency in cardiovascular aging: Recent advances. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 599–610. [Google Scholar] [CrossRef]
- Rivas-Campo, Y.; Garcia-Garro, P.A.; Aibar-Almazan, A.; Martinez-Amat, A.; Vega-Avila, G.C.; Afanador-Restrepo, D.F.; Leon-Morillas, F.; Hita-Contreras, F. The Effects of High-Intensity Functional Training on Cognition in Older Adults with Cognitive Impairment: A Systematic Review. Healthcare 2022, 10, 670. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Franco-Martin, M.; Parra-Vidales, E.; Gonzalez-Palau, F.; Bernate-Navarro, M.; Solis, A. The influence of physical exercise in the prevention of cognitive deterioration in the elderly: A systematic review. Rev. Neurol. 2013, 56, 545–554. [Google Scholar]
- Garcia-Garro, P.A.; Hita-Contreras, F.; Martinez-Amat, A.; Achalandabaso-Ochoa, A.; Jimenez-Garcia, J.D.; Cruz-Diaz, D.; Aibar-Almazan, A. Effectiveness of A Pilates Training Program on Cognitive and Functional Abilities in Postmenopausal Women. Int. J. Environ. Res. Public Health 2020, 17, 3580. [Google Scholar] [CrossRef]
- Kawada, T. Which reduces the risk of cognitive impairment: Physical activity or daytime nap? Psychogeriatrics 2022, 22, 772. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Qiu, Q.; Li, W.; Nie, J.; Zhang, J.; Li, X.; Xiao, S. The Beneficial Effect of Physical Exercise on Cognitive Function in a Non-dementia Aging Chinese Population. Front. Aging Neurosci. 2019, 11, 238. [Google Scholar] [CrossRef]
- Ramirez-Velez, R.; Saez De Asteasu, M.L.; Martinez-Velilla, N.; Zambon-Ferraresi, F.; Garcia-Hermoso, A.; Recarey, A.E.; Fernandez-Irigoyen, J.; Santamaria, E.; Palomino-Echeverria, S.; Izquierdo, M. Circulating Cytokines and Lower Body Muscle Performance in Older Adults at Hospital Admission. J. Nutr. Health Aging 2020, 24, 1131–1139. [Google Scholar] [CrossRef]
- Zeng, D.; Ling, X.Y.; Fang, Z.L.; Lu, Y.F. Optimal exercise to improve physical ability and performance in older adults with sarcopenia: A systematic review and network meta-analysis. Geriatr. Nurs. 2023, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, F.; Cheng, J.; Huang, Y.; Ren, Z.; Ye, R.; Fan, J.; Li, L.; Gao, Y. The relationship between physical activity and subjective cognitive decline: Evidence from the behavioral risk factor surveillance system (BRFSS). J. Affect. Disord. 2023, 328, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Noguchi-Shinohara, M.; Yokoyama, K.; Komatsu, J.; Masuda, K.; Kouno, M.; Yoshita, M.; Ono, K. Exercise program to reduce the risk of cognitive decline and physical frailty in older adults: Study protocol for an open label double-arm clinical trial. Front. Aging Neurosci. 2023, 15, 1162765. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Cheng, C. Anxiety, Depression, Chronic Pain, and Quality of Life Among Older Adults in Rural China: An Observational, Cross-Sectional, Multi-Center Study. J. Community Health Nurs. 2022, 39, 202–212. [Google Scholar] [CrossRef]
- Segura-Cardona, A.; Cardona-Arango, D.; Segura-Cardona, A.; Garzon-Duque, M. Risk of depression and associated factors in older adults. Antioquia, Colombia. 2012. Rev. Salud Publica 2015, 17, 184–194. [Google Scholar] [CrossRef]
- Su, H.; Zhou, Y.; Sun, Y.; Cai, Y. The relationship between depression and subjective cognitive decline in older adults of China: The mediating role of general self-efficacy. Psychol. Health Med. 2023, 28, 1057–1067. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Zheng, X.; Sun, S.; Zhao, J.; Long, Y.; Mao, Y. Acidic oligosaccharide sugar chain combined with hyperbaric oxygen delays D-galactose-induced brain senescence in mice via attenuating oxidative stress and neuroinflammation. Neurosci. Res. 2022, 185, 40–48. [Google Scholar] [CrossRef]
- Grigoriou, S.S.; Krase, A.A.; Karatzaferi, C.; Giannaki, C.D.; Lavdas, E.; Mitrou, G.I.; Bloxham, S.; Stefanidis, I.; Sakkas, G.K. Long-term intradialytic hybrid exercise training on fatigue symptoms in patients receiving hemodialysis therapy. Int. Urol. Nephrol. 2021, 53, 771–784. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Montagne, A.; Zhao, Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell Mol. Life Sci. 2021, 78, 4907–4920. [Google Scholar] [CrossRef]
- Greendale, G.A.; Karlamangla, A.S.; Maki, P.M. The Menopause Transition and Cognition. JAMA 2020, 323, 1495–1496. [Google Scholar] [CrossRef]
- Merghati-Khoei, E.; Sheikhan, F.; Shamsalizadeh, N.; Haghani, H.; Yousofnia Pasha, Y.R.; Killeen, T. Menopause negatively impacts sexual lives of middle-aged Iranian women: A cross-sectional study. J. Sex. Marital. Ther. 2014, 40, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrezuelo, I.; Aibar-Almazan, A.; Martinez-Amat, A.; Fabrega-Cuadros, R.; Diaz-Mohedo, E.; Wangensteen, R.; Hita-Contreras, F. Female Sexual Function and Its Association with the Severity of Menopause-Related Symptoms. Int. J. Environ. Res. Public Health 2020, 17, 7235. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Li, L. Urban-rural differences in epidemiology and risk factors of menopause syndrome in middle-aged Chinese women. Menopause 2023, 30, 306–316. [Google Scholar] [CrossRef]
- Aibar-Almazan, A.; Hita-Contreras, F.; Cruz-Diaz, D.; de la Torre-Cruz, M.; Jimenez-Garcia, J.D.; Martinez-Amat, A. Effects of Pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: A randomized controlled trial. Maturitas 2019, 124, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Carcelen-Fraile, M.D.C.; Aibar-Almazan, A.; Martinez-Amat, A.; Cruz-Diaz, D.; Diaz-Mohedo, E.; Redecillas-Peiro, M.T.; Hita-Contreras, F. Effects of Physical Exercise on Sexual Function and Quality of Sexual Life Related to Menopausal Symptoms in Peri- and Postmenopausal Women: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2680. [Google Scholar] [CrossRef] [PubMed]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Won, J.; Lee, S.; Hong, Y.; Kim, J.H.; Hong, Y. Benefits of Physical Exercise for Individuals with Fragile X Syndrome in Humans. J. Lifestyle Med. 2015, 5, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, N.; De Teresa, C.; Cano, A.; Godoy, D.; Hita-Contreras, F.; Lapotka, M.; Llaneza, P.; Manonelles, P.; Martinez-Amat, A.; Ocon, O.; et al. Benefits of physical exercise in postmenopausal women. Maturitas 2016, 93, 83–88. [Google Scholar] [CrossRef]
- Yu, R.; Leung, G.; Woo, J. Randomized Controlled Trial on the Effects of a Combined Intervention of Computerized Cognitive Training Preceded by Physical Exercise for Improving Frailty Status and Cognitive Function in Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 1396. [Google Scholar] [CrossRef]
- Ritchie, S.; Lawrence, V.; Jones, J.; Corbett, A. Engaging older adults in an online physical activity programme to improve cognition: A qualitative study. Int. J. Geriatr. Psychiatry 2021, 36, 1942–1949. [Google Scholar] [CrossRef]
- Borsky, P.; Holmannova, D.; Fiala, Z.; Borska, L.; Hruska, L.; Kucera, O. Physiology of ageing. Cas. Lek. Cesk 2022, 161, 11–16. [Google Scholar] [PubMed]
- Li, Q.; Gong, B.; Zhao, Y.; Wu, C. Effect of Exercise Cognitive Combined Training on Physical Function in Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis. J. Aging Phys. Act. 2023, 31, 155–170. [Google Scholar] [CrossRef]
- Li, X.; Karpac, J. Adaptive physiology drives ageing plasticity in locusts. Nat. Ecol. Evol. 2023, 7, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, F.; Poppe, L.; DeSmet, A.; Swinnen, S.; Cardon, G.; De Bourdeaudhuij, I.; Chastin, S.; Fias, W. Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 63. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Llopis-Cardona, F.; Armero, C.; Sanfelix-Gimeno, G. Estimating disease incidence rates and transition probabilities in elderly patients using multi-state models: A case study in fragility fracture using a Bayesian approach. BMC Med. Res. Methodol. 2023, 23, 40. [Google Scholar] [CrossRef]
- NIH to require both sexes in preclinical studies. Cancer Discov. 2014, 4, 860. [CrossRef]
- Rich-Edwards, J.W.; Kaiser, U.B.; Chen, G.L.; Manson, J.E.; Goldstein, J.M. Sex and Gender Differences Research Design for Basic, Clinical, and Population Studies: Essentials for Investigators. Endocr. Rev. 2018, 39, 424–439. [Google Scholar] [CrossRef]
- Shansky, R.M.; Murphy, A.Z. Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci. 2021, 24, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Carmody, C.; Duesing, C.G.; Kane, A.E.; Mitchell, S.J. Is Sex as a Biological Variable Still Being Ignored in Preclinical Aging Research? J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- INE. Indicadores de Crecimiento y Estructura de la Población. Available online: https://www.ine.es/dynt3/inebase/es/index.htm?padre=1161&dh=1 (accessed on 24 October 2023).
