Abstract
The objective of this work was to evaluate the effects of three commercial diets for ornamental fish (BIOMAA Spiruflake® (T1), Tetra TetraMin Tropical Flakes® (T2), LOMAS tropical fish flakes® (T3)) compared to a balanced feed for trout (Silver Cup TM (T4)) on the growth, survival, digestive enzyme activity, and intestinal and liver morphology of guppy (Poecilia reticulata) fry after 31 days of feeding. The highest growth was in T2 (0.101 ± 0.004 g) and T4 (0.084 ± 0.008 g) (p < 0.05). Fish fed with T1, T2, and T4 presented the greatest total length. T4 (98 ± 2.828%) and T1 (96 ± 5.656%) had the highest survival. Fish fed with T2 and T4 showed the best growth index values (AWG, SGR, FCR). T4 showed greater alkaline protease, trypsin, and chymotrypsin activity and better in vitro digestibility. The fish fed the T4 diet had the lowest intestinal fold height and the smallest hepatocyte diameter. A cheaper aquaculture diet (T4) significantly improved guppy juvenile nutrition compared to ornamental feeds. Further research should focus on developing species-specific ornamental diets for enhanced fish welfare.
1. Introduction
The term “ornamental fish” involves many aquatic species kept in aquariums or ponds for aesthetic and decorative purposes [1]. Their diversity in size, shape, coloration, and behavior makes them attractive and appreciated by aquarists [2]. Unlike fish intended for consumption or sport fishing, ornamental fish are valued primarily for their beauty and ability to enrich the environment. Guppies (Poecilia reticulata) are fish native to South and Central America, but they have been introduced worldwide due to their attractive appearance and easy handling, cultivation, and rapid development [3]. These characteristics make them stand out as one of the most popular ornamental fish worldwide. In addition, guppies are known for their varied range of colors, making them an ideal choice in the aquarium [4]. On the other hand, these organisms have also been used as model organisms in research [5,6], which makes them of great commercial and scientific importance.
The feed supply for ornamental fish is mainly based on diets formulated for various species; in other words, they are not specific, so their nutritional composition may be incorrect. However, nutrition is essential for good animal growth and well-being. Therefore, the nutritional requirements of each species must be studied. In this sense, the food industry has manufactured commercial feed for aquarium fish using different technologies for its production, such as pelleting, extrusion, and lamination [7,8,9]. It is important to emphasize that nutritionally, fish require high percentages of protein to replenish tissues and to synthesize enzymes, hormones, or somatic tissue [10]. Likewise, amino acids play an important role as a metabolic source of energy, functioning in chemical reactions, lipid metabolism, and protein and vitamin synthesis [11]. Most fish require vitamin supplements. However, the amount and type of amino acids depend on several aspects, such as the species, stage of development, and environmental factors, among others [12]. In the case of ornamental fish, they need a balanced diet that includes proteins, lipids, carbohydrates, vitamins, and minerals; nevertheless, each species has a specific requirement, which must be determined, so studies on their digestive capacity are required [10,13]. In this regard, it has been reported that the protein requirement for P. reticulata is 30–40% [14,15]. However, they are commonly fed with general formulations for tropical species, so many times, ornamental fish have a poor diet, which can cause intestinal diseases, nutritional deficiency, and generate diseases in the organism. The situation is very different in aquaculture species (e.g., trout and tilapia), where commercial diets are formulated under specific requirements and the age of the species. This situation determines the debate of this work.
Therefore, the objective of this study was to evaluate the effect of four commercial diets in P. reticulata juveniles on growth, survival rate, digestive enzyme activity, in vitro digestibility, and digestive morphology to determine whether commercial feeds formulated for ornamental fish and aquaculture cover their nutritional requirements.
2. Materials and Methods
2.1. Obtaining Organisms
A total of 300 juveniles of P. reticulata were used, with an average weight of 0.006 ± 0.001 g and 0.325 ± 0.014 cm in total length. They were obtained from the Laboratorio de Fisiología en Recursos Acuáticos (LAFIRA) in División Académica de Ciencias Biológicas de la Universidad Juárez Autónoma de Tabasco, Villahermosa, Mexico. The parental population consisted of 90 females and 30 males (ratio 1:3). Breeding took place in a 500 L community tank connected to a 1000 L recirculating biological filter system with controlled parameters. All juveniles were collected within a two-day window of birth to ensure age uniformity.
2.2. Experimental Diets
Based on the availability of commercial diets in the region, three brands used for feeding ornamental fish were selected: BIOMAA Spiruflake® (Mascotas y Acuariofilia S.A. de C.V., Jilotzingo, Mexico) (T1), Tetra TetraMin Tropical Flakes® (Tetra GmbH, Melle, Germany) (T2), and LOMAS Tropical Fish Flakes® (Tetra GmbH, Melle, Germany) (T3), as well as a balanced trout feed Silver Cup TM (Alimentos de Alta Calidad El Pedregal, S.A. de C.V., Toluca, México) (T4) (Table 1).

Table 1.
Nutrient analysis of different commercial diets.
2.3. Experimental Design
For the experiment, 25 juveniles were stocked per experimental unit (per triplicate), which were distributed in fiberglass tanks with a capacity of 8 L, connected to a recirculation system by a 0.5 HP water pump (Jacuzzi, JWPA5D-230A, Delavan, WI, USA) and to an 800 L water reservoir with a biological filter. The organisms were fed to satiety thrice daily (9:00, 13:00, and 17:00) for 31 days. After each feeding, a partial water exchange (30%) was performed using the siphoning technique. The physicochemical parameters were monitored daily, obtaining an average temperature of 28.4 ± 0.8 °C, a dissolved oxygen level of 5.8 ± 0.3 mg mg/L (YSI 85, Yellow Springs, OH, USA), and a pH of 7.9 ± 0.3, which was quantified with a potentiometer (HANNA HI 991001, Woonsocket, RI, USA).
2.4. Evaluation of Growth and Survival Rates
Biometry was performed at the beginning of the experiment, and on days 15 and 30 to determine the individual wet weight using an analytical balance (Ohaus mod. CS2000, Shanghai, China), and the total length was obtained by photographing the organisms using ImageJ 1.51j8 software (U.S. National Institutes of Health, Bethesda, MD, USA). At the end of the experiment, the following production parameters were determined: survival (S): (final number of fish/initial number of fish) × 100; feed intake (FI): total feed intake per experimental unit/number of days of rearing; absolute weight gain (AWG): final weight (g) − initial weight (g); specific growth rate (SGR): [(log of final weight − log of initial weight)/days] × 100; feed conversion rate (FCR): (feed intake, g of dry matter)/(weight gain of fish (g)); and condition factor (K): [wet weight (g) × total length − 3 (cm)] × 100.
2.5. Sample Collection
At the end of the experiment, nine fish per replicate were sacrificed by thermal shock after being placed in clove oil (1 mL·L−1). Due to the size of the organisms (<0.15 g), the euthanasia process was almost immediate (<5 s). To determine the enzymatic activity, digestibility, and histological variations, three fish per each replicate were used. The fish were dissected, and the intestine and stomach were obtained to quantify digestive enzyme activity and in vitro digestibility with the pH Stat equipment. The organ samples were kept at a temperature of −80 °C until use. On the other hand, for histological analysis, the whole fish were preserved in Davidson solution at room temperature until analysis. This study was conducted under the Declaration of Helsinki and the protocol authorized by the Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA), Mexico, NOM-062-Z00-1999 [16], and this experiment had the approval (UJAT-CIEI-2025-059) of the Institutional Commission of Ethics in Research of the División Académica de Ciencias Biológicas de la Universidad Juárez Autónoma de Tabasco, Mexico, guaranteeing adequate handling and treatment of the organisms following the ethical guidelines for research with animals.
