pH-Sensitive Sensors at Work on Poultry Meat Degradation Detection: From the Laboratory to the Supermarket Shelf
Abstract
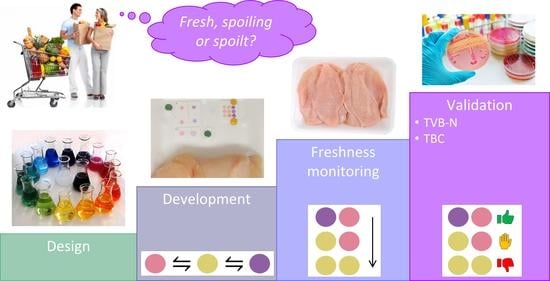
:1. Introduction
2. Materials and Methods
2.1. Optodes Preparation: From Synthesis to Miniaturization
2.2. Sensors Thickness Selection
2.3. Experimental Set-Up for Vapors Analysis
2.4. Pictures Acquisition and Multivariate Data Elaboration
2.5. TVB-N Quantification
2.6. Microbiological Analysis
3. Results and Discussion
3.1. Sensing Approach for 3-Step Degradation Detection
3.2. Sensors’ Components Selection
3.3. Sensors Effective Thickness Selection
3.4. Detection Kinetic of Acid–Base Analytes in Vapor Phase
3.5. Sensors’ Stability
3.6. Application on Real Samples: Chicken Breast Slices Freshness Monitoring during Chilled Storage
- Freshness (F): both the PC1 and PC2 score values remain constant, meaning that none of the sensors is changing its color (Day 1–Day 3). In this step, the dual-sensor array still presents its starting coloration—violet for b-CR-EVOH@ and pink for a-CR-EVOH@.
- Early spoilage (ES): PC1 score values undergo a steady increase related to b-CR-EVOH@ color transition from violet to yellow as a consequence of acidic volatile by-products’ detection (Day 4–Day 6). A slight increase is observed also for PC2 score values.
- Spoilage (S): PC1 score values’ increase becomes much less evident, while PC2 score values significantly lower as a consequence of a-CR-EVOH@ detection of a slightly more alkaline environment, and consequently, the color turns from pink to yellow. (Day 7–10).
3.7. CR-EVOH@ Dual-Sensor Array Corroboration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavigne, J.J.; Anslyn, E.V. Sensing A Paradigm Shift in the Field of Molecular Recognition: From Selective to Differential Receptors. Angew. Chem. Int. Ed. 2001, 40, 3118–3130. [Google Scholar] [CrossRef]
- Diehl, K.L.; Anslyn, E.V. Array sensing using optical methods for detection of chemical and biological hazards. Chem. Soc. Rev. 2013, 42, 8596. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Goncalves Dias Diniz, P.H. Chemometrics-assisted color histogram-based analytical systems. J. Chemom. 2020, 34, 3242. [Google Scholar] [CrossRef]
- Lopez-Ruiz, N.; Curto, V.F.; Erenas, M.M.; Benito-Lopez, F.; Diamond, D.; Palma, A.J.; Capitan-Vallvey, L.F. Smartphone-Based Simultaneous pH and Nitrite Colorimetric Determination for Paper Microfluidic Devices. Anal. Chem. 2014, 86, 9554–9562. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical pH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef]
- Hoang, A.T.; Cho, Y.B.; Kim, Y.S. A strip array of colorimetric sensors for visualizing a concentration level of gaseous analytes with basicity. Sens. Actuators B Chem. 2017, 251, 1089–1095. [Google Scholar] [CrossRef]
- Kim, S.D.; Koo, Y.; Yun, Y. A Smartphone-Based Automatic Measurement Method for Colorimetric pH Detection Using a Color Adaptation Algorithm. Sensors 2017, 17, 1604. [Google Scholar] [CrossRef] [Green Version]
- Craig, R.L.; Peterson, P.K.; Nandy, L.; Lei, Z.; Hossain, M.A.; Camarena, S.; Dodson, R.A.; Cook, R.D.; Dutcher, C.S.; Ault, A.P. Direct Determination of Aerosol pH: Size-Resolved Measurements of Submicrometer and Supermicrometer Aqueous Particles. Anal. Chem. 2018, 90, 11232–11239. [Google Scholar] [CrossRef]
- Duong, H.D.; Shin, Y.; Rhee, J.I. Development of novel optical pH sensors based on coumarin 6 and nile blue A encapsulated in resin particles and specific support materials. Mater. Sci. Eng. 2020, 107, 110323. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Badocco, D.; Cappellin, L.; Pastore, A. Enhancement of the pH measurement of a PVDF-supported colorimetric sensor by tailoring hue changes with the addition of a second dye. Microchem. J. 2020, 154, 104552. [Google Scholar] [CrossRef]
