Comparative Study of Various Graphene Oxide Structures as Efficient Drug Release Systems for Ibuprofen
Abstract
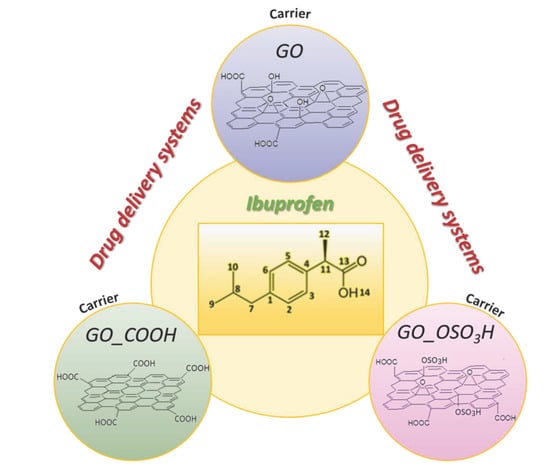
:1. Introduction
2. Materials and Methods
2.1. Preparations of Materials
2.1.1. Preparation of Graphite Oxide (GO)
2.1.2. Preparation of Sulfonated Graphite Oxide (GO_OSO3H)
2.1.3. Preparation of Carboxylated Graphite Oxide (GO_COOH)
2.1.4. Intercalation of Ibuprofen into Graphite Oxide (GO), Sulfonated Graphite Oxide (GO_OSO3H) and Carboxylated Graphite Oxide (GO_COOH)
2.2. In Vitro Drug Release of Ibuprofen
2.3. Characterization Techniques
2.4. Computational Methods
3. Results
3.1. Material Characterization
3.2. Theoretical Calculations
3.3. In Vitro Drug Release of Ibuprofen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Bonthagarala, B.; Lakshmi Sai, P.D.L.; Venkata, S.K.; Rao, B.N.; Dasari, V. Enhancement of dissolution rate of Clofibrate BCS Class II drug by using liquisolid compact technology. Int. J. Biomed. Adv. Res. 2015, 6, 288. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.-J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-based drug delivery carriers for cancer therapy. Arch. Pharmacal Res. 2014, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. J. Cell. Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C 2019, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Shah, N.J.; Drake, A.C.; DeMuth, P.C.; Lee, J.B.; Chen, J.; Hammond, P.T. Graphene Multilayers as Gates for Multi-Week Sequential Release of Proteins from Surfaces. ACS Nano 2012, 6, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Tavares Ferreira, J.; Pinto, L.A.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Silva, G.A.; Bitoque, D.B.; Ferreira, J.; Pinto, L.A.; Morgado, J.; Ferreira, Q. Self-Assembled Multilayer Films for Time-Controlled Ocular Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Batchelor-McAuley, C.; Rasche, B.; Johnston, C.; Hindle, N.; Compton, R.G. Surface area measurements of graphene and graphene oxide samples: Dopamine adsorption as a complement or alternative to methylene blue? Appl. Mater. Today 2020, 18, 100506. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Mojgan, N.; Fahimeh, C.; Mohammad, R. Graphene as multifunctional delivery platform in cancer therapy. J. Biomed. Mater. Res. Part A 2017, 105, 2355–2367. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Y.; Shen, Q.; Ma, D.; Huang, L.; Cai, X.; Tan, S. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater. Sci. Eng. C 2017, 79, 185–190. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; Sun, W.; Gu, Z. ATP-responsive DNA-graphene hybrid nanoaggregates for anticancer drug delivery. Biomaterials 2015, 50, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Hussein, M.; Kura, A.; Fakurazi, S.; Masarudin, M.; Ahmad Saad, F. Graphene Oxide as a Nanocarrier for a Theranostics Delivery System of Protocatechuic Acid and Gadolinium/Gold Nanoparticles. Molecules 2018, 23, 500. [Google Scholar] [CrossRef] [Green Version]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Dembereldorj, U.; Kim, M.; Kim, S.; Ganbold, E.-O.; Lee, S.Y.; Joo, S.-W. A spatiotemporal anticancer drug release platform of PEGylated graphene oxide triggered by glutathione in vitro and in vivo. J. Mater. Chem. 2012, 22, 23845–23851. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Yang, Y.; Ying-Ming, Z.; Yong, C.; Di, Z.; Jia-Tong, C.; Yu, L. Construction of a Graphene Oxide Based Noncovalent Multiple Nanosupramolecular Assembly as a Scaffold for Drug Delivery. Chem. Eur. J. 2012, 18, 4208–4215. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Urbas, K.; Perużyńska, M.; Kurzawski, M.; Droździk, M.; Mijowska, E. Covalent conjugation of graphene oxide with methotrexate and its antitumor activity. Chem. Phys. Lett. 2013, 568–569, 151–156. [Google Scholar] [CrossRef]