- Luchsinger, J.A.; Kazemi, E.J.; Sanchez, D.L.; Larkin, M.E.; Valencia, W.M.; Desouza, C.; Carlson, A.L.; Pop-Busui, R.; Seaquist, E.R.; Florez, H.J.; et al. BMI, insulin sensitivity, and cognition in early type 2 diabetes: The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study. Obesity 2023, 31, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Strehler, B.L. Aging: A challenge to science, society, and the individual. Clin. Geriatr. Med. 1985, 1, 5–13. [Google Scholar] [CrossRef]
- Vina, J.; Borras, C.; Miquel, J. Theories of ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Ong, A.D.; Ram, N. Fragile and Enduring Positive Affect: Implications for Adaptive Aging. Gerontology 2017, 63, 263–269. [Google Scholar] [CrossRef]
- The Lancet Healthy, L. Ageing populations: Unaffordable demography. Lancet Healthy Longev. 2022, 3, e804. [Google Scholar] [CrossRef]
- Dai, Y.; Hsu, Y.C.; Fernandes, B.S.; Zhang, K.; Li, X.; Enduru, N.; Liu, A.; Manuel, A.M.; Jiang, X.; Zhao, Z. Alzheimer’s Disease Neuroimaging Initiative. Disentangling Accelerated Cognitive Decline from the Normal Aging Process and Unraveling Its Genetic Components: A Neuroimaging-Based Deep Learning Approach. J. Alzheimer’s Dis. 2024, 97, 1807–1827. [Google Scholar] [CrossRef]
- Tampi, R.R. Diabetes, Cognition, and Mortality. Am. J. Geriatr. Psychiatry 2023, 31, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Nigam, Y.; Knight, J.; Bhattacharya, S.; Bayer, A. Physiological changes associated with aging and immobility. J. Aging Res. 2012, 2012, 468469. [Google Scholar] [CrossRef] [PubMed]
- Katusic, Z.S.; Austin, S.A. Neurovascular Protective Function of Endothelial Nitric Oxide—Recent Advances. Circ. J. 2016, 80, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, C.F.; Ledo, A.; Barbosa, R.M.; Laranjinha, J. Neurovascular-neuroenergetic coupling axis in the brain: Master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic. Biol. Med. 2017, 108, 668–682. [Google Scholar] [CrossRef]
- Pitsikas, N. The role of nitric oxide in the object recognition memory. Behav. Brain Res. 2015, 285, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.R.; Mangini, L.D.; Thelus-Jean, R.; Hayward, M.D. Life-course origins of the ages at menarche and menopause. Adolesc. Health Med. Ther. 2013, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, M.; Heidari, M. Quality of Life in Postmenopausal Female Members and Non-members of the Elderly Support Association. J. Menopausal Med. 2016, 22, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 2015, 11, 393–405. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; Group, S.C. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Armeni, A.; Armeni, E.; Augoulea, A.; Stergiotis, S.; Kaparos, G.; Alexandrou, A.; Eleftheriadis, M.; Georgopoulos, N.; Vlahos, N.; Lambrinoudaki, I. Climacteric symptoms, age, and sense of coherence are associated with sexual function scores in women after menopause. J. Sex. Med. 2023, 20, 313–323. [Google Scholar] [CrossRef]
- Fritz, M.A.; Speroff, L. Clinical Gynecologic Endocrinology and Infertility, 8th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. 684. 1439p. [Google Scholar]
- Rhoades, R.; Bell, D.R. Medical Physiology: Principles for Clinical Medicine, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; p. xvii. 860p. [Google Scholar]
- Wecker, L.; Taylor, D.A.; Theobald, R.J. Brody’s Human Pharmacology: Molecular to Clinical, 6th ed.; Elsevier, Inc.: Philadelphia, PA, USA, 2019. [Google Scholar]
- Brinton, R.D. Estrogen-induced plasticity from cells to circuits: Predictions for cognitive function. Trends Pharmacol. Sci. 2009, 30, 212–222. [Google Scholar] [CrossRef]
- McEwen, B.S.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012, 126, 4–16. [Google Scholar] [CrossRef]
- Nilsson, S.; Koehler, K.F.; Gustafsson, J.A. Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discov. 2011, 10, 778–792. [Google Scholar] [CrossRef]
- Mattson, M.P.; Magnus, T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006, 7, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Rettberg, J.R.; Dang, H.; Hodis, H.N.; Henderson, V.W.; St John, J.A.; Mack, W.J.; Brinton, R.D. Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: Potential for detecting an at-Alzheimer’s risk metabolic phenotype. Neurobiol. Aging 2016, 40, 155–163. [Google Scholar] [CrossRef]
- Yin, F.; Yao, J.; Sancheti, H.; Feng, T.; Melcangi, R.C.; Morgan, T.E.; Finch, C.E.; Pike, C.J.; Mack, W.J.; Cadenas, E.; et al. The perimenopausal aging transition in the female rat brain: Decline in bioenergetic systems and synaptic plasticity. Neurobiol. Aging 2015, 36, 2282–2295. [Google Scholar] [CrossRef]
- Anderson, D.; Yoshizawa, T.; Gollschewski, S.; Atogami, F.; Courtney, M. Menopause in Australia and Japan: Effects of country of residence on menopausal status and menopausal symptoms. Climacteric 2004, 7, 165–174. [Google Scholar] [CrossRef]
- Blumel, J.E.; Chedraui, P.; Baron, G.; Belzares, E.; Bencosme, A.; Calle, A.; Danckers, L.; Espinoza, M.T.; Flores, D.; Gomez, G.; et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause 2011, 18, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Dias-da-Costa, J.S.; Olinto, M.T.; Gigante, D.P.; Menezes, A.M.; Macedo, S.; Daltoe, T.; Santos Ida, S.; Fuchs, S.C. Use of outpatient services in Pelotas, Rio Grande do Sul State, Brazil: Factors related to above-average number of physician visits. Cad. Saude Publica 2008, 24, 353–363. [Google Scholar] [CrossRef]
- Politi, M.C.; Schleinitz, M.D.; Col, N.F. Revisiting the duration of vasomotor symptoms of menopause: A meta-analysis. J. Gen. Intern. Med. 2008, 23, 1507–1513. [Google Scholar] [CrossRef]
