Abstract
The yellow-flowering plant Cladanthus arabicus (L.) Cass., commonly called Arabian Cladanthus or palm springs daisy, is typical of the West Mediterranean region and is particularly abundant in Morocco. The plant is used in traditional Moroccan medicine for the treatment of diabetes and other ailments. Over the past 20 years, this abundant wild plant has been neglected from a phytochemical viewpoint. For the first time, the present review provides a survey of the pharmacological properties reported from extracts of C. arabicus and from essential oils derived from the aerial parts, mainly antimicrobial, antioxidant, and anti-inflammatory properties. The main bioactive natural products are discussed, with a focus on two rare sesquiterpenes of major interest, which are abundant in the stems and leaves: the 6,12-guaianolide cladantholide and the germacranolide sintenin. These sesquiterpene lactones and their analogues are presented to highlight their properties, extraction or total synthesis, and their therapeutic benefits. They both represent convenient biosourced precursors for the synthesis of derivatives. Sintenin may be used as a starting material for the design of hemi-synthetic germacradienolide-type costunolide or parthenolide derivatives. The 6,12-guaianolide scaffold of cladantholide offers opportunities to design novel arglabin derivatives. The therapeutic potential of the neglected and under-utilized plant Cladanthus arabicus and its original phytochemicals shall be explored further.
Keywords:
cladantholide; Cladanthus; Cladanthus arabicus; germacranolide; guaianolide; sesquiterpene; sintenin 1. Introduction
Cladanthus is a small genus of twelve species with an accepted name, which are all native of the Mediterranean region and southwestern Europe (www.worldfloraonline.org accessed on 9 November 2023). They are characteristic of the sunflower family. Five species are found in Morocco: Cladanthus mixtus (L.) Chevall, Cladanthus arabicus (L.) Cass, Cladanthus scariosus (Ball) Oberpr. and Vogt, Cladanthus eriolepis (Maire) Oberpr. and Vogt, and Cladanthus flahaultii (Emb). Oberpr. and Vogt. With feathery leaves and a daisy-like appearance with gold or white flowers, in general, these Mediterranean plants do not go unnoticed in fields and gardens. The prevalent species in Morocco are C. scariosus which is endemic in the High Atlas area [1,2] and the flavonoid-rich species C. mixtus in other areas [3]. The latter Cladanthus species is a prominent source of polyphenols and flavonoids, such as the glycosyl-flavone acetylisospinosin, which has recently been identified together with many other flavonoids [4,5]. Here we have centered our analysis on the less-studied species Cladanthus arabicus (L.) Cass., 1817 (hereafter designated C. arabicus) which is well distributed in Morocco, notably in the anti-Atlas region and presents interesting phytochemical properties.
C. arabicus (Figure 1) displays feathery leaves and a daisy-like appearance with gold flowers. It is commonly called Arabian Cladanthus or criss-cross, golden of Araby, golden crown, palm springs daisy, or Moroccan sunshine, as well as wild Moroccan chamomile although this later trivial name is more often associated with the species C. mixtus [6]. C. arabicus (L.) Cass. is the commonly used name and the accepted botanical name. There are several synonyms, such as C. maroccanus Gand. and Anthemis arabica L., but they are rarely used in the scientific literature.

Figure 1.
The plant Cladanthus arabicus (L.) Cass., also called (synonyms): Anthemis arabica L.; Anthemis prolifera Pers.; Anthemis sessilis Salisb.; Chamaemelum cladanthus E.H.L.Krause; Chamaemelum proliferum Moench; Cladanthus ifniensis Caball.; Cladanthus maroccanus Gand.; Cladanthus proliferus DC. (a,b) Spring pastures of the C. arabicus. (c,d) Flowers. (e) Distribution map for C. arabicus.
As a perennial herb, C. arabicus represents an attractive flowering plant, native to the Ibero–Maghreb region (Algeria, Libya, Morocco, Sicily, Spain, and Tunisia). It is a hardy or half-hardy and annual and can self-seed in light, sandy soils. The plant, very branched and up to 80 cm high, grows well on ordinary, drained soil in full sun. It behaves as a bushy and spreading plant forming a colored mound of pleasantly scented feathery foliage. All summer, it provides a profusion of large, fragrant, golden-yellow flowers. The main flower on each stem is followed by further flowers on stems arising directly under the main one (Figure 1). The ferny foliage gives rise to many golden discs, and generally each new stem radiates from the edge of the faded flower. The plant is not considered edible but the flowers can be used to garnish dishes. In Morocco, the flowers (called Tâfs (or Tafsse) in the Arabic language, and Aourzid in the vernacular Amazigh language) are used by the Messiwa people to decorate dishes and are consumed [7,8]. The plant is eaten by wild herbivores, notably by Moroccan dorcas gazelle in west-central Morocco [9] and by camels but with a risk of toxicity in this later case [10].