- Li, G.; Su, H.; Ma, N.; Zheng, G.; Kuhn, U.; Li, M.; Klimach, T.; Pöschl, U.; Cheng, Y. Multifactor colorimetric analysis on pH-indicator papers: An optimized approach for direct determination of ambient aerosol pH. Atmos. Meas. Tech. 2020, 13, 6053–6065. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Vieira Craveiro, D.; Oliveira Lima, K.; Leão Gouveia Costa, H.; Rubim Lopes, D.; Prentice, C. Intelligent Packaging with pH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.-W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality and safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Recent Advances in Intelligent Food Packaging Applications Using Natural Food Colorants. ACS Food Sci. Technol. 2021, 1, 124–138. [Google Scholar] [CrossRef]
- Rodrigues, C.; Lauriano Souza, V.G.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Kuswandi, M.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Contr. 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Yuk, H.-G. Detection of volatile organic compounds as markers of chicken breast spoilage using HS-SPME-GC/MS-FASST. Food Sci. Biotechnol. 2015, 24, 361–372. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Ukuku, D.O.; Yuk, H.-G. Identification and Quantification of Volatile Chemical Spoilage Indexes Associat-ed with Bacterial Growth Dynamics in Aerobically Stored Chicken. J. Food Sci. 2016, 81, M2006–M2014. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.-J.E.; Skadamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Dainty, R.H. Chemical/biochemical detection of spoilage. Int. J. Food. Microbiol. 1996, 33, 19–33. [Google Scholar] [CrossRef]
- Erim, F.B. Recent analytical approaches to the analysis of biogenic amines in food samples. Trends Analyt. Chem. 2013, 52, 239–247. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Alberti, G.; Quadrelli, P.; Biesuz, R. Development of a dye-based device to assess the poultry meat spoilage. Part I: Building and testing the sensitive array. J. Agric. Food Chem. 2020, 68, 12702–12709. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Alberti, G.; Capone, F.; Zanoni, C.; Mannucci, B.; Quadrelli, P.; Biesuz, R. Development of a dye-based device to assess the poultry meat spoilage. Part II: Array on act. J. Agric. Food Chem. 2020, 68, 12710–12718. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Capone, F.; Zanoni, C.; Alberti, G.; Quadrelli, P.; Biesuz, R. Colorimetric sensor array for monitoring, modelling and comparing spoilage processes of different meat and fish foods. Foods 2020, 9, 684. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Alberti, G.; Milanese, C.; Quadrelli, P.; Biesuz, R. Naked-Eye Food Freshness Detection: Innovative Polymeric Optode for High-Protein Food Spoilage Monitoring. ACS Food Sci. Technol. 2021, 1, 165–175. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Capone, F.; Alberti, G.; Zanoni, C.; Mannucci, B.; Quadrelli, P.; Biesuz, R. EVOH-based pH-sensitive Optodes Array & Chemometrics: From Naked-eye Analysis to Predictive Modelling to Detect Milk Freshness. ACS Food Sci. Technol. 2021, 1, 819–828. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Zanoni, C.; Alberti, G.; Quadrelli, P.; Biesuz, R. Towards intelligent packaging: BCP-EVOH@ optode for milk freshness measurement. Talanta 2022, 241, 123230. [Google Scholar] [CrossRef] [PubMed]
- Biesuz, R.; Quadrelli, P.; Magnaghi, L.R. Sensori per la Valutazione della Qualità di Prodotti Alimentari a Base di Carne. Italian Patent 10201900000464, 19 March 2019. [Google Scholar]
- Biesuz, R.; Quadrelli, P.; Magnaghi, L.R. Sensors for the Evaluation of the Quality of Meat-Based Food. WIPO PCT/IB2020/052998, 30 March 2020. [Google Scholar]
- Biesuz, R.; Quadrelli, P.; Magnaghi, L.R. Sensors for the Evaluation of the Quality of Meat-Based Food. U.S. Patent 17599196, 28 September 2021. [Google Scholar]
- Biesuz, R.; Quadrelli, P.; Magnaghi, L.R. Sensors for the Evaluation of the Quality of Meat-Based Food. EURO-PCT 20721299.4, 30 March 2020. [Google Scholar]
- GNU Image Manipulation Program (GIMP). Available online: https://www.gimp.org/ (accessed on 1 January 2022).