- Whiteley, J.; Wagner, J.S.; Bushmakin, A.; Kopenhafer, L.; Dibonaventura, M.; Racketa, J. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity. Menopause 2013, 20, 518–524. [Google Scholar] [CrossRef]
- Woods, N.F.; Mitchell, E.S. Symptoms during the perimenopause: Prevalence, severity, trajectory, and significance in women’s lives. Am. J. Med. 2005, 118 (Suppl. 12B), 14–24. [Google Scholar] [CrossRef]
- da Silva, A.A.; de Mello, R.G.; Schaan, C.W.; Fuchs, F.D.; Redline, S.; Fuchs, S.C. Sleep duration and mortality in the elderly: A systematic review with meta-analysis. BMJ Open 2016, 6, e008119. [Google Scholar] [CrossRef]
- Goldman, S.E.; Stone, K.L.; Ancoli-Israel, S.; Blackwell, T.; Ewing, S.K.; Boudreau, R.; Cauley, J.A.; Hall, M.; Matthews, K.A.; Newman, A.B. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep 2007, 30, 1317–1324. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Zagalaz-Anula, N.; Martinez-Amat, A.; Cruz-Diaz, D.; Sanchez-Montesinos, I.; Aibar-Almazan, A.; Lomas-Vega, R. Sleep quality and its association with postural stability and fear of falling among Spanish postmenopausal women. Menopause 2018, 25, 62–69. [Google Scholar] [CrossRef]
- Mulhall, S.; Andel, R.; Anstey, K.J. Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas 2018, 108, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Naufel, M.F.; Frange, C.; Andersen, M.L.; Girao, M.; Tufik, S.; Beraldi Ribeiro, E.; Hachul, H. Association between obesity and sleep disorders in postmenopausal women. Menopause 2018, 25, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Shaver, J.L.; Woods, N.F. Sleep and menopause: A narrative review. Menopause 2015, 22, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Ding, P.; Tian, L.; Kuang, X.; Huang, L.; Shi, H. Sleep duration trajectories and all-cause mortality among Chinese elderly: A community-based cohort study. BMC Public Health 2023, 23, 1095. [Google Scholar] [CrossRef] [PubMed]
- Hita-Contreras, F.; Martinez-Amat, A.; Lomas-Vega, R.; Alvarez, P.; Mendoza, N.; Romero-Franco, N.; Aranega, A. Relationship of body mass index and body fat distribution with postural balance and risk of falls in Spanish postmenopausal women. Menopause 2013, 20, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet. Neuronal Interact. 2009, 9, 186–197. [Google Scholar] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Heinemann, K.; Ruebig, A.; Potthoff, P.; Schneider, H.P.; Strelow, F.; Heinemann, L.A.; Do, M.T. The Menopause Rating Scale (MRS) scale: A methodological review. Health Qual. Life Outcomes 2004, 2, 45. [Google Scholar] [CrossRef]
- Heinemann, L.A.; Potthoff, P.; Schneider, H.P. International versions of the Menopause Rating Scale (MRS). Health Qual. Life Outcomes 2003, 1, 28. [Google Scholar] [CrossRef]
- Potthoff, P.; Heinemann, L.A.; Schneider, H.P.; Rosemeier, H.P.; Hauser, G.A. The Menopause Rating Scale (MRS II): Methodological standardization in the German population. Zentralblatt Gynakol. 2000, 122, 280–286. [Google Scholar]
- Csipo, T.; Lipecz, A.; Ashpole, N.M.; Balasubramanian, P.; Tarantini, S. Astrocyte senescence contributes to cognitive decline. Geroscience 2020, 42, 51–55. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Haapasalo, A.; Hiltunen, M.; Soininen, H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur. J. Neurosci. 2011, 34, 3–11. [Google Scholar] [CrossRef]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 2012, 7, e45069. [Google Scholar] [CrossRef]
- Godbout, J.P.; Johnson, R.W. Age and neuroinflammation: A lifetime of psychoneuroimmune consequences. Immunol. Allergy Clin. N. Am. 2009, 29, 321–337. [Google Scholar] [CrossRef]
- Pertusa, M.; Garcia-Matas, S.; Rodriguez-Farre, E.; Sanfeliu, C.; Cristofol, R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem. 2007, 101, 794–805. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Souza, D.G.; Bellaver, B.; Raupp, G.S.; Souza, D.O.; Quincozes-Santos, A. Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem. Int. 2015, 90, 93–97. [Google Scholar] [CrossRef]
- Cortese, G.P.; Burger, C. Neuroinflammatory challenges compromise neuronal function in the aging brain: Postoperative cognitive delirium and Alzheimer’s disease. Behav. Brain Res. 2017, 322, 269–279. [Google Scholar] [CrossRef]
- Sala, G.; Nishita, Y.; Tange, C.; Tomida, M.; Gondo, Y.; Shimokata, H.; Otsuka, R. No Appreciable Effect of Education on Aging-Associated Declines in Cognition: A 20-Year Follow-Up Study. Psychol. Sci. 2023, 34, 527–536. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, C.; Li, X.; Zhang, J.; Chen, K.; Liu, Z.; Li, H.; Fan, J.; Qin, T.; Luo, L.; et al. The positive impacts of early-life education on cognition, leisure activity, and brain structure in healthy aging. Aging 2019, 11, 4923–4942. [Google Scholar] [CrossRef] [PubMed]
- Maltais, M.; de Souto Barreto, P.; Bowman, G.L.; Smith, A.D.; Cantet, C.; Andrieu, S.; Rolland, Y. Omega-3 Supplementation for the Prevention of Cognitive Decline in Older Adults: Does It Depend on Homocysteine Levels? J. Nutr. Health Aging 2022, 26, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Scher, C.; Nepomnyaschy, L.; Amano, T. Comparison of Cognitive and Physical Decline as Predictors of Depression Among Older Adults. J. Appl. Gerontol. 2023, 42, 387–398. [Google Scholar] [CrossRef]
- Stephan, B.C.M.; Siervo, M.; Brayne, C. How can population-based studies best be utilized to reduce the global impact of dementia? Recommendations for researchers, funders, and policymakers. Alzheimers Dement. 2020, 16, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Cagney, K.; Choi, Y. Predictors of cognitive functioning trajectories among older Americans: A new investigation covering 20 years of age- and non-age-related cognitive change. PLoS ONE 2023, 18, e0281139. [Google Scholar] [CrossRef]