The objectives of the study, presented here as a narrative literature survey, are to underline the ethnobotanical usages of the plant C. arabicus in Morocco, the pharmacological effects reported with plant extracts, and to identify the main active phytoconstituents and their mechanism of actions. For that, an extensive analysis of the scientific information was performed via the analysis of all general databases (essentially, Pubmed Central, Scopus, and Google Scholar) and specific databases from publishers (such as Oxford University Press, SAGE journals, ScienceDirect, Springer, Thieme, Wiley, and others) covering the literature (mostly in English, or occasionally French languages) from 1970 to October 2023. The search was conducted using keywords such as Cladanthus, phytochemicals, and sesquiterpenes. More than 160 articles were analyzed. A phytochemical analysis of C. arabicus is reported, pointing out the unique presence of the two rare sesquiterpenes cladantholide and sintenin. This is the first and unique review on the little-known species C. arabicus. commonly found in semi-arid Mediterranean landscapes and in Morocco in particular.
2. Use of C. arabicus in Traditional Medicine
Ensuring a sustainable supply of affordable medicines for the world’s fast-growing population (which now surpass 8 billion people) is a major challenge in today’s economy. The challenges are even broader for people with low resources living in rural areas. There is a major need to improve access to and the affordability of health care in rural communities [11,12]. The World Health Organization (WHO) has estimated that about 80% of the world’s population use traditional medicine or rely on plant-based therapy for their primary care needs [13]. Plants have long been used as traditional remedies to help fight human diseases and remain largely used today to combat parasitic and virus infections, cardiovascular, mental, and inflammatory diseases, cancers, and many other pathologies [14,15]. Moreover, the demand for health-promoting products is increasing [16]. Plants still represent a large untapped source of structurally novel compounds that might serve as a lead in the development of novel drugs [17]. Many medicines of plant origin with analgesic and anti-nociceptive activity have been used for a long time without any major adverse effects [18,19]. Similarly, traditional herbal medicines and natural plant products contribute considerably to the treatment of cancers [20,21]. The bioprospection of medicinal plants remains a valid approach to identify new molecules which could be active against cancer and inflammatory diseases [22,23,24].
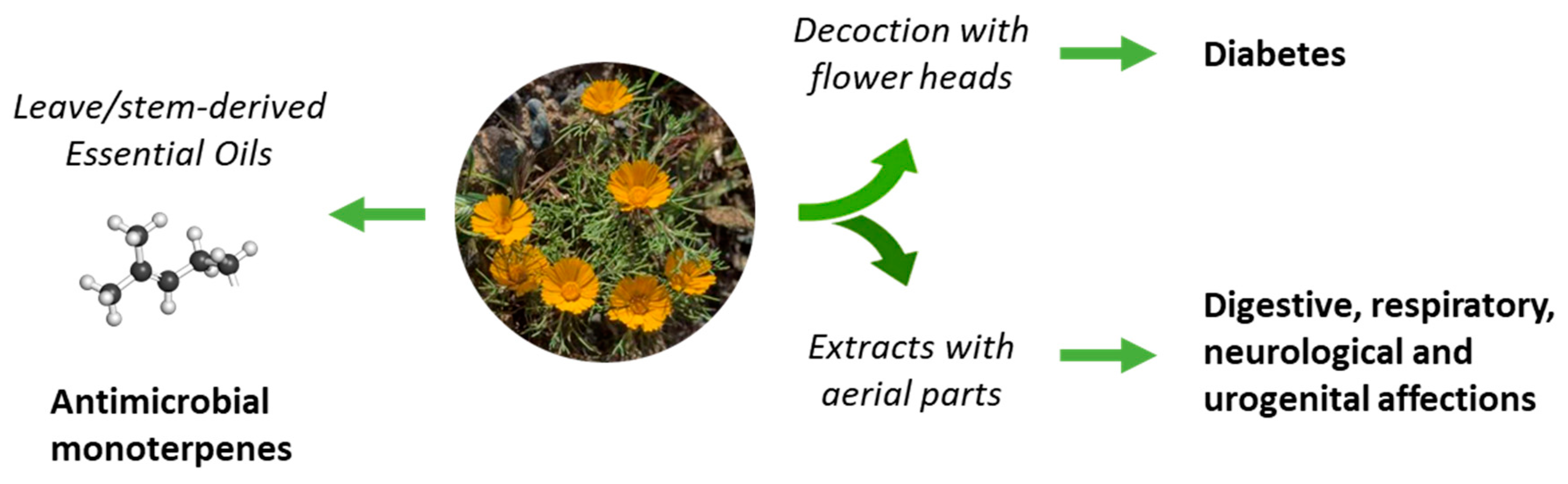
The use of C. arabicus in traditional medicine is not largely documented, with only sparse citations in scientific reviews. The plant is known in Morocco for the treatment of diabetes [25]. Apparently, an infusion prepared from the flower heads of the plant, drunk twice a day, could be useful to combat type 2 diabetes [26,27] (Figure 2). C. arabicus can be used alone or combined with other plants, such as Rubia peregrina, Corrigiola telephiifolia, or Ridolfia segetum, to prepare an antidiabetic decoction [28]. There are other reports primarily citing the use of the species C. mixtus (L.) Chevall [29] and C. scariosus (Ball) Oberpr. and Vogt [30], but not C. arabicus, for the treatment of diabetes. Other anti-diabetic Moroccan Asteraceae are cited also [31,32] and could be combined with C. arabicus. Beyond diabetes, the uses of C. arabicus for the treatment of digestive disorders, neurological troubles, and respiratory and urogenital affections have been mentioned, without much detail [33]. Apparently, the plant is “good for stomach and anemia” but robust experimental evidence to support these claims is lacking [7].