- Hu, H.; Tang, C.; Yin, C. Folate conjugated trimethyl chitosan/graphene oxide nanocomplexes as potential carriers for drug and gene delivery. Mater. Lett. 2014, 125, 82–85. [Google Scholar] [CrossRef]
- Dong, H.; Dai, W.; Ju, H.; Lu, H.; Wang, S.; Xu, L.; Zhou, S.-F.; Zhang, Y.; Zhang, X. Multifunctional Poly(l-lactide)–Polyethylene Glycol-Grafted Graphene Quantum Dots for Intracellular MicroRNA Imaging and Combined Specific-Gene-Targeting Agents Delivery for Improved Therapeutics. ACS Appl. Mater. Interfaces 2015, 7, 11015–11023. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, K.; Calvaresi, M.; Diamanti, E.K.; Tsoufis, T.; Gournis, D.; Rudolf, P.; Zerbetto, F. Graphite Oxide and Aromatic Amines: Size Matters. Adv. Funct. Mater. 2015, 25, 263–269. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Dai, L. Sulfated Graphene Oxide as a Hole-Extraction Layer in High-Performance Polymer Solar Cells. J. Phys. Chem. Lett. 2012, 3, 1928–1933. [Google Scholar] [CrossRef]
- Ciobotaru, C.C.; Damian, C.M.; Matei, E.; Iovu, H. Covalent Functionalization of Graphene Oxide with Cisplatin. Mater. Plast. 2014, 51, 75–80. [Google Scholar]
- Zheng, J.P.; Luan, L.; Wang, H.Y.; Xi, L.F.; Yao, K.D. Study on ibuprofen/montmorillonite intercalation composites as drug release system. Appl. Clay Sci. 2007, 36, 297–301. [Google Scholar] [CrossRef]
- Lee, S.W.; Condrate, R.A. The infrared and Raman spectra of ZrO2-SiO2 glasses prepared by a sol-gel process. J. Mater. Sci. 1988, 23, 2951–2959. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dobbs, K.D.; Hehre, W.J. Molecular orbital theory of the properties of inorganic and organometallic compounds 4. Extended basis sets for third-and fourth-row, main-group elements. J. Comput. Chem. 1986, 7, 359–378. [Google Scholar] [CrossRef]
- Namur, J.; Wassef, M.; Pelage, J.P.; Lewis, A.; Manfait, M.; Laurent, A. Infrared microspectroscopy analysis of Ibuprofen release from drug eluting beads in uterine tissue. J. Control. Release 2009, 135, 198–202. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.-T. Preparation and Characterization of Graphene Oxide. J. Nanomater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Dékány, I.; Krüger-Grasser, R.; Weiss, A. Selective liquid sorption properties of hydrophobized graphite oxide nanostructures. Colloid Polym. Sci. 1998, 276, 570–576. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zygouri, P.; Spyrou, K.; Papayannis, D.K.; Asimakopoulos, G.; Dounousi, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Comparative Study of Various Graphene Oxide Structures as Efficient Drug Release Systems for Ibuprofen. AppliedChem 2022, 2, 93-105. https://doi.org/10.3390/appliedchem2020006
Zygouri P, Spyrou K, Papayannis DK, Asimakopoulos G, Dounousi E, Stamatis H, Gournis D, Rudolf P. Comparative Study of Various Graphene Oxide Structures as Efficient Drug Release Systems for Ibuprofen. AppliedChem. 2022; 2(2):93-105. https://doi.org/10.3390/appliedchem2020006
Chicago/Turabian StyleZygouri, Panagiota, Konstantinos Spyrou, Demetrios K. Papayannis, Georgios Asimakopoulos, Evangelia Dounousi, Haralambos Stamatis, Dimitrios Gournis, and Petra Rudolf. 2022. "Comparative Study of Various Graphene Oxide Structures as Efficient Drug Release Systems for Ibuprofen" AppliedChem 2, no. 2: 93-105. https://doi.org/10.3390/appliedchem2020006
APA StyleZygouri, P., Spyrou, K., Papayannis, D. K., Asimakopoulos, G., Dounousi, E., Stamatis, H., Gournis, D., & Rudolf, P. (2022). Comparative Study of Various Graphene Oxide Structures as Efficient Drug Release Systems for Ibuprofen. AppliedChem, 2(2), 93-105. https://doi.org/10.3390/appliedchem2020006






_Stamatis.png)