- Cassani, R.; Estarellas, M.; San-Martin, R.; Fraga, F.J.; Falk, T.H. Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment. Dis. Markers 2018, 2018, 5174815. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Gagliardi, C.; Papa, R.; Postacchini, D.; Giuli, C. Association between Cognitive Status and Physical Activity: Study Profile on Baseline Survey of the My Mind Project. Int. J. Environ. Res. Public Health 2016, 13, 585. [Google Scholar] [CrossRef]
- Henley, D.B.; Dowsett, S.A.; Chen, Y.F.; Liu-Seifert, H.; Grill, J.D.; Doody, R.S.; Aisen, P.; Raman, R.; Miller, D.S.; Hake, A.M.; et al. Alzheimer’s disease progression by geographical region in a clinical trial setting. Alzheimers Res. Ther. 2015, 7, 43. [Google Scholar] [CrossRef]
- Blair, C.K.; Folsom, A.R.; Knopman, D.S.; Bray, M.S.; Mosley, T.H.; Boerwinkle, E.; Investigators, A.S. genotype and cognitive decline in a middle-aged cohort. Neurology 2005, 64, 268–276. [Google Scholar] [CrossRef]
- Anstey, K.J.; von Sanden, C.; Salim, A.; O’Kearney, R. Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. Am. J. Epidemiol. 2007, 166, 367–378. [Google Scholar] [CrossRef]
- Bangen, K.J.; Beiser, A.; Delano-Wood, L.; Nation, D.A.; Lamar, M.; Libon, D.J.; Bondi, M.W.; Seshadri, S.; Wolf, P.A.; Au, R. APOE Genotype Modifies the Relationship between Midlife Vascular Risk Factors and Later Cognitive Decline. J. Stroke Cerebrovasc. 2013, 22, 1361–1369. [Google Scholar] [CrossRef]
- Carmelli, D.; Swan, G.E.; Reed, T.; Miller, B.; Wolf, P.A.; Jarvik, G.P.; Schellenberg, G.D. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology 1998, 50, 1580–1585. [Google Scholar] [CrossRef]
- Dintica, C.S.; Hoang, T.; Allen, N.; Sidney, S.; Yaffe, K. The Metabolic Syndrome Is Associated With Lower Cognitive Performance and Reduced White Matter Integrity in Midlife: The CARDIA Study. Front. Neurosci. 2022, 16, 942743. [Google Scholar] [CrossRef]
- Emery, C.F.; Finkel, D.; Pedersen, N.L. Pulmonary Function as a Cause of Cognitive Aging. Psychol. Sci. 2012, 23, 1024–1032. [Google Scholar] [CrossRef]
- Finkel, D.; Ernsth-Bravell, M.; Pedersen, N.L. Temporal Dynamics of Motor Functioning and Cognitive Aging. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Allerhand, M.; Deary, I.J.; Team, H.S. Is there a bidirectional relationship between depressive symptoms and cognitive ability in older people? A prospective study using the English Longitudinal Study of Ageing. Psychol. Med. 2012, 42, 2057–2069. [Google Scholar] [CrossRef]
- Knopman, D.S.; Mosley, T.H.; Catellier, D.J.; Coker, L.H.; Atherosclerosis Risk in Communities Study Brain MRI Study. Fourteen-year longitudinal study of vascular risk factors, genotype, and cognition: The ARIC MRI Study. Alzheimers Dement. 2009, 5, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Radler, K.H.; Chapman, S.; Zdrodowska, M.A.; Dowd, H.N.; Liu, X.H.; Huey, E.D.; Cosentino, S.; Louis, E.D. Physical Activity as a Predictor of Cognitive Decline in an Elderly Essential Tremor Cohort: A Prospective, Longitudinal Study. Front. Neurol. 2021, 12, 658527. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Schneider, J.A.; Boyle, P.A.; Arnold, S.E.; Tang, Y.; Bennett, D.A. Chronic distress and incidence of mild cognitive impairment. Neurology 2007, 68, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Blackwell, T.; Gore, R.; Sands, L.; Reus, V.; Browner, W.S. Depressive symptoms and cognitive decline in nondemented elderly women—A prospective study. Arch. Gen. Psychiatry 1999, 56, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Chuang, I.C.; Liao, W.W.; Wu, C.Y.; Yeh, T.T.; Chen, C.L.; Lin, C.H.; Huang, T.H.; Pei, Y.C. Baseline Global Cognitive Function Affects Cognitive and Functional Outcomes of Combined Physical and Cognitive Training Among Older Adults With Cognitive Decline. Am. J. Occup. Ther. 2022, 76, 7602205140. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casares, N.; Fuentes, P.G.; Barbancho, M.A.; Lopez-Gigosos, R.; Garcia-Rodriguez, A.; Gutierrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642. [Google Scholar] [CrossRef]
- Teixeira, C.V.L.; Weiler, M.; Vicentini, J.E.; Junqueira, T.; Cendes, F.; Balthazar, M.L.F. Physical Activity and Hippocampus Volume in Alzheimer Disease, Mild Cognitive Impairment and Normal Cognitive Elderly. Med. Sci. Sports Exerc. 2015, 47, 457–458. [Google Scholar] [CrossRef]
- Koutsonida, M.; Koskeridis, F.; Markozannes, G.; Kanellopoulou, A.; Mousas, A.; Ntotsikas, E.; Ioannidis, P.; Aretouli, E.; Tsilidis, K.K. Metabolic syndrome and cognitive deficits in the Greek cohort of Epirus Health Study. Neurol. Sci. 2023, 44, 3523–3533. [Google Scholar] [CrossRef]
- Koutsonida, M.; Markozannes, G.; Bouras, E.; Aretouli, E.; Tsilidis, K.K. Metabolic syndrome and cognition: A systematic review across cognitive domains and a bibliometric analysis. Front. Psychol. 2022, 13, 981379. [Google Scholar] [CrossRef]
- Pillai, J.A.; Bena, J.; Bekris, L.; Kodur, N.; Kasumov, T.; Leverenz, J.B.; Kashyap, S.R.; Init, A.D.N. Metabolic syndrome biomarkers relate to rate of cognitive decline in MCI and dementia stages of Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 54. [Google Scholar] [CrossRef]
- Stirland, L.E.; O’Shea, C.I.; Russ, T.C. Passive smoking as a risk factor for dementia and cognitive impairment: Systematic review of observational studies. Int. Psychogeriatr. 2018, 30, 1177–1187. [Google Scholar] [CrossRef]
- Tzimourta, K.D.; Christou, V.; Tzallas, A.T.; Giannakeas, N.; Astrakas, L.G.; Angelidis, P.; Tsalikakis, D.; Tsipouras, M.G. Machine Learning Algorithms and Statistical Approaches for Alzheimer’s Disease Analysis Based on Resting-State EEG Recordings: A Systematic Review. Int. J. Neural Syst. 2021, 31, 2130002. [Google Scholar] [CrossRef]