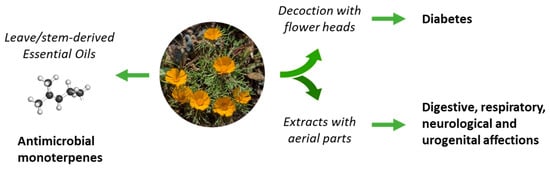
Figure 2.
The uses of C. arabicus in traditional medicine. Decoctions prepared from flowers heads are used to treat diabetes and total extracts are used to combat a variety of diseases. Essential oils from the leaves and stems provide remedies to treat microbial infections.
3. Pharmacological Activities of C. arabicus Extracts
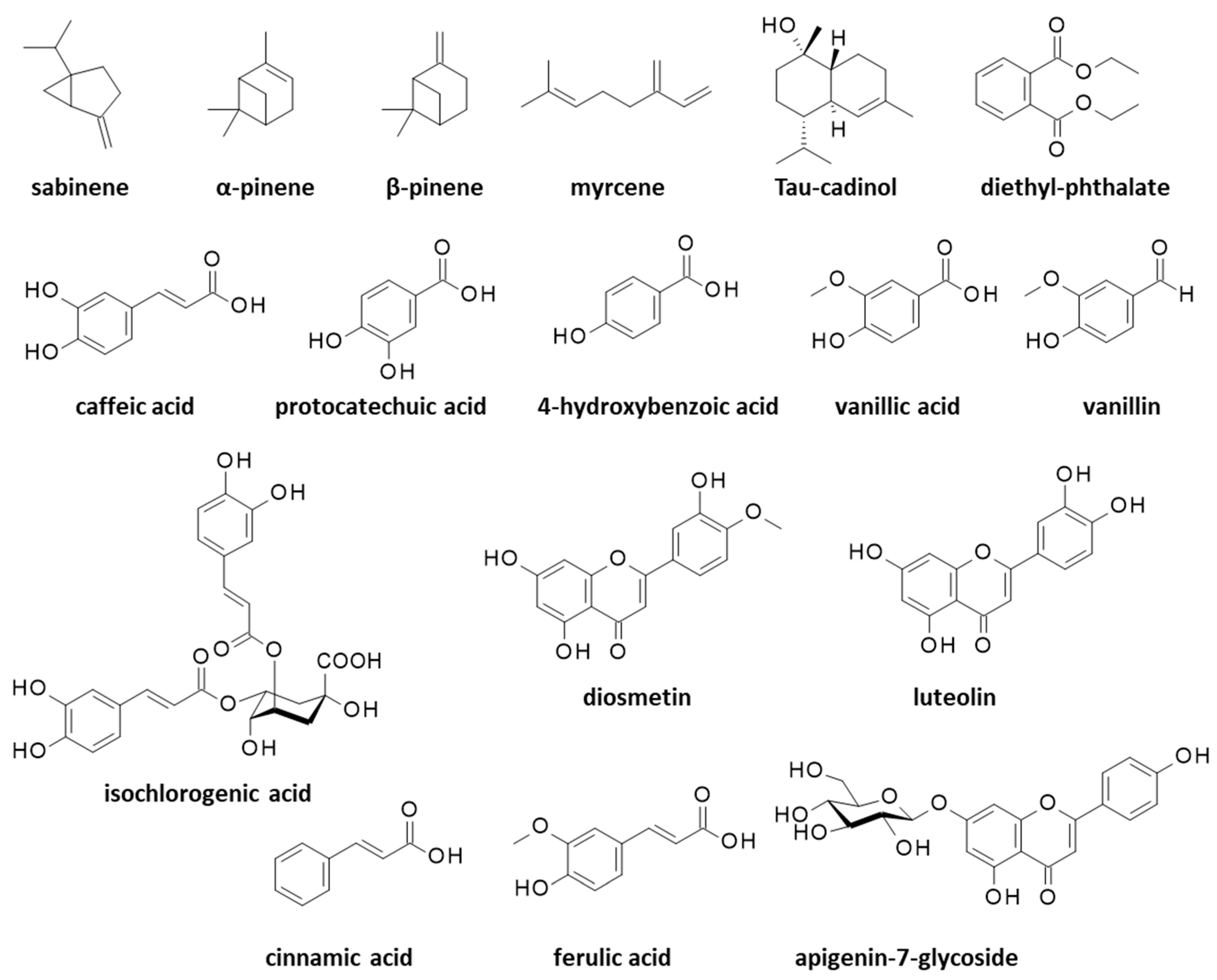
Essential oils (EO) extracted from the aerial parts of C. arabicus have revealed the marked antimicrobial activities associated with a rich monoterpenic compounds content (Figure 2). In particular, an essential oil derived from the plant stems and leaves showed antibacterial activities against the opportunistic human pathogen Bacillus cereus and Enterococcus faecalis which is at the origin of nosocomial infections, but it showed no effect against the hard-to-treat pathogen Pseudomonas aeruginosa. This leaf/stem-derived EO principally contained the monoterpenes sabinene (13%), α-pinene (8%), β-pinene (12%), myrcene (7%), and many other volatile terpenes in smaller proportions (36 monoterpenes were identified) (Figure 3).
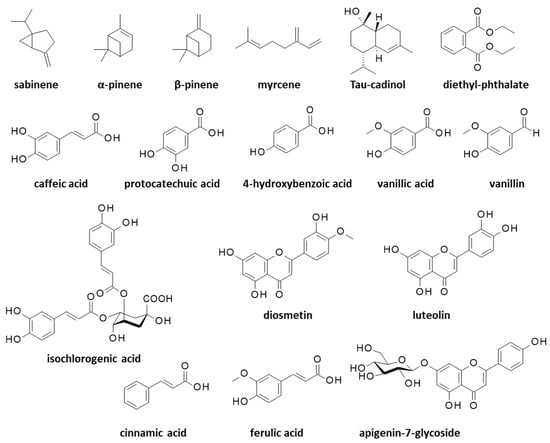
Figure 3.
Structures of various natural products identified from essential oils or alcoholic extracts of Cladanthus arabicus.
An EO prepared under identical conditions from the plant’s flowers revealed a much poorer monoterpenes content, essentially only sabinene (23%) and β-pinene (32%) [34]. The composition of the essential oil can vary significantly from one study to another, depending on the process, the plant origin and its growth, or the collecting season. For example, an EO made from C. arabicus’ aerial parts was found to contain up to 60 terpenic compounds, principally sabinene (31%), β-pinene (17%), myrcene (12%) and α-pinene (5%). In this case, the EO was characterized for its strong antioxidant activity (IC50 = 55.4 μg/mL in a DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay) and its antimicrobial effects, notably against Micrococcus luteus bacterium and the pathogenic fungus Candida albicans (MIC = 0.187 mg/mL in both cases) [35]. In a subsequent study, the same authors further tested this EO against other microbes and found activities against Escherichia coli (strain ATCC 25922), Klebsiella pneumoniae (strain S12b/16), and Enterobacter cloacae (strain S5/16), but at significantly higher doses than were measured for reference antibiotics such as amoxicillin and neomycin. Interestingly, the EO showed a synergistic activity with amoxicillin against Proteus mirabilis (strain S32/16), a Gram-negative bacterium that is frequently implicated in urinary tract infections [36]. The potent antibacterial activity of C. arabicus EO and its strong interaction with amoxicillin warrant further investigation. Apparently, the terpene content of C. arabicus EO is quite variable from one preparation to another. Recently, Mziouid and coworkers [37] reported the antioxidant activity of an EO from C. arabicus which contained α-pinene (5.7%) and β-pinene (23.6%), but also a large proportion of tau-cadinol (9.5%) (Figure 3), a sesquiterpene commonly found in EOs but not mentioned in the aforementioned preparations using C. arabicus. T-cadinol is known to be an anti-trypanosomal agent which is able to induce a mitochondrial impairment in Trypanosoma cruzi parasites responsible for the Chagas disease [38]. The C. arabicus EO also contained diethyl phthalate (DEP, 7.9%) which is an unwanted estrogenic endocrine-disrupting chemical (Figure 3). In this case, the EO exhibited a modest antioxidant activity (IC50 = 1.33 mg/mL in the DPPH assay) [37]. A well-established, robust process is needed to prepare an EO in a reproducible manner with a constant, stable composition, which is free from toxic chemicals (following the recommendations of the European Pharmacopoeia, for example).