2.6. Quantification of Digestive Enzyme Activity
Stomach and intestine samples from each biological replicate were cold macerated with 100 mM glycine–HCl pH 2 buffer for stomach samples, and 50 mM Tris-HCl pH 7.5 was used as a buffer solution for intestine samples. All samples were centrifuged at 16,000× g at 4 °C for 15 min. Supernatants were stored at −80 °C. Soluble protein was quantified using the Bradford technique [17]. Anson’s method [18] determined acid protease activity in stomach samples using 0.5% hemoglobin as a substrate solubilized in 100 mM glycine-HCl, pH 2, and measured at 280 nm. Alkaline protease activity was quantified in intestinal samples using 0.5% casein as substrate and solubilized in 50 mM Tris-HCl and 10 mM CaCl2 at pH 9 [19]. Samples were incubated at 37 °C for both techniques, and absorbance was measured at 280 nm. Trypsin activity was determined according to the method of Erlanger, Kokowsky, and Cohen [20] using 1 mM BAPNA (Nα-benzoyl-DL arginine-P-nitroanilide) as a substrate, dissolved in 50 mM Tris HCl and 10 mM CaCl2 at pH 8.2, and quantified at 410 nm. Chymotrypsin was determined using 5 mM BTEE (N-benzoyl-L-tyrosine ethyl ester) as a substrate solubilized in 44.4 mM Tris-HCl, 55.5 mM CaCl2, pH 7.8 at 256 nm absorbance [21].
2.7. In Vitro Digestibility Analysis
For the analysis of the degree of hydrolysis (pH Stat technique), an enzyme extract was prepared from stomach and intestinal tissues, respectively, homogenized in distilled water (5:1, water: wet mass) with a tissue disperser (ULTRA TURRAX® IKA T18 Basic, IKA; Staufen, Germany). The samples were centrifuged at 16,000× g for 15 min at 4 °C, and the supernatant was adjusted to pH 3.5 for acid digestibility and pH 8.0 for alkaline digestibility. The samples were stored at −80 °C until use. The degree of hydrolysis was determined using 5 mL of an aqueous solution containing each feedstuff per mL, pH 3.5, for the acid phase [22]. In the pH Stat titration system (Titrino 918, Methrom, Herisau, Switzerland), 50·U mL−1 of protease activity from the crude stomach extract was added to an aqueous solution, keeping the mixture under continuous stirring at 37 °C for 15 min. For the alkaline phase, the pH of the aqueous solution was adjusted to 8 with NaOH (0.1 N). Then, 50 U·mL−1 of crude intestine extract was added to the pH system, keeping it under continuous stirring for 45 min at 37 °C. The amount of hydrochloric acid (HCl, 0.1 N) and sodium hydroxide (NaOH, 0.1 N) used was recorded in both cases. The degree of hydrolysis (DH) was calculated from the volume of HCl needed to maintain the pH at 3.5 or the volume of NaOH required to keep the pH at 8.0 after digestion.
2.8. Histological Analysis
Whole organisms were fixed in Davidson solution and then dehydrated with an alcohol gradient (70%, 80%, 90%, 96%, and 100%), ending with paraffin embedding. Samples were then cut at a thickness of 7 µm and stained with hematoxylin and eosin (H&E). Photographs were taken with an Axiocam ERc 5s camera (Carl Zeiss Microscopy GmbH, Jena, Germany) connected to a Zeiss Primo Star optical microscope (Primo Star, Suzhou, China). The diameter of hepatocytes (µm) in the liver was measured. In the intestine, the height of the enterocytes (µm) and the length of the folds (µm) were measured and analyzed using ZEN 2.3 Lite software (Carl Zeiss AG). Each parameter was quantified at least 30 times for each fish.
2.9. Statistical Analysis
Growth parameters and enzymatic activity data were analyzed using normality tests (Kolmogorov–Smirnov) and homoscedasticity (Bartlett). The ANOVA test was used, followed by a Tukey’s post hoc test. All values were analyzed using Prism GraphPad V. 9.0 software, with a significance value of 0.05.
3. Results
3.1. Growth and Survival Rates
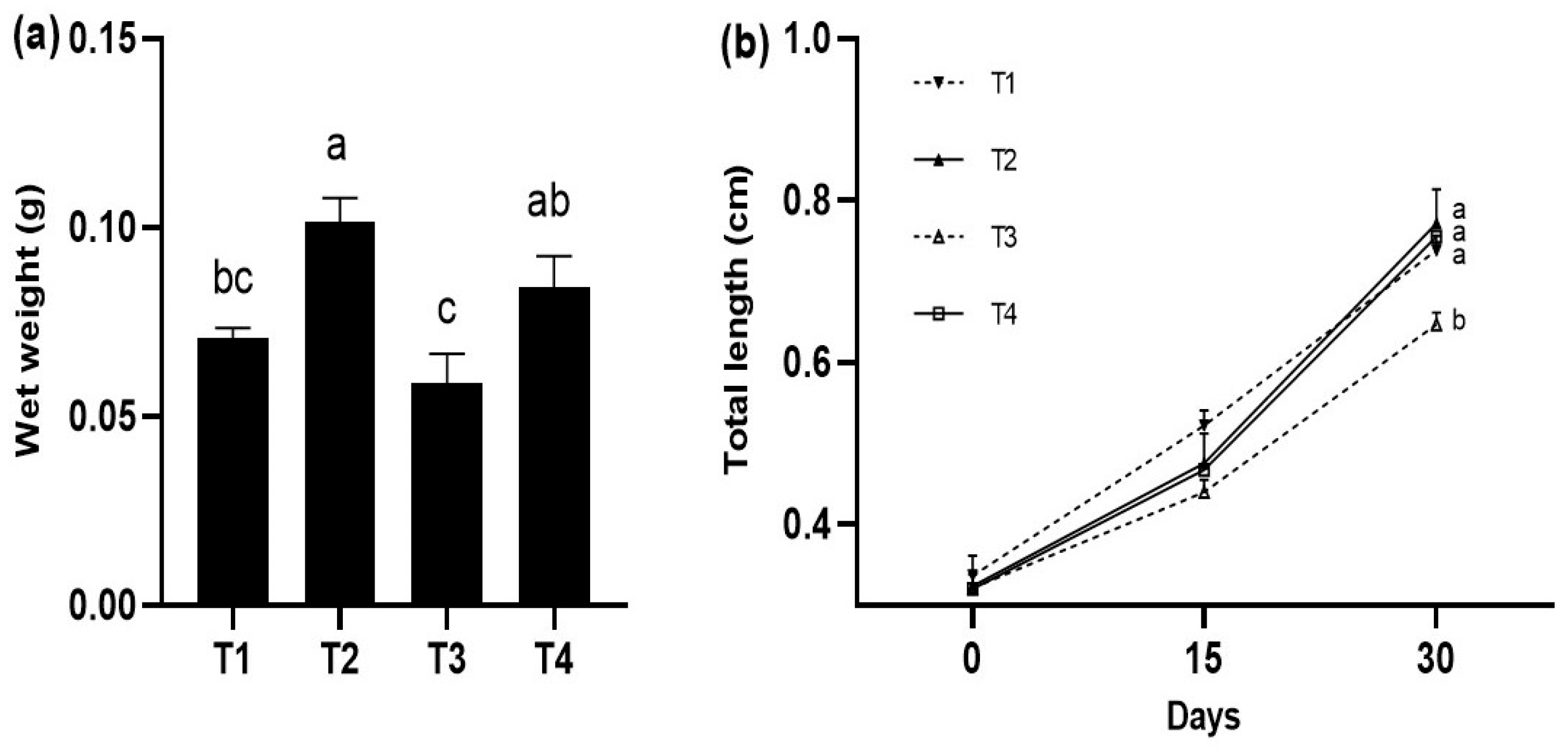
At the end of the experiment, the fish with the highest growth were those fed with the T2 treatment (0.101 ± 0.004 g), followed by the fish fed with the T4 diet without significant differences (p > 0.05) between them; however, the fish fed with the T2 diet showed significant differences with the rest of the treatments (p < 0.05) (Figure 1a). The greatest total length was quantified in the fish fed with the T1, T2, and T4 diets (0.783 ± 0.062 cm, 0.771 ± 0.042 cm, and 0.755 ± 0.004 cm, respectively), which were significantly larger (p < 0.05) compared to the T3 (Figure 1b).