- Lewis, E.D.; Apostol, M.; Langston, J.; Parker, A.; Evans, M. A Multi-Center, Open-Label Exploratory Study to Assess Cognitive Function Response to Lifestyle Changes Plus Supplementation in Healthy Adults with Risk Factors Associated with Cognitive Decline. Appl. Sci. 2023, 13, 2818. [Google Scholar] [CrossRef]
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The worldwide costs of dementia in 2019. Alzheimers Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Chin, S.; Sedai, A.K. The welfare cost of late-life depression. J. Econ. Behav. Organ. 2022, 204, 15–36. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Liu, Z.J. Temporal relationship between depression and cognitive decline in the elderly: A two-wave cross-lagged study in a Chinese sample. Aging Ment. Health 2023, 27, 2179–2186. [Google Scholar] [CrossRef]
- Hwang, I.C.; Ahn, H.Y. The effect of depression on cognitive decline among Korean retirees. J. Res. Med. Sci. 2023, 28, 33. [Google Scholar] [CrossRef]
- Soares, B.; Kanevsky, G.; Teng, C.T.; Pérez-Esparza, R.; Bonetto, G.G.; Lacerda, A.L.T.; Uribe, E.S.; Cordoba, R.; Lupo, C.; Samora, A.M.; et al. Prevalence and Impact of Treatment-Resistant Depression in Latin America: A Prospective, Observational Study. Psychiatr. Q. 2021, 92, 1797–1815. [Google Scholar] [CrossRef] [PubMed]
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2018, 48, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Sampath, D.; Sathyanesan, M.; Newton, S.S. Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatr. Dis. Treat. 2017, 13, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Jackowski, A.; Salas, R.; Gupta, S.; Sato, J.R.; Mao, X.; Coplan, J.D.; Shungu, D.C.; Mathew, S.J. The Nucleus Accumbens and Ketamine Treatment in Major Depressive Disorder. Neuropsychopharmacology 2017, 42, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, G.M.; Zhang, J.; Thomas, M.; Banasr, M.; Ma, X.; Pittman, B.; Bristow, L.; Schaeffer, E.; Duman, R.S.; Rothman, D.L.; et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol. Psychiatry 2017, 22, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M.A. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61, 4–6. [Google Scholar] [PubMed]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major Depressive Disorder: New Clinical, Neurobiological, and Treatment Perspectives. Focus 2016, 14, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A., Jr. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chen, C.A.; Hsu, F.Y.; Hsiao, Y.H. Elevated Hippocampal CRMP5 Mediates Chronic Stress-Induced Cognitive Deficits by Disrupting Synaptic Plasticity, Hindering AMPAR Trafficking, and Triggering Cytokine Release. Int. J. Mol. Sci. 2023, 24, 4898. [Google Scholar] [CrossRef]
- Lopez-Munoz, F.; D’Ocon, P.; Romero, A.; Guerra, J.A.; Alamo, C. Role of serendipity in the discovery of classical antidepressant drugs: Applying operational criteria and patterns of discovery. World J. Psychiatry 2022, 12, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997, 17, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Perez-Esparza, R. Ketamine for Treatment-Resistant Depression: A New Advocate. Rev. Investig. Clin. 2018, 70, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Esparza, R.; Kobayashi-Romero, L.F.; García-Mendoza, A.M.; Lamas-Aguilar, R.M.; Fonseca-Perezamador, A. Promises and concerns regarding the use of ketamine and esketamine in the treatment of depression. Acta Psychiatr. Scand. 2019, 140, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.; Buchmann, A.; Muller, S.T.; Volleberg, M.; Haynes, M.; Ghisleni, C.; Noeske, R.; Tuura, R.; Hasler, G. Evaluation of Prefrontal gamma-Aminobutyric Acid and Glutamate Levels in Individuals with Major Depressive Disorder Using Proton Magnetic Resonance Spectroscopy. JAMA Psychiatry 2022, 79, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Banasr, M. From pathophysiology to novel antidepressant drugs: Glial contributions to the pathology and treatment of mood disorders. Biol. Psychiatry 2013, 73, 1172–1179. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Liu, W.; Karatsoreos, I.N.; Ren, Y.; Feng, J.; McEwen, B.S.; Yan, Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol. Psychiatry 2011, 16, 156–170. [Google Scholar] [CrossRef]
- Giacometti, L.L.; Barker, J.M. Sex differences in the glutamate system: Implications for addiction. Neurosci. Biobehav. Rev. 2020, 113, 157–168. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Rizzo, S.; Laudani, S.; Ieraci, A.; Drago, F.; Leggio, G.M. Acute stress alters recognition memory and AMPA/NMDA receptor subunits in a sex-dependent manner. Neurobiol. Stress 2023, 25, 100545. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.S.; Gobinath, A.R.; Galea, L.A.M. Sex differences in depression: Insights from clinical and preclinical studies. Prog. Neurobiol. 2019, 176, 86–102. [Google Scholar] [CrossRef]
- Labaka, A.; Goni-Balentziaga, O.; Lebena, A.; Perez-Tejada, J. Biological Sex Differences in Depression: A Systematic Review. Biol. Res. Nurs. 2018, 20, 383–392. [Google Scholar] [CrossRef]
- Jett, S.; Dyke, J.P.; Boneu Yepez, C.; Zarate, C.; Carlton, C.; Schelbaum, E.; Jang, G.; Pahlajani, S.; Williams, S.; Diaz Brinton, R.; et al. Effects of sex and APOE epsilon4 genotype on brain mitochondrial high-energy phosphates in midlife individuals at risk for Alzheimer’s disease: A 31Phosphorus MR spectroscopy study. PLoS ONE 2023, 18, e0281302. [Google Scholar] [CrossRef] [PubMed]
- Sauty, B.; Durrleman, S. Impact of sex and APOE-epsilon4 genotype on patterns of regional brain atrophy in Alzheimer’s disease and healthy aging. Front. Neurol. 2023, 14, 1161527. [Google Scholar] [CrossRef]