The antioxidant activity is more pronounced when a total plant extract is used instead of an EO. For example, the antioxidant IC50 value dropped from 1.33 mg/mL to 0.23 mg/mL when a methanolic extract was used in place of the EO from C. arabicus, due to the high phenolic content of the extract [37]. A phytochemical analysis of an alcoholic extract of the aerial parts of C. arabicus has revealed the presence of two major components, caffeic acid (4.9 mg/kg) and protocatechuic acid (4.7 mg/kg), followed with other polyphenols such as ferulic acid (1.8 mg/kg), 4-hydroxybenzoic acid (1.5 mg/kg), vanillin, and vanillic acid (both 1.6 mg/kg), plus a series of minor components including flavonoids which are sometimes glycosylated (diosmetin, luteolin, apigenin-7-glucoside) (Figure 3). The high polyphenols content, notably caffeic acid, suggests a possible use of the plant in combatting adverse hematologic events such as thrombocytopenia [39]. Caffeic acid is a strong antioxidant and an anti-inflammatory agent with cardioprotective and hepatoprotective effects. This common natural product is considered to be beneficial in limiting the progression of diabetes mellitus and its associated complications [40]. Protocatechuic acid is also a chemoprotective agent which is notably able to protect cardiomyocytes from oxidative damages [41]. These two polyphenols are commonly found in fruits, vegetables, grains, and herbal medicine; thus, they are definitely not specific to C. arabicus. Nevertheless, these compounds are important. Polyphenols from Mediterranean plants have benefits in the prevention and treatment of various diseases, notably skin diseases such as atopic dermatitis, psoriasis, and chronic urticaria [42].