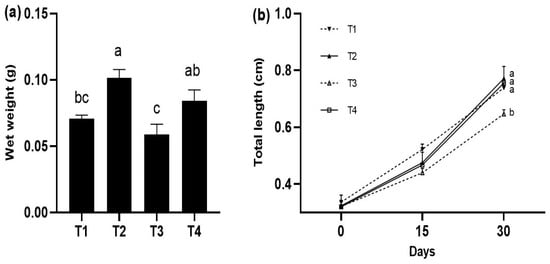
Figure 1.
Growth in weight (g) and total length (cm) (mean ± standard deviation, SD) in juveniles of P. reticulata fed different commercial diets. (a) wet weight in P. reticulata juveniles at the end of the experiment. (b) total length of P. reticulata juveniles at the end of the experiment. Significant differences between treatments are indicated by different letters (p < 0.05).
Guppies in T2 and T4 obtained the highest values in AWG and SGR without showing differences (p > 0.05). The highest survival was obtained in fish fed with the T4 (98.0 ± 2.8%) and T1 (96.0 ± 5.7%) diets, showing significant differences with fish fed the T2 diet (p < 0.05). The highest FCR was obtained in T3 (3.3 ± 0.4 g), which showed significant differences (p < 0.05) with fish fed the T2 and T4 diets, respectively. The treatment with the highest K value was presented in T3 (23.31 ± 0.05 g/cm), which showed significant differences (p < 0.05) compared to the rest of the treatments. Finally, FI values showed no significant difference between treatments (p > 0.05) (Table 2).

Table 2.
Productive performance indices (mean ± standard deviation, SD) in juvenile guppies (Poecilia reticulata) fed different commercial diets.
3.2. Digestive Enzyme Activity
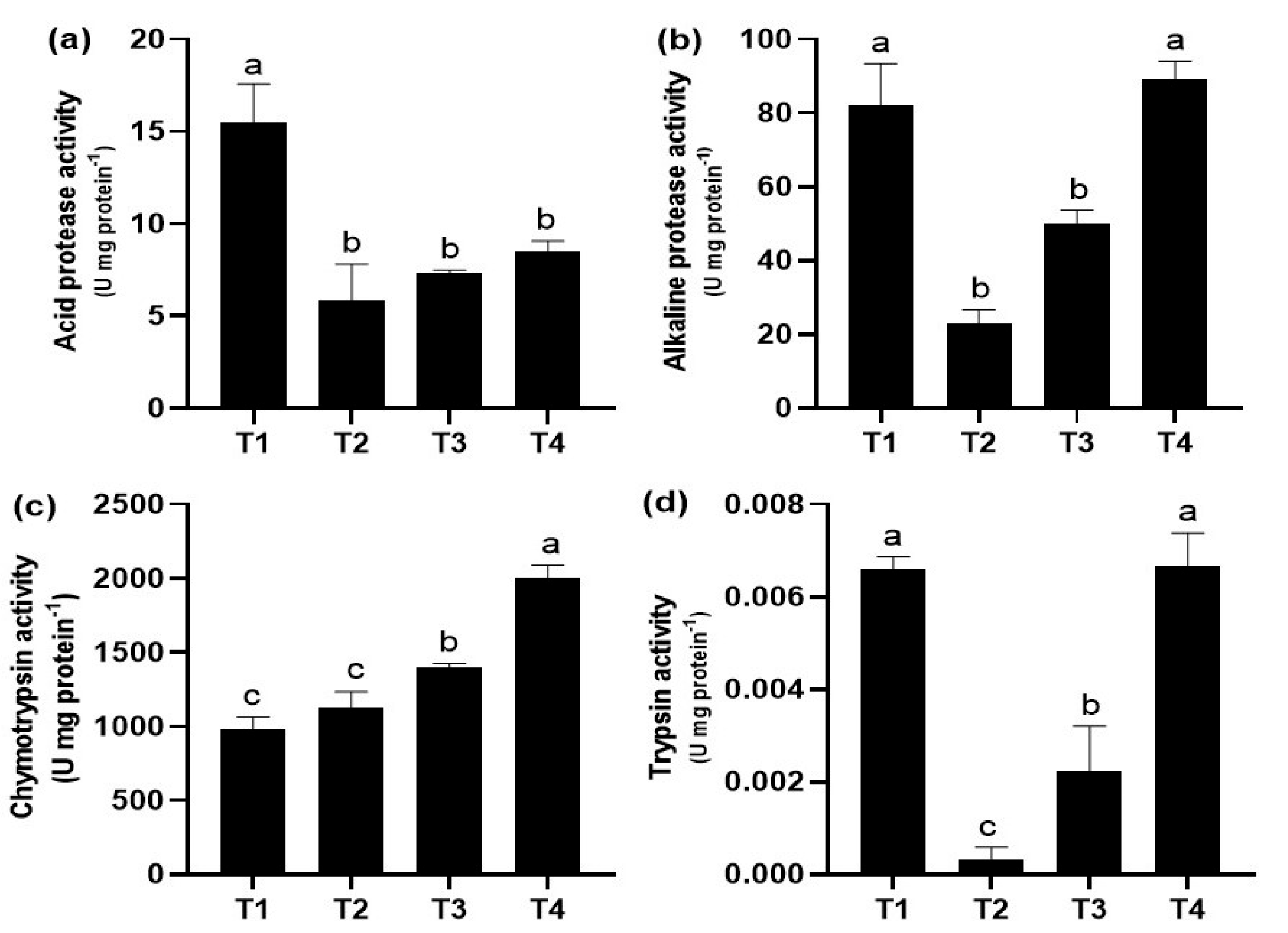
The effect of the commercial diets on digestive enzyme activity in juvenile guppies is shown in Figure 2. Fish fed the T1 diet had the highest acid protease activity (p < 0.05), which was significantly different from the rest of the treatments (Figure 2a). The highest alkaline protease and trypsin activity was present in T4 and T1, which were statistically different (p < 0.05) compared to the rest of the treatments (Figure 2b,d). Finally, fish fed T4 diet showed the highest chymotrypsin activity with significant differences (p < 0.05) compared to the rest of the treatments (Figure 2c).