- Dawkins, E.; Small, D.H. Insights into the physiological function of the beta-amyloid precursor protein: Beyond Alzheimer’s disease. J. Neurochem. 2014, 129, 756–769. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Q.; Zhang, Y.W.; Xu, H. Proteolytic processing of Alzheimer’s beta-amyloid precursor protein. J. Neurochem. 2012, 120 (Suppl. 1), 9–21. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Choi, J.K.; Na, Y.J.; Jang, B.; Wasco, W.; Buxbaum, J.D.; Kim, Y.S.; Choi, E.K. Calsenilin regulates presenilin 1/gamma-secretase-mediated N-cadherin epsilon-cleavage and beta-catenin signaling. FASEB J. 2011, 25, 4174–4183. [Google Scholar] [CrossRef]
- Jin, J.K.; Choi, J.K.; Wasco, W.; Buxbaum, J.D.; Kozlowski, P.B.; Carp, R.I.; Kim, Y.S.; Choi, E.K. Expression of calsenilin in neurons and astrocytes in the Alzheimer’s disease brain. Neuroreport 2005, 16, 451–455. [Google Scholar] [CrossRef]
- Rivas, M.; Villar, D.; González, P.; Dopazo, X.M.; Mellstrom, B.; Naranjo, J.R. Building the DREAM interactome. Sci. China Life Sci. 2011, 54, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Lusin, J.D.; Vanarotti, M.; Dace, A.; Li, C.M.; Valiveti, A.; Ames, J.B. NMR structure of DREAM: Implications for Ca -dependent DNA binding and protein dimerization. Biochemistry 2008, 47, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, F.; Barski, J.J.; Farina, C.; Meyer, M. Mouse DREAM/Calsenilin/KChIP3: Gene structure, coding potential, and expression. Mol. Cell Neurosci. 2001, 17, 1–16. [Google Scholar] [CrossRef]
- Misiura, M.B.; Butts, B.; Hammerschlag, B.; Munkombwe, C.; Bird, A.; Fyffe, M.; Hemphill, A.; Dotson, V.M.; Wharton, W. Intersectionality in Alzheimer’s Disease: The Role of Female Sex and Black American Race in the Development and Prevalence of Alzheimer’s Disease. Neurotherapeutics 2023, 20, 1019–1036. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Tran, M.; Maki, P.M.; Bondi, M.W. Sex differences in the association between apolipoprotein E epsilon4 allele and Alzheimer’s disease markers. Alzheimers Dement. 2018, 10, 438–447. [Google Scholar] [CrossRef]
- Arnold, M.; Nho, K.; Kueider-Paisley, A.; Massaro, T.; Huynh, K.; Brauner, B.; MahmoudianDehkordi, S.; Louie, G.; Moseley, M.A.; Thompson, J.W.; et al. Sex and APOE epsilon4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun. 2020, 11, 1148. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Boueiz, A.; Abougergi, M.S.; Kitner-Triolo, M.H.; Beydoun, H.A.; Resnick, S.M.; O’Brien, R.; Zonderman, A.B. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol. Aging 2012, 33, 720–731.e4. [Google Scholar] [CrossRef]
- Gamache, J.; Yun, Y.; Chiba-Falek, O. Sex-dependent effect of APOE on Alzheimer’s disease and other age-related neurodegenerative disorders. Dis. Model. Mech. 2020, 13, dmm045211. [Google Scholar] [CrossRef]
- Hou, X.; Adeosun, S.O.; Zhang, Q.; Barlow, B.; Brents, M.; Zheng, B.; Wang, J. Differential contributions of ApoE4 and female sex to BACE1 activity and expression mediate Abeta deposition and learning and memory in mouse models of Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 207. [Google Scholar] [CrossRef]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. [Google Scholar] [CrossRef]
- Shinohara, M.; Murray, M.E.; Frank, R.D.; Shinohara, M.; DeTure, M.; Yamazaki, Y.; Tachibana, M.; Atagi, Y.; Davis, M.D.; Liu, C.C.; et al. Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. 2016, 132, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Tensil, M.; Hessler, J.B.; Gutsmiedl, M.; Riedl, L.; Grimmer, T.; Diehl-Schmid, J. Sex Differences in Neuropsychological Test Performance in Alzheimer’s Disease and the Influence of the ApoE Genotype. Alzheimer Dis. Assoc. Disord. 2018, 32, 145–149. [Google Scholar] [CrossRef]
- Toro, C.A.; Zhang, L.; Cao, J.; Cai, D. Sex differences in Alzheimer’s disease: Understanding the molecular impact. Brain Res. 2019, 1719, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, L.; Sikkes, S.A.M.; van den Hout, A.; Handels, R.; Bos, I.; van der Flier, W.M.; Kern, S.; Ousset, P.J.; Maruff, P.; Skoog, I.; et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019, 15, 888–898. [Google Scholar] [CrossRef]
- Zhao, N.; Ren, Y.; Yamazaki, Y.; Qiao, W.; Li, F.; Felton, L.M.; Mahmoudiandehkordi, S.; Kueider-Paisley, A.; Sonoustoun, B.; Arnold, M.; et al. Alzheimer’s Risk Factors Age, APOE Genotype, and Sex Drive Distinct Molecular Pathways. Neuron 2020, 106, 727–742.e726. [Google Scholar] [CrossRef]
- Puertas, M.C.; Martinez-Martos, J.M.; Cobo, M.P.; Carrera, M.P.; Mayas, M.D.; Ramirez-Exposito, M.J. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp. Gerontol. 2012, 47, 625–630. [Google Scholar] [CrossRef]
- Ayaz, M.; Mosa, O.F.; Nawaz, A.; Hamdoon, A.A.E.; Elkhalifa, M.E.M.; Sadiq, A.; Ullah, F.; Ahmed, A.; Kabra, A.; Khan, H.; et al. Neuroprotective potentials of Lead phytochemicals against Alzheimer’s disease with focus on oxidative stress-mediated signaling pathways: Pharmacokinetic challenges, target specificity, clinical trials and future perspectives. Phytomedicine 2024, 124, 155272. [Google Scholar] [CrossRef]
- Barth, C.; Crestol, A.; de Lange, A.G.; Galea, L.A.M. Sex steroids and the female brain across the lifespan: Insights into risk of depression and Alzheimer’s disease. Lancet Diabetes Endocrinol. 2023, 11, 926–941. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Carroll, J.C.; Morgan, T.E.; Lin, S.; Zhao, L.; Arimoto, J.M.; Murphy, M.P.; Beckett, T.L.; Finch, C.E.; Brinton, R.D.; et al. 17beta-estradiol and progesterone regulate expression of beta-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology 2012, 153, 5467–5479. [Google Scholar] [CrossRef]