In C. arabicus extracts, the polyphenol content was rich, whereas the levels of heavy metals (Cd, As and Pb) were low, and the extract revealed a marked antibacterial activity against Escherichia coli strain S33/16 (MIC = 0.125 mg/mL) [43]. The same hydro-methanolic extract has revealed the presence of diverse flavonoids and phenolic acids, including isochlorogenic acid and cinnamic acid (Figure 3). All together, these compounds are responsible for the marked antioxidant activity of the extract, as well as its modest inhibitory activity toward cholinesterase, acetylcholinesterase, tyrosinase, and α-glucosidase enzymes [44].
4. Phytochemical Analysis
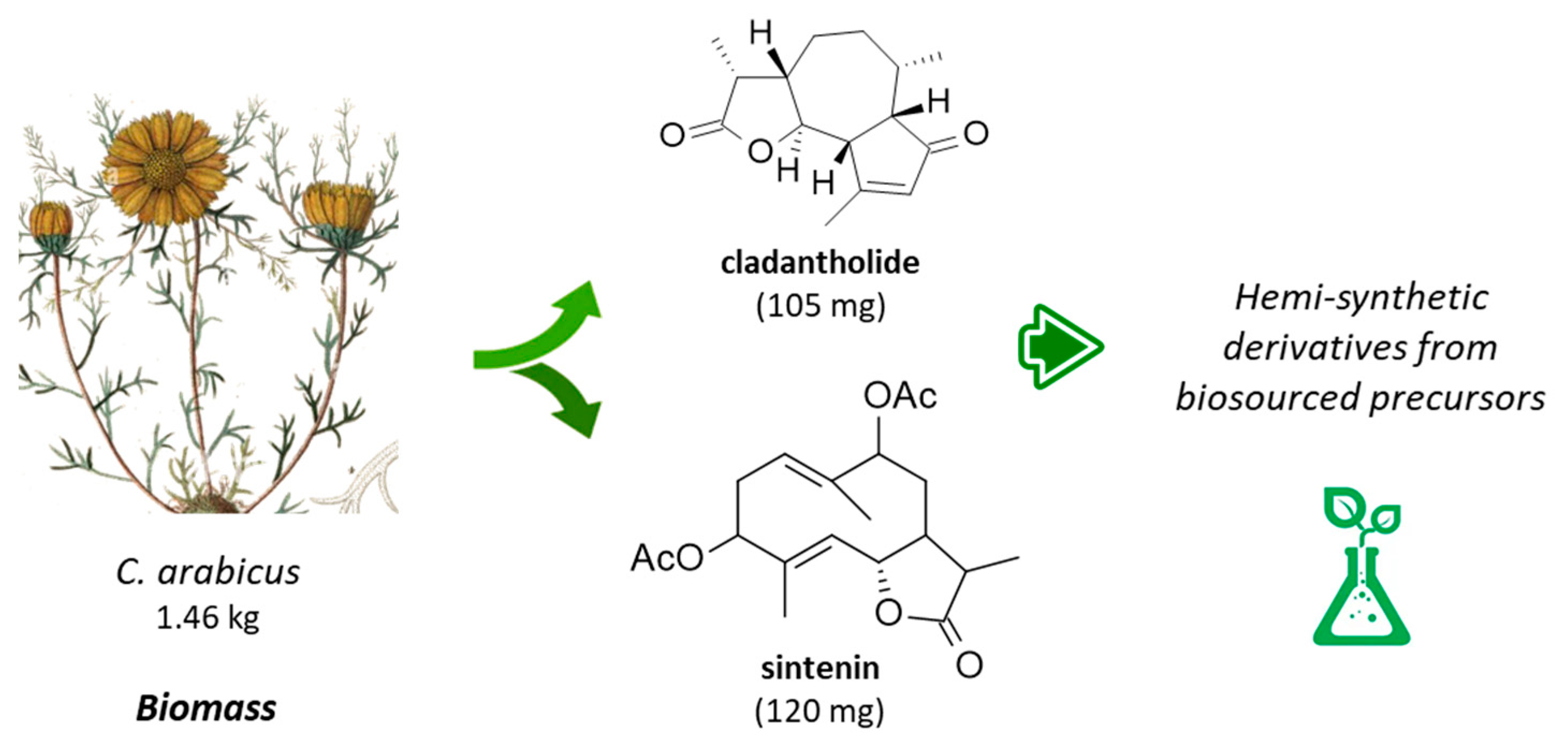
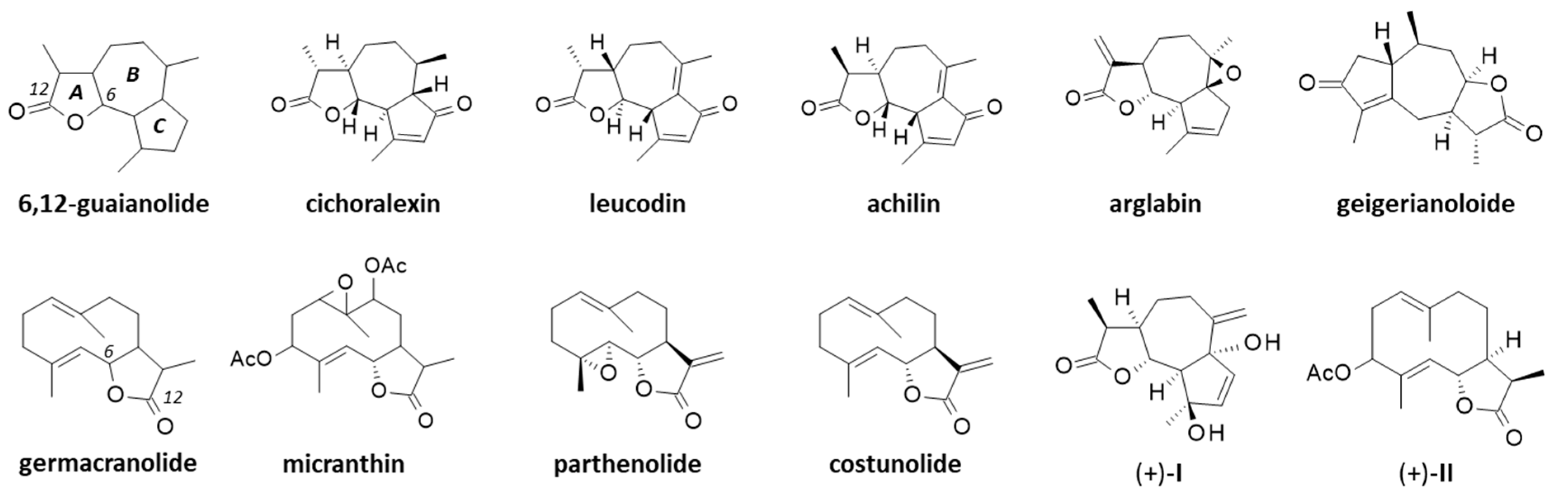
The first phytochemical analysis of C. arabicus was reported 30 years ago, when Daniewski and coworkers reported the isolation of a new sesquiterpene they called cladantholide from a methanolic extract of the total dried aerial parts (without flowers) of a sample of C. arabicus cultivated locally [45]. The product is abundant in the plant. Over 100 mg of the newly purified product was obtained from an initial 1.46 kg of the dried plant material. Cladantholide corresponds to a three-ring system with a central seven-membered ring fused to planar a cyclo-pentenone ring on one side and a five-membered lactone ring on the other side (Figure 4). It is an isomer of cichoralexin (first isolated from chicory [Cichorium intybus] also known as Carpesia lactone [46]) and is a close analogue to the two sesquiterpene lactones leucodin and achillin (Figure 5) found in Achillea species. These two products display antihypertensive and vasorelaxant effects [47]. They also exhibit anti-inflammatory properties via the inhibition of cyclooxygenase 2 (COX-2) expression and activity. They are considered to be of potential interest in the treatment of pain and inflammation and for cardiometabolic diseases [48]. Leucodin has revealed a high anti-hypercholesterolemic potential [49,50] coupled with smooth muscle relaxant activity [47,51]. This type of 6,12-guaianolide structure (Figure 5) has been found in diverse Achillea species (Asteraceae) notably those used to prepare chamomile herbal tea [52].