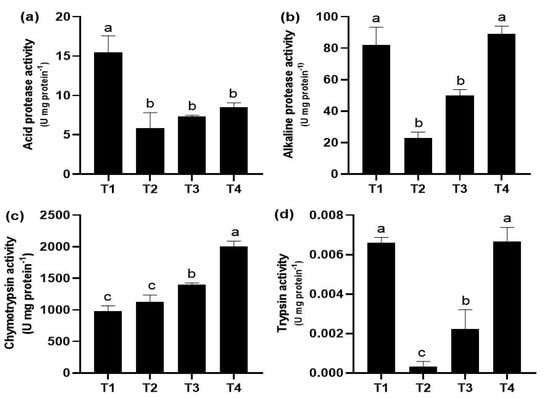
Figure 2.
Digestive enzyme activities (mean ± standard deviation, SD) in juveniles of P. reticulata fed with different commercial diets. (a) acid protease activity; (b) alkaline protease activity; (c) chymotrypsin activity; (d) trypsin activity. Significant differences between treatments are indicated by different letters (p < 0.05).
3.3. In Vitro Digestibility
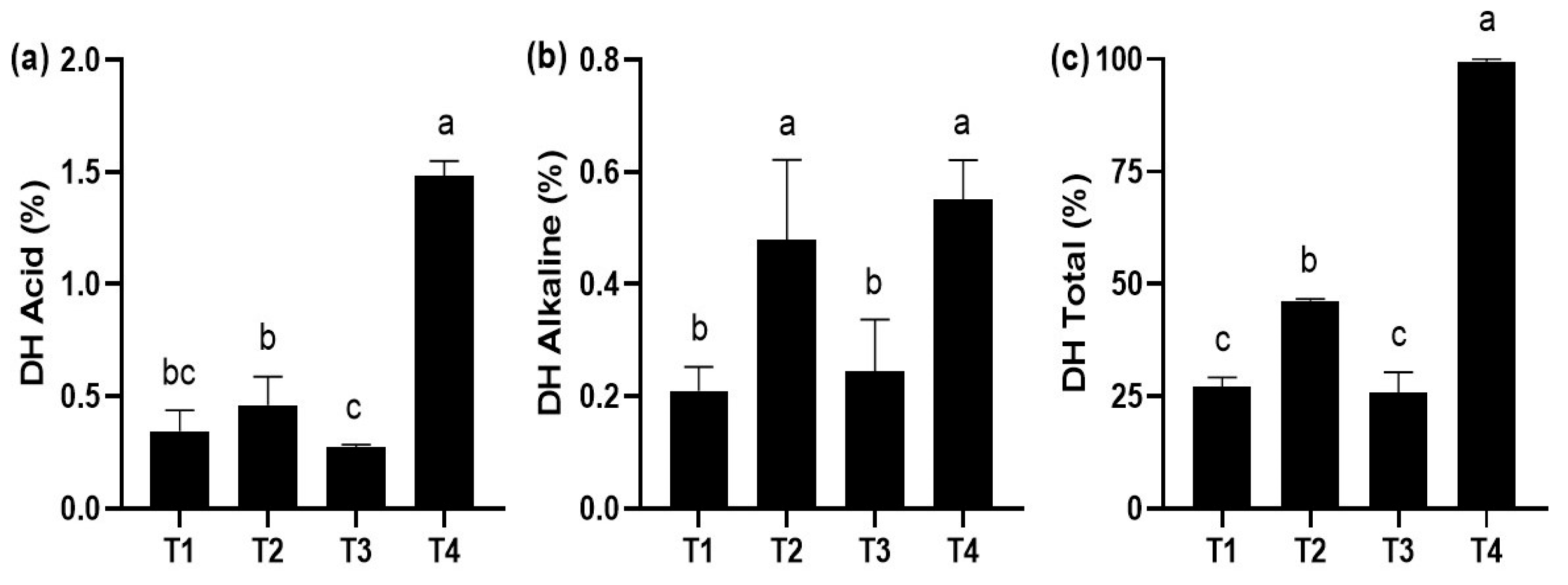
The in vitro digestibility technique showed that the highest degree of acid hydrolysis occurred with the T4 diet, being statistically different (p < 0.05) compared to the rest of the treatments (Figure 3a). The highest degree of alkaline hydrolysis occurred in T4 and T2, which were significantly different (p < 0.05) compared to the rest of the treatments (Figure 3b). Finally, the highest degree of total hydrolysis occurred with the T4 diet, being significantly different (p < 0.05) compared to the rest of the treatments (Figure 3c).
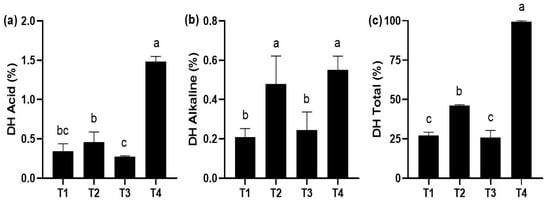
Figure 3.
In vitro digestibility (mean ± standard deviation, SD) in juveniles of P. reticulata fed with different commercial diets. (a) acid hydrolysis degree; (b) alkaline hydrolysis degree; (c) total hydrolysis degree. Significant differences between treatments are indicated by different letters (p < 0.05).
3.4. Histological Analysis
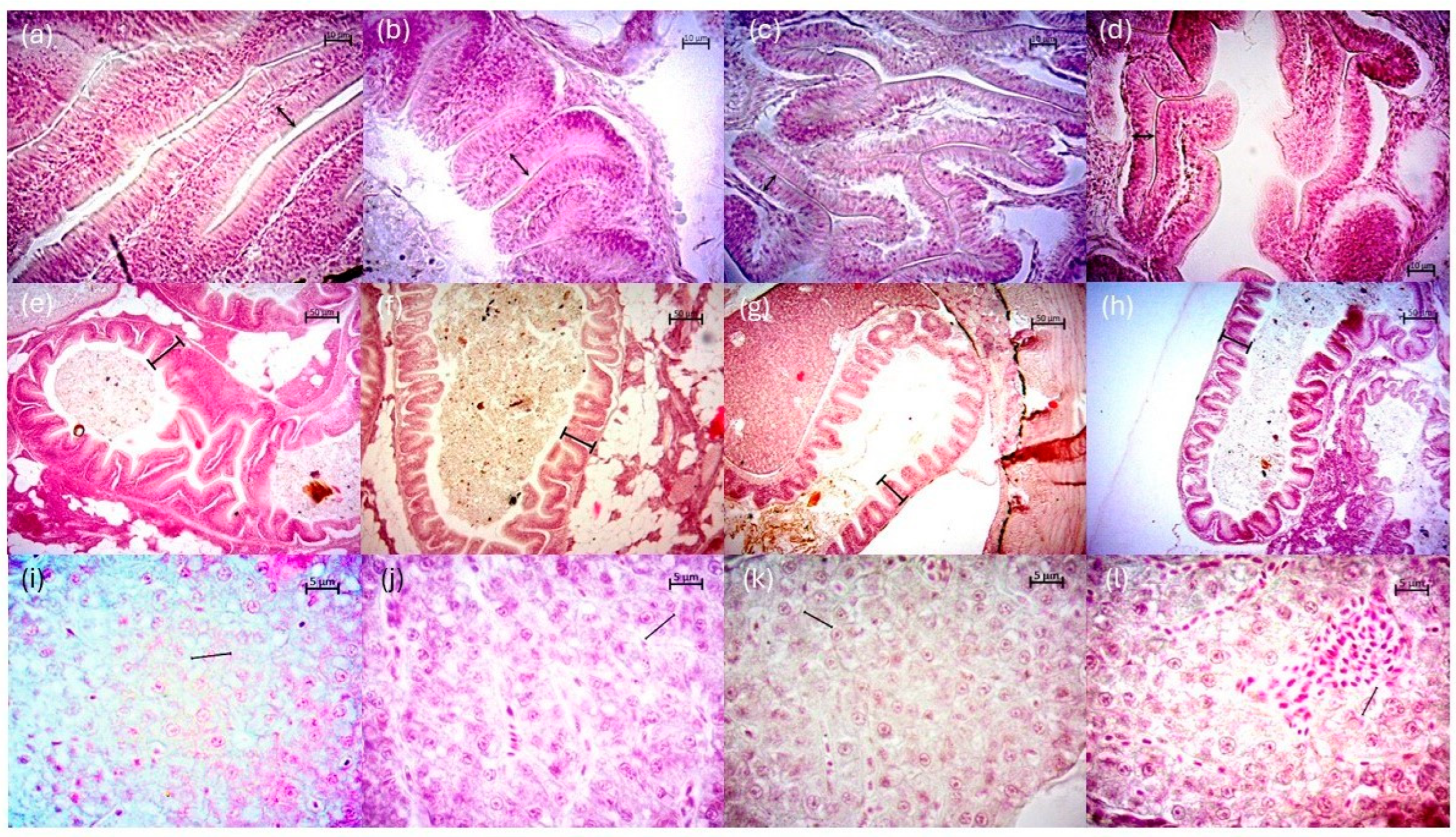
Fish fed with T1 had the highest enterocyte height at the intestinal level, although no significant differences were detected compared to T4 (p > 0.05). However, significant differences were shown (p < 0.05) between fish fed with T1 compared to fish fed with T3 (Table 3). The highest fold height was presented in fish with T1 and T2 without significant differences between the two treatments (p > 0.05). The lowest height was recorded in T4 with a difference from T1 (p < 0.05). In the liver, the largest hepatocyte diameter was observed in fish with T1, T2, and T3, respectively, showing significant differences with T4 (p < 0.05) (Table 3) (Figure 4).