- Pike, C.J. Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 671–680. [Google Scholar] [CrossRef]
- Poling, M.C.; Kauffman, A.S. Organizational and activational effects of sex steroids on kisspeptin neuron development. Front. Neuroendocrinol. 2013, 34, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Rosario, E.R.; Pike, C.J. Androgen regulation of beta-amyloid protein and the risk of Alzheimer’s disease. Brain Res. Rev. 2008, 57, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Tublin, J.M.; Adelstein, J.M.; Del Monte, F.; Combs, C.K.; Wold, L.E. Getting to the Heart of Alzheimer Disease. Circ. Res. 2019, 124, 142–149. [Google Scholar] [CrossRef]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Guerrero-González, C.; Martínez-Martos, J.M. Alzheimer’s disease: Is there a relationship between brain renin-angiotensin system, estradiol and glucose transporter-4 (GLUT-4)? AIMS Mol. Sci. 2023, 10, 37–51. [Google Scholar] [CrossRef]
- Puertas Mdel, C.; Martinez-Martos, J.M.; Cobo, M.; Lorite, P.; Sandalio, R.M.; Palomeque, T.; Torres, M.I.; Carrera-Gonzalez, M.P.; Mayas, M.D.; Ramirez-Exposito, M.J. Plasma renin-angiotensin system-regulating aminopeptidase activities are modified in early stage Alzheimer’s disease and show gender differences but are not related to apolipoprotein E genotype. Exp. Gerontol. 2013, 48, 557–564. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R.; Martinez-Dominguez, S.J.; Lajusticia, H.; Chedraui, P.; Health Outcomes Systematic Analyses, P. Effects of programmed exercise on depressive symptoms in midlife and older women: A meta-analysis of randomized controlled trials. Maturitas 2017, 106, 38–47. [Google Scholar] [CrossRef]
- Ravindran, A.V.; Balneaves, L.G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R.L.; Ravindran, L.; Yatham, L.N.; Kennedy, S.H.; Lam, R.W.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Focus 2018, 16, 85–94. [Google Scholar] [CrossRef]
- Byrnes, K.; Wu, P.J.; Whillier, S. Is Pilates an effective rehabilitation tool? A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, C. Research on exercise fatigue estimation method of Pilates rehabilitation based on ECG and sEMG feature fusion. BMC Med. Inform. Decis. Mak. 2022, 22, 67. [Google Scholar] [CrossRef]
- Pinheiro, É.P.; do Espirito Santo, R.C.; Dos Santos, L.P.; Goncalves, W.V.; Júnior, L.A.F.; Xavier, R.M.; Filippin, L.I. Multicomponent or Resistance Training for Nursing Home Residents: A Systematic Review with Meta-Analysis. J. Am. Med. Dir. Assoc. 2022, 23, 1926.e1–1926.e10. [Google Scholar] [CrossRef]
- Li, L.; Liu, M.; Zeng, H.; Pan, L. Multi-component exercise training improves the physical and cognitive function of the elderly with mild cognitive impairment: A six-month randomized controlled trial. Ann. Palliat. Med. 2021, 10, 8919–8929. [Google Scholar] [CrossRef]
- Herold, F.; Torpel, A.; Schega, L.; Muller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, X.; Li, B.; Cai, Y.; Zhang, S.; Wan, Q.; Yu, F. Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: A systematic review and network meta-analysis. J. Sports Health Sci. 2022, 11, 212–223. [Google Scholar] [CrossRef]
- Etgen, T.; Sander, D.; Bickel, H.; Forstl, H. Mild cognitive impairment and dementia: The importance of modifiable risk factors. Dtsch. Arztebl. Int. 2011, 108, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef]
- Kirshner, D.; Spiegelhalder, K.; Shahar, R.T.; Shochat, T.; Agmon, M. The association between objective measurements of sleep quality and postural control in adults: A systematic review. Sleep Med. Rev. 2022, 63, 101633. [Google Scholar] [CrossRef]
- Bergamin, M.; Gobbo, S.; Bullo, V.; Zanotto, T.; Vendramin, B.; Duregon, F.; Cugusi, L.; Camozzi, V.; Zaccaria, M.; Neunhaeuserer, D.; et al. Effects of a Pilates exercise program on muscle strength, postural control and body composition: Results from a pilot study in a group of post-menopausal women. Age 2015, 37, 118. [Google Scholar] [CrossRef]
- Di Lorenzo, C.E. Pilates: What is it? Should it be used in rehabilitation? Sports Health 2011, 3, 352–361. [Google Scholar] [CrossRef]
- Ben-Zeev, T.; Hirsh, T.; Weiss, I.; Gornstein, M.; Okun, E. The Effects of High-intensity Functional Training (HIFT) on Spatial Learning, Visual Pattern Separation and Attention Span in Adolescents. Front. Behav. Neurosci. 2020, 14, 577390. [Google Scholar] [CrossRef]
- Ben-Zeev, T.; Okun, E. High-Intensity Functional Training: Molecular Mechanisms and Benefits. Neuromol. Med. 2021, 23, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.; Knapp, K.; Lackie, A.; Lewry, C.; Horvey, K.; Benko, C.; Trinh, J.; Butcher, S. Multimodal high-intensity interval training increases muscle function and metabolic performance in females. Appl. Physiol. Nutr. Metab. 2015, 40, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef]
- Fisher, J.; Sales, A.; Carlson, L.; Steele, J. A comparison of the motivational factors between CrossFit participants and other resistance exercise modalities: A pilot study. J. Sports Med. Phys. Fitness 2017, 57, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Heinrich, K.M.; Patel, P.M.; O’Neal, J.L.; Heinrich, B.S. High-intensity compared to moderate-intensity training for exercise initiation, enjoyment, adherence, and intentions: An intervention study. BMC Public Health 2014, 14, 789. [Google Scholar] [CrossRef]