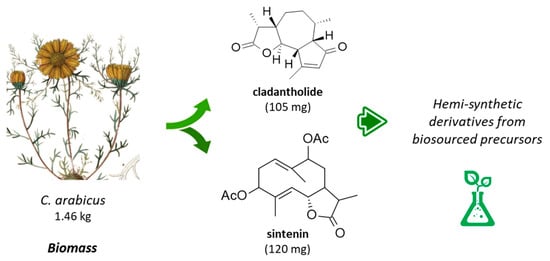
Figure 4.
Structures cladantholide and sintenin isolated from the stems and leaves of C. arabicus.
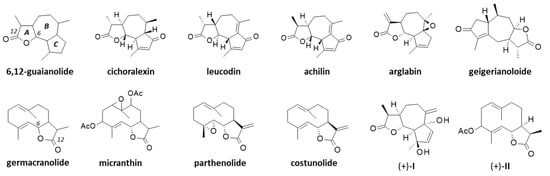
Figure 5.
Structures of several guaianolides related to cladantholide and germacranolides related to sintenin.
Cladantholide has been isolated from C. arabicus together with the germacranolide derivative sintenin (Figure 4), which is also a sesquiterpenoid found in diverse Achillea species, such as A. clavennae, A. millefolium, and others [53,54]. Sintenin is a sesquiterpene lactone initially isolated from the aerial parts of Achillea sintenisii Hub.-Mor. (Compositae) [55] and is also present in A. falcata L. [56] and A. micrantha Willd. [57]. But the sintenin content in Achillea plants is limited. For example, only 6 mg of sintenin was obtained from 1.37 kg of the dried aerial parts of A. sintenisii [55]. A much higher quantity can be obtained from the stems and leaves of C. arabicus. Recently, cladantholide has also been identified from the plant species of Scorzonera longiana Sümbül (an endemic species to Turkey), together with other terpenes and dihydroisocoumarin derivatives [58]. The ground-up dry underground parts (rootstock, 600 g) of S. longiana afforded 10.7 mg of cladantholide, which is 4-times less than the yield obtained from the dried aerial parts of C. arabicus. This latter fast-growing and easily accessible plant can provide a convenient source of cladantholide.