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: http://www.gruppochemiometria.it/index.php/software/19-download-the-rbased-chemometric-software (accessed on 1 January 2022).
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- European Commission Regulation (EC) No 2074/2005 of 5 December 2005. Total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and analysis methods to be used. Off. J. Eur. Union 2005, 338, 36–39.
- European Commission Regulation (EC) No. 2073/2005, Microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1.
- Biesuz, R.; Magnaghi, L.R. Role of Biogenic Amines in Protein Foods Sensing: Myths and Evidence. In Meat and Nutrition; Ranabhat, C.L., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review. Pol. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef] [Green Version]
- Casula, R.; Crisponi, G.; Cristiani, F.; Nurchi, V.M.; Casu, M.; Lai, A. Characterization of the ionization and spectral properties of sulfonephtalein indicators. Correlation with substituent effects and structural features. Talanta 1993, 40, 1781–1788. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Crisponi, G.; Nurchi, V.M.; Silvagni, R. Characterization of the ionization and spectral properties of sulfonephtalein indicators. Correlation with substituent effects and structural features. Part II. Talanta 1995, 42, 1157−1163. [Google Scholar] [CrossRef]
- Riley, A. 14—Plastics manufacturing processes for packaging materials. In Packaging Technology; Emblem, A., Emblem, H., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 310–360. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Growth of spoilage bacteria during storage and transport of meat. EFSA J. 2016, 14, 4523. [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnaghi, L.R.; Zanoni, C.; Bancalari, E.; Hadj Saadoun, J.; Alberti, G.; Quadrelli, P.; Biesuz, R. pH-Sensitive Sensors at Work on Poultry Meat Degradation Detection: From the Laboratory to the Supermarket Shelf. AppliedChem 2022, 2, 128-141. https://doi.org/10.3390/appliedchem2030009
Magnaghi LR, Zanoni C, Bancalari E, Hadj Saadoun J, Alberti G, Quadrelli P, Biesuz R. pH-Sensitive Sensors at Work on Poultry Meat Degradation Detection: From the Laboratory to the Supermarket Shelf. AppliedChem. 2022; 2(3):128-141. https://doi.org/10.3390/appliedchem2030009
Chicago/Turabian StyleMagnaghi, Lisa Rita, Camilla Zanoni, Elena Bancalari, Jasmine Hadj Saadoun, Giancarla Alberti, Paolo Quadrelli, and Raffaela Biesuz. 2022. "pH-Sensitive Sensors at Work on Poultry Meat Degradation Detection: From the Laboratory to the Supermarket Shelf" AppliedChem 2, no. 3: 128-141. https://doi.org/10.3390/appliedchem2030009
APA StyleMagnaghi, L. R., Zanoni, C., Bancalari, E., Hadj Saadoun, J., Alberti, G., Quadrelli, P., & Biesuz, R. (2022). pH-Sensitive Sensors at Work on Poultry Meat Degradation Detection: From the Laboratory to the Supermarket Shelf. AppliedChem, 2(3), 128-141. https://doi.org/10.3390/appliedchem2030009