- Jimenez-Garcia, J.D.; Martinez-Amat, A.; De la Torre-Cruz, M.J.; Fabrega-Cuadros, R.; Cruz-Diaz, D.; Aibar-Almazan, A.; Achalandabaso-Ochoa, A.; Hita-Contreras, F. Suspension Training HIIT Improves Gait Speed, Strength and Quality of Life in Older Adults. Int. J. Sports Med. 2019, 40, 116–124. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Williamson, C.; Bechke, E.; McKenzie, M.; Hoffstetter, W. Autonomic response to a short and long bout of high-intensity functional training. J. Sports Sci. 2018, 36, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Klemp, A.; Dolan, C.; Maharaj, A.; Huang, C.J.; Khamoui, A.V.; Trexler, E.T.; Whitehurst, M.; Zourdos, M.C. Impact of resistance training program configuration on the circulating brain-derived neurotrophic factor response. Appl. Physiol. Nutr. Metab. 2020, 45, 667–674. [Google Scholar] [CrossRef]
- Wilke, J. Functional high-intensity exercise is more effective in acutely increasing working memory than aerobic walking: An exploratory randomized, controlled trial. Sci. Rep. 2020, 10, 12335. [Google Scholar] [CrossRef] [PubMed]
- Hita-Contreras, F.; Martinez-Amat, A.; Cruz-Diaz, D.; Perez-Lopez, F.R. Fall prevention in postmenopausal women: The role of Pilates exercise training. Climacteric 2016, 19, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Harmer, P.; Fisher, K.J.; McAuley, E.; Chaumeton, N.; Eckstrom, E.; Wilson, N.L. Tai Chi and fall reductions in older adults: A randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C.; American College of Sports, M.; American Heart, A. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1094–1105. [Google Scholar] [CrossRef]
- de Oliveira, R.D.J.; de Oliveira, R.G.; de Oliveira, L.C.; Santos-Filho, S.D.; Sa-Caputo, D.C.; Bernardo-Filho, M. Effectiveness of whole-body vibration on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2023, 34, 29–52. [Google Scholar] [CrossRef]
- Figueroa, A.; Kalfon, R.; Madzima, T.A.; Wong, A. Whole-body vibration exercise training reduces arterial stiffness in postmenopausal women with prehypertension and hypertension. Menopause 2014, 21, 131–136. [Google Scholar] [CrossRef]
- Guedes De Aguiar, E.O.; Moreira Marconi, E.; Monteiro-Oliveira, B.B.; Gomes-Santos, A.C.; Coelho Oliveira, A.C.; Paineiras-Domingos, L.L.; Sa-Caputo, D.C.; Bernardo Filho, M. Whole-Body Vibration Exercise Improves the Functionality in Postmenopausal Women: A Systematic Review. Iran. J. Public Health 2023, 52, 476–487. [Google Scholar] [CrossRef]
- Marin-Cascales, E.; Alcaraz, P.E.; Ramos-Campo, D.J.; Martinez-Rodriguez, A.; Chung, L.H.; Rubio-Arias, J.A. Whole-body vibration training and bone health in postmenopausal women: A systematic review and meta-analysis. Medicine 2018, 97, e11918. [Google Scholar] [CrossRef]
- Matsubara, T.; Miyaki, A.; Akazawa, N.; Choi, Y.; Ra, S.G.; Tanahashi, K.; Kumagai, H.; Oikawa, S.; Maeda, S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H348–H355. [Google Scholar] [CrossRef] [PubMed]
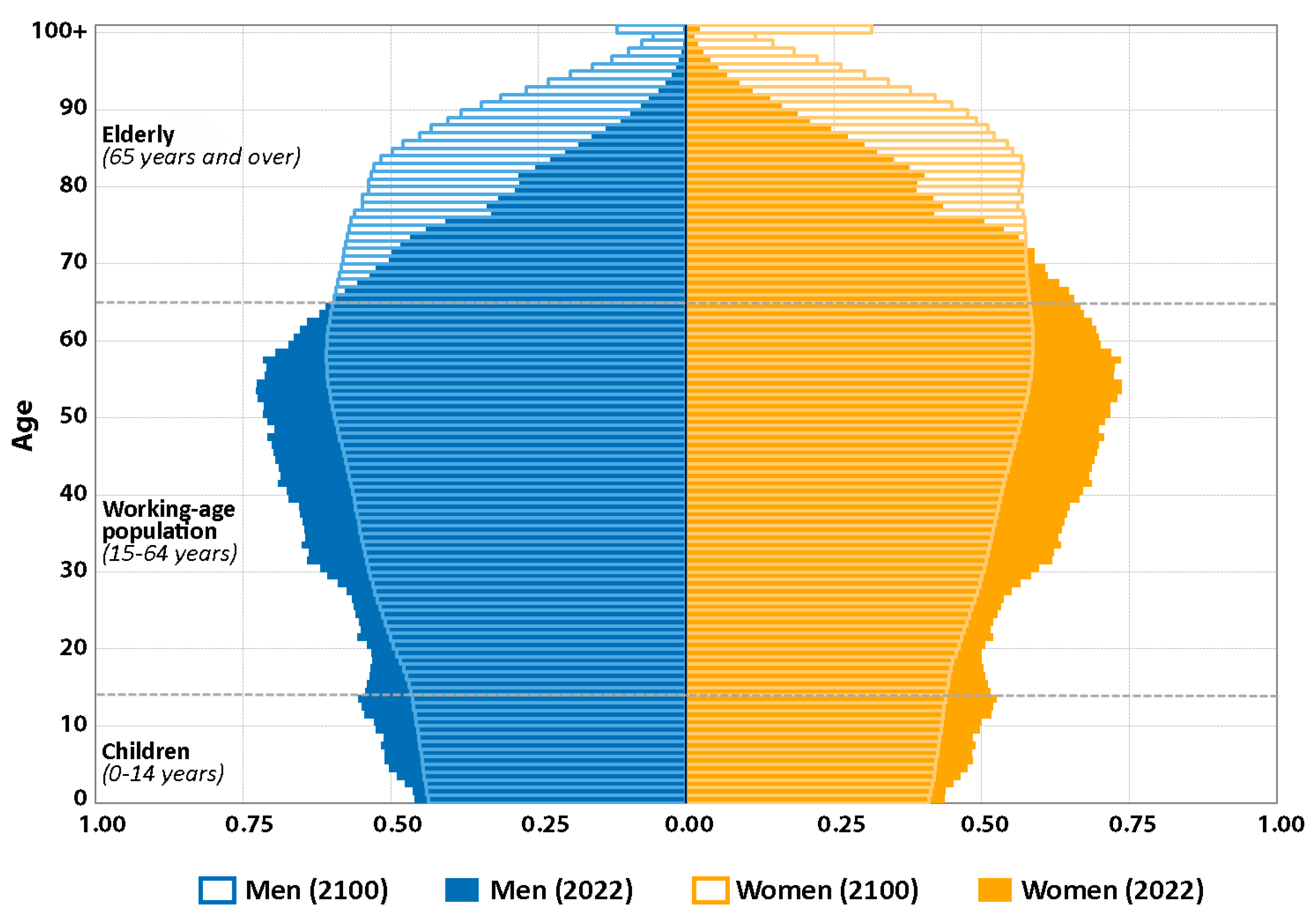
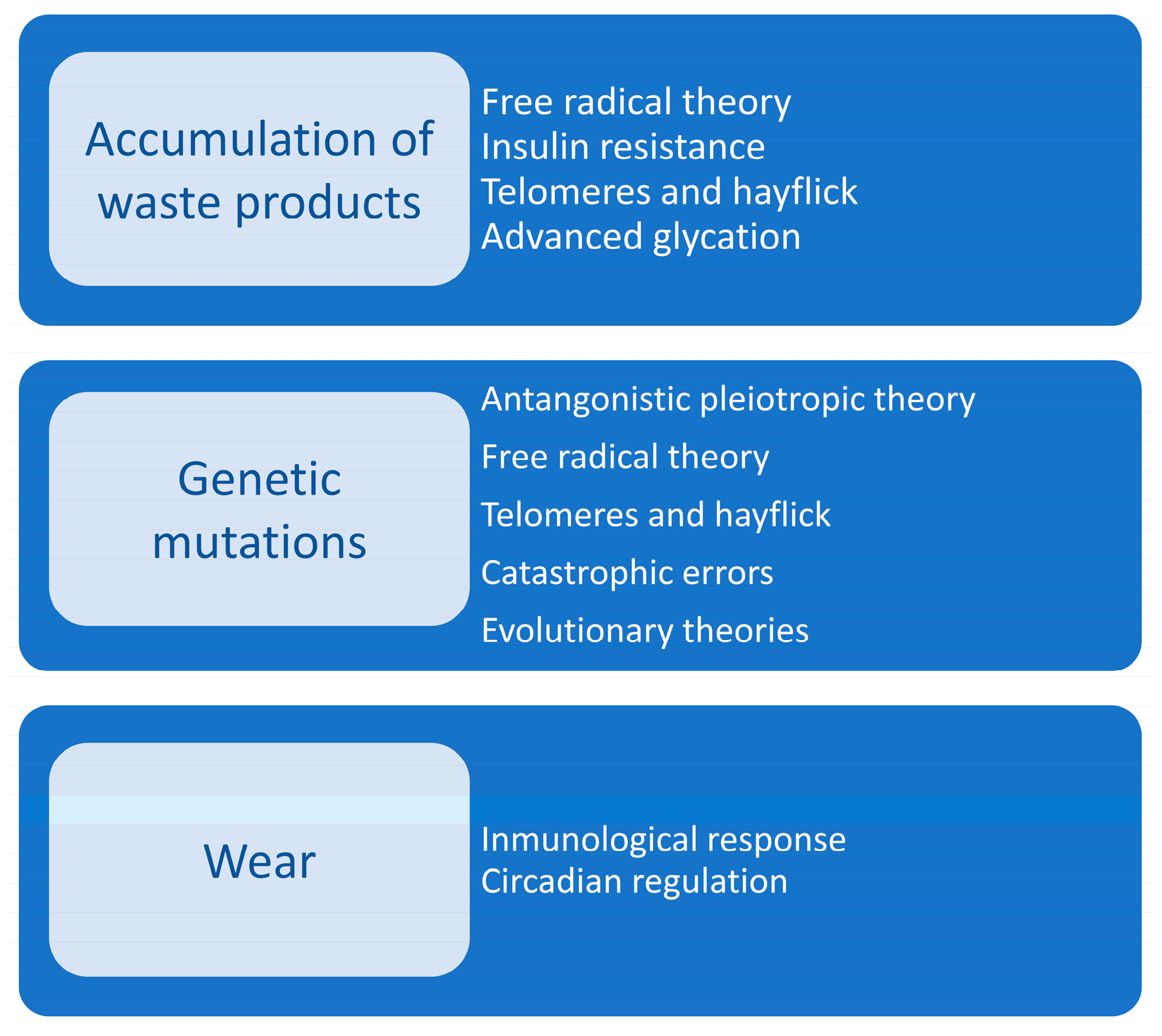
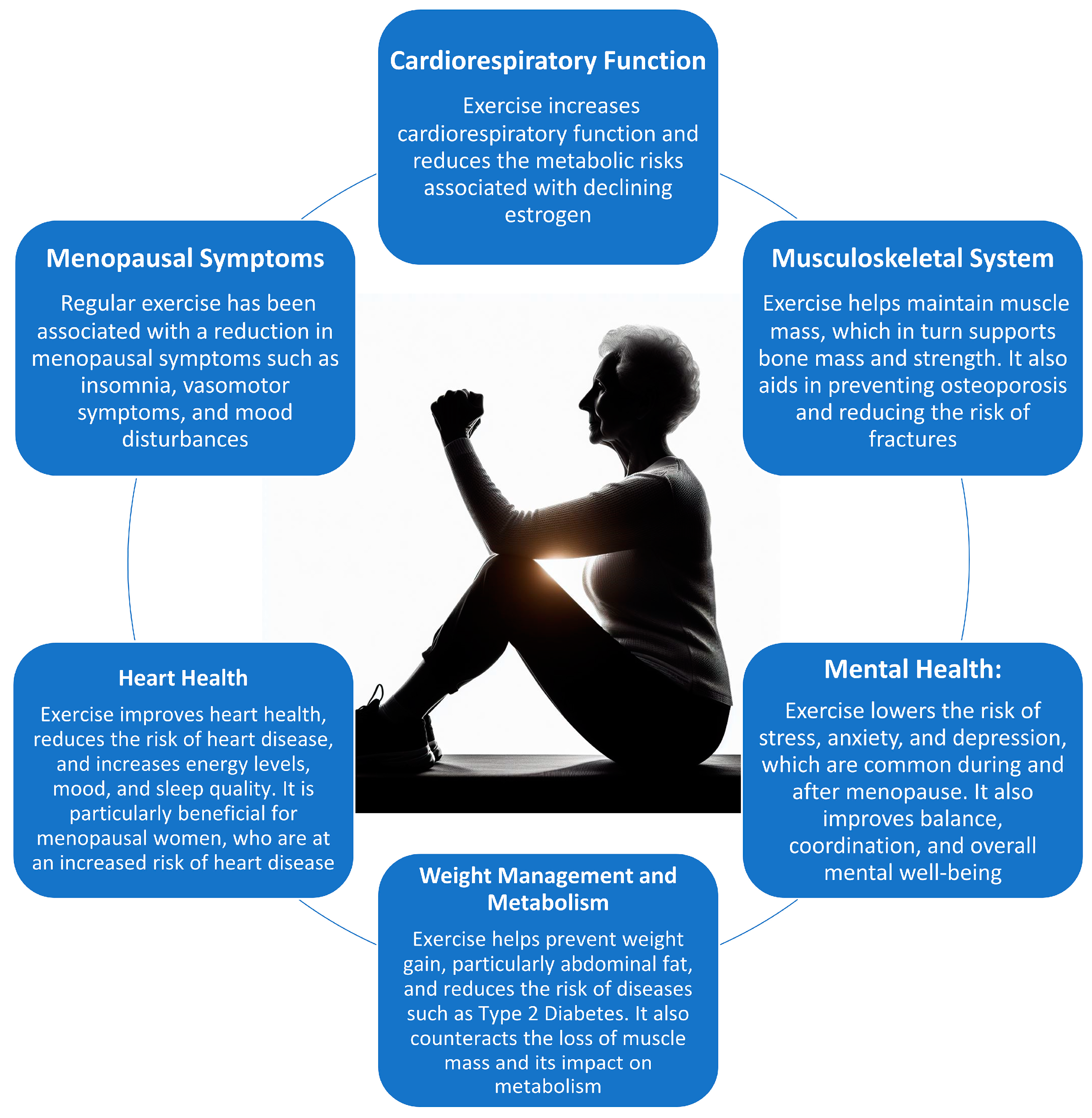
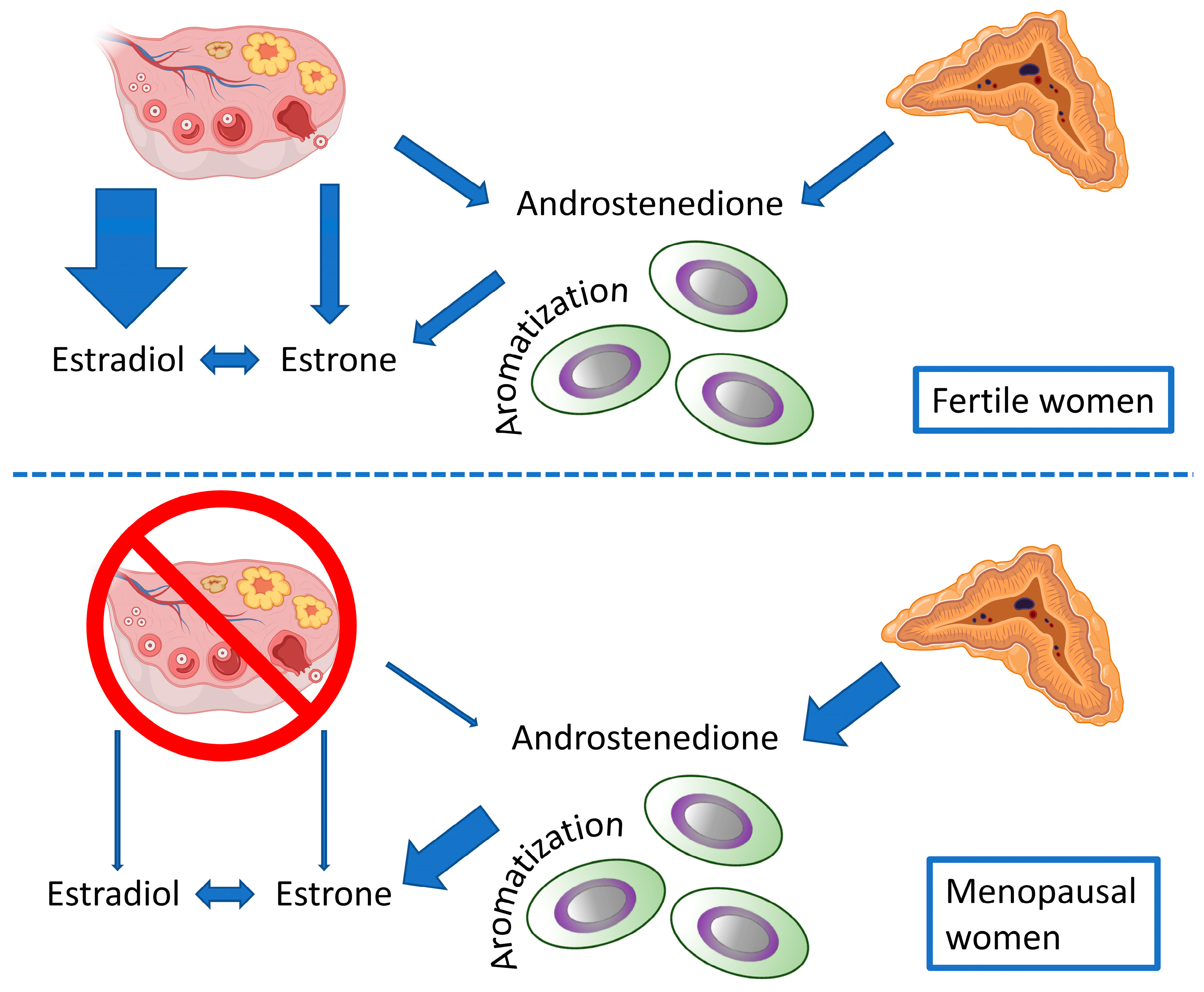
| Physiological System | Functional Changes | Morphological Changes |
|---|---|---|
| Glucose metabolism | -Increased insulin resistance and predisposition to diabetes. -Increased production of adipokines and inflammatory factors. | -Lower β-pancreatic cell mass. -Fatty infiltration of tissues. -Increased visceral fat. |
| Endocrine | -Decrease in growth hormone (GH). -Decreased sensitivity to hormones. | -Decreases cell size, weight and number of cells. -Increases fibrous content. -Decreases the number and/or affinity of receptors. |
| Renal | -Lower hydroxylation of vitamin D. -Lower levels of renin and aldosterone. -Reduced ability to concentrate urine. | -Thickening of glomerular basement membrane. -Sclerosis of glomerular arteries, due to the formation of atheromatous plaques at this level. -Thinning renal cortex and decreased nephrons number. |
| Locomotor | -Bone fragility due to loss of muscle mass. -Decrease in strength. | -Morphological changes in the bone system: osteoporosis. -Fatty infiltration of the muscle. -Loss of muscle mass. |
| Cardiovascular | -Increased risk of arrhythmias. -Endothelial dysfunction. -Preservation of ejection fraction. -Vascular and cardiac stiffness. | -Decreased cardiomyocytes and increased extracellular matrix. -Cardiac hypertrophy with thickening of the septum and especially of the left ventricle. -Loss of elastin fibers. -Increase of collagenous matrix in tunica media. |
| Central nervous system | -Reduced motor skills. -Decreased recent memory. -Slower processing speed. -Proprioceptive sensitivity is altered. | -Minimal neuronal loss, focal. -Increased cerebrospinal fluid. -Lower brain mass: decrease in volume and weight. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-González, C.; Cueto-Ureña, C.; Cantón-Habas, V.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Healthy Aging in Menopause: Prevention of Cognitive Decline, Depression and Dementia through Physical Exercise. Physiologia 2024, 4, 115-138. https://doi.org/10.3390/physiologia4010007
Guerrero-González C, Cueto-Ureña C, Cantón-Habas V, Ramírez-Expósito MJ, Martínez-Martos JM. Healthy Aging in Menopause: Prevention of Cognitive Decline, Depression and Dementia through Physical Exercise. Physiologia. 2024; 4(1):115-138. https://doi.org/10.3390/physiologia4010007
Chicago/Turabian StyleGuerrero-González, Carmen, Cristina Cueto-Ureña, Vanesa Cantón-Habas, María Jesús Ramírez-Expósito, and José Manuel Martínez-Martos. 2024. "Healthy Aging in Menopause: Prevention of Cognitive Decline, Depression and Dementia through Physical Exercise" Physiologia 4, no. 1: 115-138. https://doi.org/10.3390/physiologia4010007
APA StyleGuerrero-González, C., Cueto-Ureña, C., Cantón-Habas, V., Ramírez-Expósito, M. J., & Martínez-Martos, J. M. (2024). Healthy Aging in Menopause: Prevention of Cognitive Decline, Depression and Dementia through Physical Exercise. Physiologia, 4(1), 115-138. https://doi.org/10.3390/physiologia4010007