Table 3.
Histological analysis (mean ± standard deviation, SD) in juveniles of P. reticulata fed with different commercial diets.
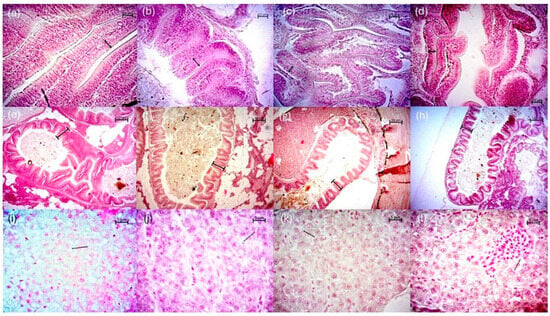
Figure 4.
Representative histological images of the liver and intestine in P. reticulata juveniles. Morphological analysis of the intestine (enterocyte height: double sided arrows): (a) T1, (b) T2, (c) T3, (d) T4 (scale bar = 10 µm). Fold height (marks): (e) T1, (f) T2, (g) T3, (h) T4 (scale bar = 50 µm). And, in liver (hepatocyte diameter: lines): (i) T1, (j) T2, (k) T3, (l) T4 (scale bar = 5 µm).
4. Discussion
4.1. Growth and Feed Indexes
Guppies (Poecilia reticulata) have omnivorous feeding habits, which allow them to consume various food sources (filamentous algae, diatoms, mosquitoes, and detritus) [23]. In this regard, the high commercialization of guppies (P. reticulata) has allowed various studies to be carried out on their nutrition, which has led to experimentation with different products and commercial and experimental diets, applied mainly to promote growth, the immune system, resistance to stress, coloration improvement, or reproduction [24,25,26,27]. Studies focused on the nutrition of P. reticulata have allowed us to determine the optimum macro and micronutrients by evaluating three diets with different percentages of protein (18.26%, 29.27%, and 43.60%) and lipids (4.17%, 4.55%, and 9.47%), where fish fed with higher protein and lipids presented a higher specific growth rate [27]. On the other hand, the addition of vitamins and minerals has also been evaluated [28]; for example, two concentrations of vitamin C were evaluated (1000 mg/kg or 2000 mg/kg), where it was shown that the inclusion of this vitamin considerably decreased the stress of the guppy when exposed to sudden changes in salinity [25]. Similarly, Shim and Ng [29] evaluated different concentrations of magnesium (0.0 g/kg, 0.18 g/kg, 0.36 g/kg, 0.54 g/kg, 0.72 g/kg, and 0.90 g/kg) in P. reticulata and determined that the optimum concentration for best growth was 0.54 g/kg. Similarly, three concentrations of calcium (0.03%, 0.59%, and 1.28%) and phosphorus (0.05%, 0.53%, and 1.23%) were evaluated for 12 weeks in P. reticulata. However, no correlation was found between growth and calcium concentrations, but optimal growth levels were found when using 0.53% and 1.23% phosphorus [30].
One aspect to highlight in our study is that the commercial diets T2 and T3 showed the percentages of micronutrients contained in their formulation; however, the other two treatments only indicated the presence of various vitamins and minerals without specifying their concentrations, an aspect that may be related to the lower growth shown by the juveniles of P. reticulata possibly caused by low or zero concentrations of some of the vitamins and minerals essential for this species. In this regard, research to improve diets for P. reticulata has led to the implementation of various ingredients or additives, where, in some cases, the results significantly improved production parameters. For example, using microalgae (Parietochloris incisa) significantly improved the survival rate and stress resistance in P. reticulata [31]. Also, the inclusion of garlic (Allium sativum) improved digestive enzyme activity, survival, and the presence of sex steroids (estradiol, testosterone, 17α-hydroxyprogesterone, and progesterone) [24]. However, although additives were not tested in this research, our results provided evidence that the commercial diet for trout (T4), although not specific for ornamental fish (formulated for trout, an aquaculture species), promotes greater digestive enzyme activity (alkaline protease, trypsin, and chymotrypsin). This digestive enzyme improvement can be attributed to the high quality of the ingredients or additives used in its manufacture, which promote greater digestion and absorption of amino acids and essential nutrients and, consequently, better growth in juvenile P. reticulata. Likewise, when evaluating P. reticulata, several experimental diets that contained various types of high-quality protein and lipid ingredients (fish meal, shrimp meal, meat and bone meal, wheat flour, corn flour, starch, and soybean oil) and a commercial diet, the experimental diet that contained fish meal as the basis of dietary protein, resulted in better productive parameters, growth, and survival [32].
On the other hand, three commercial diets (Prima diet, Galaxy diet, and shrimp feed) were evaluated in juvenile P. reticulata, where the results showed that shrimp feed allowed for better growth and reproductive performance [33]. Likewise, Sharon et al. [34] evaluated a commercial diet (BernAqua) in three presentations (Regular, Ornamental, Premium) compared to experimental diets, and at the end of the 57 days of the experiment, the juvenile P. reticulata fed with BernAqua Ornamental obtained a moderate weight gain, greater resistance to parasitic infection, and did not show muscular dystrophy but did show insignificant deformity. These results coincide with those obtained in this investigation, in which no significant differences in growth were found between the T1, T2, and T4 treatments. However, the fish fed with the T4 diet had the highest survival rate. Considering the above, Harpaz et al. [35] have shown that the presentation of food can modify the growth of fish, where two presentations (powder and flakes) were evaluated in P. reticulata, which resulted in greater growth in the fish fed with the powder presentation as opposed to the fish fed with flakes. Hence, the authors suggested that the powder presentation facilitates the capture and consumption of food by fish and decreases the leaching of nutrients from the feed. Likewise, the inclusion of 40% spirulina (Spirulina platensis) in the feed allowed for the obtaining of significant values in growth and conversion rates in the juveniles of P. reticulata [36]. Our results indicated that the conversion rate of treatments T3 and T1 (containing spirulina) showed the highest values. However, these treatments did not improve intestinal morphology, unlike the fish fed with the T4 diet, in addition to better survival.
4.2. Nutrition in the Aquarium Hobby
According to the latest report, the aquarium industry is valued at approximately 15 to 30 billion dollars per year [37], from which other sectors emerge, such as the manufacture of ornamental fish diets. In recent years, the application of concepts such as traceability, the quality of ingredients, quality control during the manufacture of diets, and sustainability has increased their relevance in the aquarium hobby [37,38,39].
Although the aquarium hobby has been an independent activity from aquaculture in recent decades, various branches, such as nutrition, are essential for both industries. In this sense, as in aquaculture, the nutrition of ornamental fish has been the subject of great interest in recent years for the commercial food industry. One of the results to highlight from this work is the cost-benefit ratio, since T4 (aquaculture diet for trout) is 72 times cheaper than a specialized premium food for ornamental fish (T2: Tetra TetraMin Tropical Flakes®). When choosing food for ornamental fish, it is necessary to consider that these products are often designed to be visually appealing to owners rather than the fish, as they typically come in brightly colored flakes and are generally generic. The quality and type of ingredients used for these foods are largely unknown.
On the other hand, the manufacturing technology in most foods for ornamental fish is lacking, which makes them more susceptible to nutrient leaching and, when supplied to fish, could cause malnutrition problems [35]. Factors such as high prices, the purpose of the product, and the diversity of the species in the culture to which these foods are directed have allowed the rapid growth of the industry that manufactures foods for ornamental fish. However, it must be questioned whether the nutritional formulation of these foods meets the nutritional requirements for all the species to which the commercial brands indicate they are directed towards by being generic, since it is possible that for some species, it is adequate by covering their requirements, but for other species, deficiencies occur, which would imply the death of those individuals [40]. Likewise, the different technologies used in preparing commercial foods (pellets, laminated, and extruded) or presentations are correct for the eating habits of various fish species. Unlike in aquaculture, ornamental fish feed can be in forms such as powdered, flakes, powdered milk, bovine heart and liver, or tubifex worms, as well as live food such as Artemia, rotifers, and Moina, considering mainly foods that allow for the coloration of the fish [12]. In the search to reduce costs, the use of alternative fishmeal (sailfin catfish) that replaces commercial fishmeal in P. reticulata shows that it can be completely replaced without compromising the productive parameters of the species [41]. These ingredients can serve as a basis for using local or regional ingredients instead of fishmeal. Nutritional requirements have been shown to differ significantly between marine and freshwater fish species [42,43], and these requirements also vary depending on the stage of development [44]. Thus, the lack of knowledge of the taxonomy and diversity of the kept ornamental species and using a single commercial feed for multiple species can generate various physiological effects [45].
4.3. Food Manufacturing and Effects on Fish
The quality of fish feed during its manufacture is essential to ensure that it has the necessary nutrients to cover its requirements, as well as the care and selection of the ingredients to be used [46]. This ingredient selection plays a key role in formulating specialized diets for each fish species in culture [47]. In this sense, the chemical characteristics of the ingredients can vary during the manufacture of the feed, so this quality must be checked before and during the experiments, which can be evaluated using various techniques such as apparent density, pellet hardness, durability, expansion rate, and starch gelatinization, which allow for adjusting the industrial procedures during its manufacture [48]. In this way, varying the temperature and humidity during the production process can generate variations in the physical characteristics of the feed and, consequently, affect the quality and quantity of the nutrients in the feed, such as fatty acids and amino acids, which can affect its digestibility [49,50]. This research measured the in vitro digestibility of three flake foods (T1, T2, T3) and one extruded feed (T4) using the stomach and intestinal enzymes of P. reticulata. This way, the highest acid (stomach) and alkaline (intestinal) digestibility was presented with the T4 diet, with significant differences compared to the flake diets. In this way, it has been shown that the manufacturing technology of balanced feed can be modified through the manufacturing process. For example, when evaluating two pelleting techniques, it was possible to adjust variables such as the expansion rate, apparent density, and water stability (humidity) [51]. Likewise, changes in the manufacturing of extruded feeds can be reflected in a better growth rate, which has been evaluated in Nile tilapia culture [52].
As mentioned, changes in the formulation or manufacturing of feeds used in aquaculture can negatively affect fish. The use of different commercial diets (Tetra flakes, Omega flakes, Tetra + Omega, and Tetra + Omega + Lyophilizate) in Neon Tetras (Paracheirodon innesi) and Glowlight Rasboras (Trigonostigma hengeli) demonstrated adverse physiological changes, specifically an apparent accumulation of lipids in the liver of T. hengeli, reflecting a shift in hepatocytes [45]. Our results indicated that the formulation and manufacturing of the feeds significantly impacted the morphology of the fish’s digestive system, where the enterocytes’ height did not show significant differences between the treatments. Still, regarding the length of the intestinal folds, the fish fed with the T4 diet presented shorter folds, suggesting greater digestion efficiency and nutrient absorption. On the other hand, the diameter of the hepatocytes was measured, and the fish fed with the T4 diet presented a smaller diameter, which implied that this feed decreases the accumulation of liver fat, unlike other foods for ornamental fish. In this way, the differences between the two manufacturing processes are fundamental, since in extrusion, greater control of the process is achieved, allowing the obtaining of products of high nutritional quality, with greater efficiency and versatility, which improves the growth and physiology of the fish [53,54], particularly for P. reticulata.
5. Conclusions
This study demonstrated that juvenile guppies (P. reticulata) fed the T4 diet had improved growth, survival, and digestive enzyme activities. This result suggested that, of the commercial foods evaluated, extruded trout food was technologically better than laminated ornamental fish foods. On the other hand, this treatment also presented better in vitro digestibility, which translates into greater protein hydrolysis, allowing for better absorption of nutrients. It is essential to highlight that none of these foods were nutritionally specific for guppies, which opens up the possibility of future research where a specific diet designed and based on the digestive physiology of this species is generated and adequately covers its nutritional requirements. On the other hand, manufacturing techniques play a key role in the nutrition of the species, which is why it is important to use extruded foods to produce ornamental fish. This work also coincides with previous work that point out that commercial foods for ornamental species are focused on meeting the caretakers’ (owners’) demands, which can compromise the nutrition and well-being of the fish. Finally, it is recommended that more studies are carried out, evaluating the nutritional profiles of commercial foods, the validation of the additives shown on the label (factors that considerably increase costs), the presentation of the product, and the form of administration.
Author Contributions
Conceptualization, Y.J.T.-S., C.A.S.-Q., C.A.Á.-G. and R.M.-G.; methodology, Y.J.T.-S., G.M.P.-J., O.M.-M., U.R.-E. and G.G.A.-A.; software, U.R.-E., G.N.-N. and C.A.S.-Q.; validation, Y.J.T.-S., G.M.P.-J., G.G.A.-A., C.A.S.-Q. and C.A.Á.-G.; formal analysis, Y.J.T.-S., L.D.J.-M., C.A.S.-Q. and C.A.Á.-G.; investigation, Y.J.T.-S., L.D.J.-M., G.N.-N., C.A.S.-Q., C.A.Á.-G. and R.M.-G.; resources, C.A.S.-Q. and C.A.Á.-G.; data curation, Y.J.T.-S., L.D.J.-M. and G.N.-N.; writing—original draft preparation, Y.J.T.-S., C.A.S.-Q. and C.A.Á.-G.; writing—review and editing, Y.J.T.-S., G.N.-N., C.A.S.-Q., C.A.Á.-G. and R.M.-G.; visualization, R.M.-G., C.A.S.-Q. and C.A.Á.-G.; supervision, R.M.-G., C.A.S.-Q. and C.A.Á.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted following the Helsinki Declaration, the protocol was approved by the Secretary of Agriculture, Livestock, Rural Development, Fisheries and Food (NOM-062-ZOO-1999, 2001) [16], and this experiment had the approval (UJAT-CIEI-2025-059) of the Institutional Commission of Ethics in Research of the División Académica de Ciencias Biológicas de la Universidad Juárez Autónoma de Tabasco, Mexico.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request to the corresponding authors.
Acknowledgments
The authors would like to thank the Laboratory of Physiology in Aquatic Resources (LAFIRA) in the Academic Division of Biological Sciences of the Autonomous Juarez University of Tabasco, Mexico, Ignacio Bautista Garcia and Yuliana Jimenez Leon. Likewise, we thank Karen Nieves Rodriguez and Jesus Bautista Regil for their recommendations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Miller, S.M.; Mitchell, M.A. ORNAMENTAL FISH; Elsevier eBooks: Cambridge, MA, USA, 2009; pp. 39–72. [Google Scholar] [CrossRef]
- Novák, J.; Kalous, L.; Patoka, J. Modern ornamental aquaculture in Europe: Early history of freshwater fish imports. Rev. Aquac. 2020, 12, 2042–2060. [Google Scholar] [CrossRef]
- Gomes-Silva, G.; Cyubahiro, E.; Wronski, T.; Riesch, R.; Apio, A.; Plath, M. Water pollution affects fish community structure and alters evolutionary trajectories of invasive guppies (Poecilia reticulata). Sci. Total Environ. 2020, 730, 138912. [Google Scholar] [CrossRef] [PubMed]
- Kodric-Brown, A.; Nicoletto, P. Female choice in the guppy (Poecilia reticulata): The interaction between male color and display. Behav. Ecol. Sociobiol. 2001, 50, 346–351. [Google Scholar] [CrossRef]
- Imai, M.; Mizoguchi, T.; Wang, M.; Li, Y.; Hasegawa, Y.; Tonoki, A.; Itoh, M. The Guppy (Poecilia reticulata) Is a Useful Model for Analyzing Age-Dependent Changes in Metabolism, Motor Function, and Gene Expression. Exp. Gerontol. 2022, 160, 111708. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.A.; Weadick, C.J.; Janowitz, I.; Rodd, F.H.; Hughes, K.A. Sequencing and Characterization of the Guppy (Poecilia reticulata) Transcriptome. BMC Genom. 2011, 12, 202. [Google Scholar] [CrossRef]
- Chaabani, A.; Labonne, L.; Durrieu, V.; Rouilly, A.; Skiba, F.; Evon, P. Preconditioner influence on twin-screw extrusion cooking of starch-based feed pellets: The example of Fish Feed. Aquac. Eng. 2022, 98, 102268. [Google Scholar] [CrossRef]
- Ebeneezar, S.; Linga Prabu, D.; Sajina, K.A. Feeds and feed management in mariculture. In Winter School on Mariculture Technologies for Income Multiplication, Employment, Livelihood and Empowerment; ICAR-Central Marine Fisheries Research Institute: Kochi, India, 2023; pp. 242–255. Available online: http://eprints.cmfri.org.in/17104/ (accessed on 24 November 2024).
- Papáček, Š.; Petera, K.; Císař, P.; Stejskal, V.; Saberioon, M. Experimental & Computational Fluid Dynamics Study of the Suitability of Different Solid Feed Pellets for Aquaculture Systems. Appl. Sci. 2020, 10, 6954. [Google Scholar] [CrossRef]
- Garzón, J.S.V.; Espinosa, M.C.G. Aspectos nutricionales de peces ornamentales de agua dulce. Rev. Politécnica 2019, 15, 82–93. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Velasco-Santamaría, Y.; Corredor-Santamaría, W. Nutritional Requirements of Freshwater Ornamental Fish: A Review. 2011. Available online: https://www.redalyc.org/articulo.oa?id=69322446003 (accessed on 18 November 2024).
- Kruger, D.; Britz, P.; Sales, J. Influence of Varying Dietary Protein Content at Three Lipid Concentrations on Growth Characteristics of Juvenile Swordtails (Xiphophorus helleri Heckel 1848). Aquar. Sci. Conserv. 2001, 3, 275–280. [Google Scholar] [CrossRef]
- Dahlgren, B.T. The effects of three different dietary protein levels on the fecundity in the guppy, Poecilia reticulata (Peters). J. Fish Biol. 1980, 16, 83–97. [Google Scholar] [CrossRef]
- Sales, J.; Janssens, G.P. Nutrient requirements of ornamental fish. Aquat. Living Resour. 2003, 16, 533–540. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Norma Oficial Mexicana: Especificaciones Técnicas Para La Producción, Cuidado Y Uso De Los Animales De Labora-Torio. Gobierno de México: Ciudad de México, Mexico, 2001. Available online: https://www.gob.mx/senasica/documentos/nom-062-zoo-1999 (accessed on 13 April 2024).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Sarath, G.; de La Motte, R.S.; Wagner, F.W. Protease assay methods. In Proteolytic Enzymes: A Practical Approach; Beynon, R.J., Bonde, J.S., Eds.; Oxford University Press: Oxford, UK, 1989; pp. 25–54. [Google Scholar]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- DelMar, E.; Largman, C.; Brodrick, J.; Geokas, M. A sensitive new substrate for chymotrypsin. Anal. Biochem. 1979, 99, 316–320. [Google Scholar] [CrossRef]
- Dimes, L.; Haard, N. Estimation of protein digestibility—I. Development of an in vitro method for estimating protein digestibility in salmonids (Salmo gairdneri). Comp. Biochem. Physiol. Part A Physiol. 1994, 108, 349–362. [Google Scholar] [CrossRef]
- Fernando, G.K.A.W.; Jayakody, S.; Wijenayake, W.M.H.K.; Galappaththy, G.N.L.; Yatawara, M.; Harishchandra, J. A comparison of the larvivorous habits of exotic Poecilia reticulata and native Aplocheilus parvus. BMC Ecol. 2018, 18, 25. [Google Scholar] [CrossRef]
- Motlagh, H.A.; Safari, O.; Selahvarzi, Y.; Baghalian, A.; Kia, E. Non-specific immunity promotion in response to garlic extract supplemented diets in female Guppy (Poecilia reticulata). Fish Shellfish Immunol. 2019, 97, 96–99. [Google Scholar] [CrossRef]
- Lim, L.C.; Dhert, P.; Chew, W.Y.; Dermaux, V.; Nelis, H.; Sorgeloos, P. Enhancement of Stress Resistance of the Guppy Poecilia reticulata through Feeding with Vitamin C Supplement. J. World Aquac. Soc. 2002, 33, 32–40. [Google Scholar] [CrossRef]
- Sultana, R.; Khatoon, H.; Rahman, M.R.; Haque, M.E.; Nayma, Z.; Mukta, F.A. Potentiality of Nannochloropsis sp. as partial dietary replacement of fishmeal on growth, proximate composition, pigment and breeding performance in guppy (Poecilia reticulata). Bioresour. Technol. Rep. 2022, 18, 101112. [Google Scholar] [CrossRef]
- Kithsiri, P.; Prakash Sharma, H.M.; Syeddain Zaidi, S.G.; Pal, A.K.; Venkateshwarlu, G. Growth and reproductive performance of female guppy, Poecilia reticulata (Peters) fed diets with different nutrient levels. Indian J. Fish. 2010, 57, 65–71. Available online: https://epubs.icar.org.in/index.php/IJF/article/view/7526 (accessed on 27 November 2024).
- Prabhu, P.A.J.; Schrama, J.W.; Kaushik, S.J. Mineral requirements of fish: A systematic review. Rev. Aquac. 2014, 8, 172–219. [Google Scholar] [CrossRef]
- Shim, K.; Ng, S. Magnesium requirement of the guppy (Poecilia reticulata Peters). Aquaculture 1988, 73, 131–141. [Google Scholar] [CrossRef]
- Shim, K.; Ho, C. Calcium and phosphorus requirements of guppy Poecilia reticulata. Nippon. Suisan Gakkaishi 1989, 55, 1953–1955. [Google Scholar] [CrossRef]
- Nath, P.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S.; Zilberg, D. Dietary supplementation with the microalgae Parietochloris incisa increases survival and stress resistance in guppy (Poecilia reticulata) fry. Aquac. Nutr. 2011, 18, 167–180. [Google Scholar] [CrossRef]
- Anka, I.Z.; Jothi, J.S.; Sarker, J.; Talukder, A.; Islam, S.M. Growth performance and survival of guppy (Poecilia reticulata): Different formulated diets effect. Asian J. Med. Biol. Res. 2016, 2, 451–457. [Google Scholar] [CrossRef][Green Version]
- Kumaratunga, P.; Radampola, K. Effect of different commercial feeds on growth and reproductive performance of Guppy, Poecilia reticulata Peters. J. Univ. Ruhuna 2019, 7, 6–11. [Google Scholar] [CrossRef]
- Sharon, G.; Fridman, S.; Reiss-Hevlin, N.; Sinai, T.; Boisot, P.; Zilberg, D. Effects of different commercial diets on growth performance, health and resistance to Tetrahymena sp. infection in guppies, Poecilia reticulata(Peters). Aquac. Res. 2014, 47, 2276–2286. [Google Scholar] [CrossRef]
- Harpaz, S.; Slosman, T.; Segev, R. Effect of feeding guppy fish fry (Poecilia reticulata) diets in the form of powder versus flakes. Aquac. Res. 2005, 36, 996–1000. [Google Scholar] [CrossRef]
- Dernekbasi, S.; Una, H.; Karayucel, I.; Aral, O. Effect of Dietary Supplementation of Different Rates of Spirulina (Spirulina platensis) on Growth and Feed Conversion in Guppy (Poecilia reticulata Peters, 1860). J. Anim. Vet. Adv. 2010, 9, 1395–1399. [Google Scholar] [CrossRef]
- Evers, H.; Pinnegar, J.K.; Taylor, M.I. Where are they all from?—Sources and sustainability in the ornamental freshwater fish trade. J. Fish Biol. 2019, 94, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.K.M.; Oliveira, T.P.R.; Rosa, I.L.; Braga-Pereira, F.; Ramos, H.A.C.; Rocha, L.A.; Alves, R.R.N. Caught in the (inter)net: Online trade of ornamental fish in Brazil. Biol. Conserv. 2021, 263, 109344. [Google Scholar] [CrossRef]
- Tangendjaja, B. Quality Control of Feed Ingredients for Aquaculture; Elsevier eBooks: Cambridge, MA, USA, 2022; pp. 165–194. [Google Scholar] [CrossRef]
- Sicuro, B. Nutrition in ornamental aquaculture: The raise of anthropocentrism in aquaculture? Rev. Aquac. 2017, 10, 791–799. [Google Scholar] [CrossRef]
- De Fonseka, R.; Radampola, K. Feasibility of using sailfin catfish meal as an alternative to commercial fishmeal in the diets of juvenile guppy (Poecilia reticulata). J. Fish. 2022, 10, 101203. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Rainuzzo, J.R.; Reitan, K.I.; Olsen, Y. The significance of lipids at early stages of marine fish: A review. Aquaculture 1997, 155, 103–115. [Google Scholar] [CrossRef]
- Kasprzak, R.; Grzeszkiewicz, A.B.; Górecka, A. Performance of Co-Housed Neon Tetras (Paracheirodon innesi) and Glowlight Rasboras (Trigonostigma hengeli) Fed Commercial Flakes and Lyophilized Natural Food. Animals 2021, 11, 3520. [Google Scholar] [CrossRef]
- Pigott, G.M.; Tucker, B.W. Special Feeds; Elsevier eBooks: Cambridge, MA, USA, 2023; pp. 651–669. [Google Scholar] [CrossRef]
- Hardy, R.W.; Barrows, F.T. Diet Formulation and Manufacture; Elsevier eBooks: Cambridge, MA, USA, 2003; pp. 505–600. [Google Scholar] [CrossRef]
- Sørensen, M. A review of the effects of ingredient composition and processing conditions on the physical qualities of extruded high-energy fish feed as measured by prevailing methods. Aquac. Nutr. 2012, 18, 233–248. [Google Scholar] [CrossRef]
- Boucher, R.L.; Chung, W.; Ng, J.; Tan, L.; Wu, C.; Lee, C. Impact of extrusion temperature and moisture incorporation on nutrient digestibility in barramundi (Lates calcarifer) diet. Aquaculture 2024, 592, 741209. [Google Scholar] [CrossRef]
- Glencross, B.; Grobler, T.; Huyben, D. Digestible nutrient and energy values of corn and wheat glutens fed to Atlantic salmon (Salmo salar) are affected by feed processing method. Aquaculture 2021, 544, 737133. [Google Scholar] [CrossRef]
- My, A.; At, M.; Jk, I. Effect of pelletizing machines on floatation and water stability of farm-made fish feeds. Int. J. Fish. Aquat. Stud. 2016, 4, 98–103. [Google Scholar]
- Epifânio, C.M.; De MDantas, F.; Fonseca, F.A.; Gonçalves, G.S.; Viegas, E.M.; Gonçalves, L.U. Effects of the extrusion process on the physical properties of micro pellets and the growth performance of juvenile Nile tilapia. Anim. Feed. Sci. Technol. 2024, 318, 116122. [Google Scholar] [CrossRef]
- Xing, S.; Liang, X.; Wang, H.; Xie, X.; Wierenga, P.A.; Schrama, J.W.; Xue, M. The impacts of physical properties of extruded feed on the digestion kinetics, gastrointestinal emptying and stomach water fluxes of spotted seabass (Lateolabrax maculatus). Aquaculture 2023, 570, 739442. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Ao, H.; Liu, L.; Chen, Y. Effects of extruded and pelleted diets with different protein levels on growth performance and nutrient retention of largemouth bass (Micropterus salmoides). Aquac. Rep. 2023, 29, 101479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).