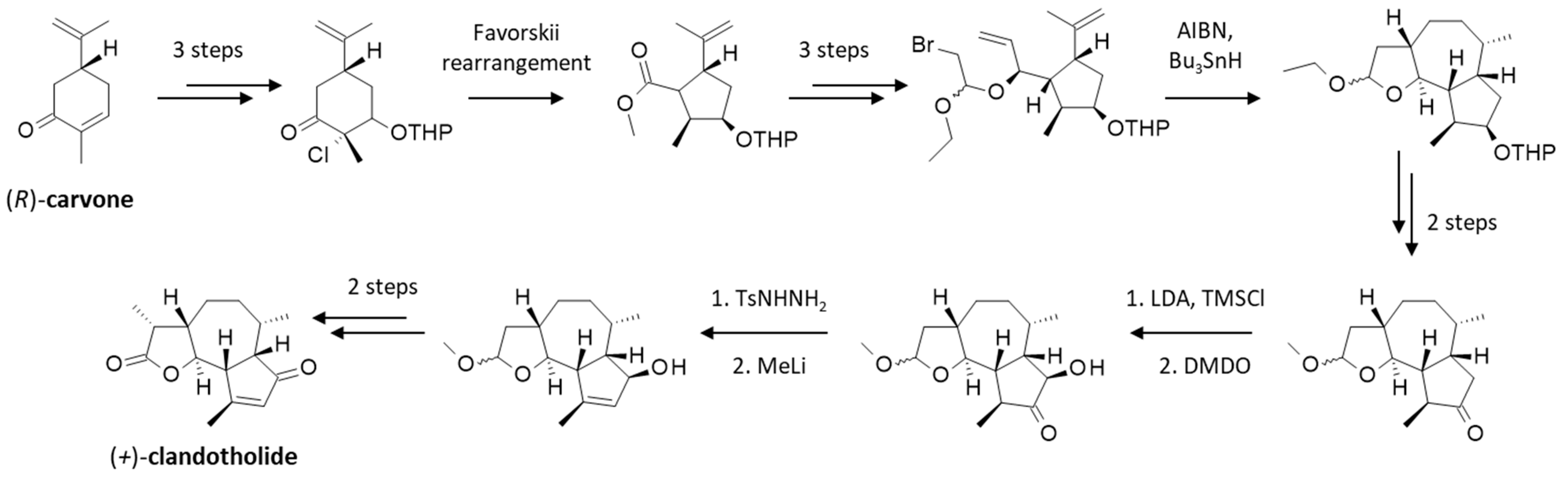
Alternatively, the product can be obtained by chemical synthesis. The total synthesis of (+)-cladantholide was reported in 1997, starting from (−)-carvone via a long and tedious procedure (16 steps, 5.0% overall yield) implicating a cis-fused hydroazulenic lactone intermediate, as depicted in Figure 6 [59]. This clever chemical work based on a radical cyclization cascade has been recognized as a remarkable synthesis of a complex guaianolide [60]. This is a technical tour-de-force and still the only stereoselective synthesis described thus far to obtain (+)-cladantholide (the (−)-isomer was not obtained). In recent years, several chemical procedures have been described to produce other 6,12-guaianolides [61], but none to specifically produce cladantholide apart from the original process described 27 years ago [59].
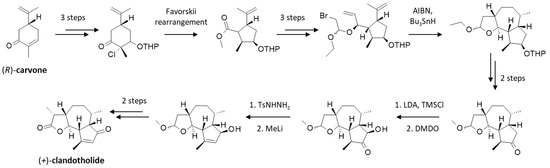
Figure 6.
Total synthesis of (+)-cladantholide, as originally reported [59] and summarized [60]. Details of the 16 steps can be found in the original reports.
Sintenin is even more abundant than cladantholide in C. arabicus [45]. A dried sample of the plant (1.46 kg) was used to obtain 105 mg of cladantholide and 120 mg of sintenin (Figure 4). Over the past 30 years, these two compounds have rarely been reported. Sintenin and its epoxide derivative micranthin have been identified in an ethyl acetate extract of the aerial parts of the plant Achillea biebersteinii Afan. and the extract was well characterized for its antidiabetic and gastric antisecretory effects [62]. It is therefore plausible that sintenin contributes to the antidiabetic effect of C. arabicus. Sintenin is a non-cytotoxic lactone, with no effect on the proliferation of different types of cancer cells [53,54]. Sesquiterpene lactones often present marked antitumor properties, such as those isolated from the Inula species for example [63,64], but it is not the case for sintenin and cladantholide, both non-cytotoxic compounds. They deserve further studies as anti-inflammatory and antidiabetic agents. Interestingly, a structural analogy can be seen between cladantholide and the sesquiterpene lactone geigerianoloide isolated from the antidiabetic plant Geigeria alata (DC) Oliv. and Hiern. (also from the Asteraceae family) [65] (Figure 5).
Parenthetically, caution must be exercised when studying the scientific literature because a totally distinct product exists which is also called sintenin, which is a naturally occurring ester, (3-(3,4-dimethoxyphenyl)propyl 3-(3,4-dimethoxyphenyl)propanoate) with cytotoxic properties, used as a template for the synthesis of derivatives endowed with antioxidative and neuroprotective activities [66,67]. This ester sintenin has nothing to see with lactone sintenin from C. arabicus which is a non-cytotoxic compound.
Beyond cladantholide and sintenin, two other sesquiterpenes designated (+)-I and (+)-II (Figure 5) have been isolated from the original methanolic extract of a sample of C. arabicus (cultivated in Poland). The corresponding structures were presented but no specific information was reported [68]. Compound I is unknown. Compound II is an analogue of sintenin lacking one of the two acetyl groups. We could not find these two compounds in chemical databanks.
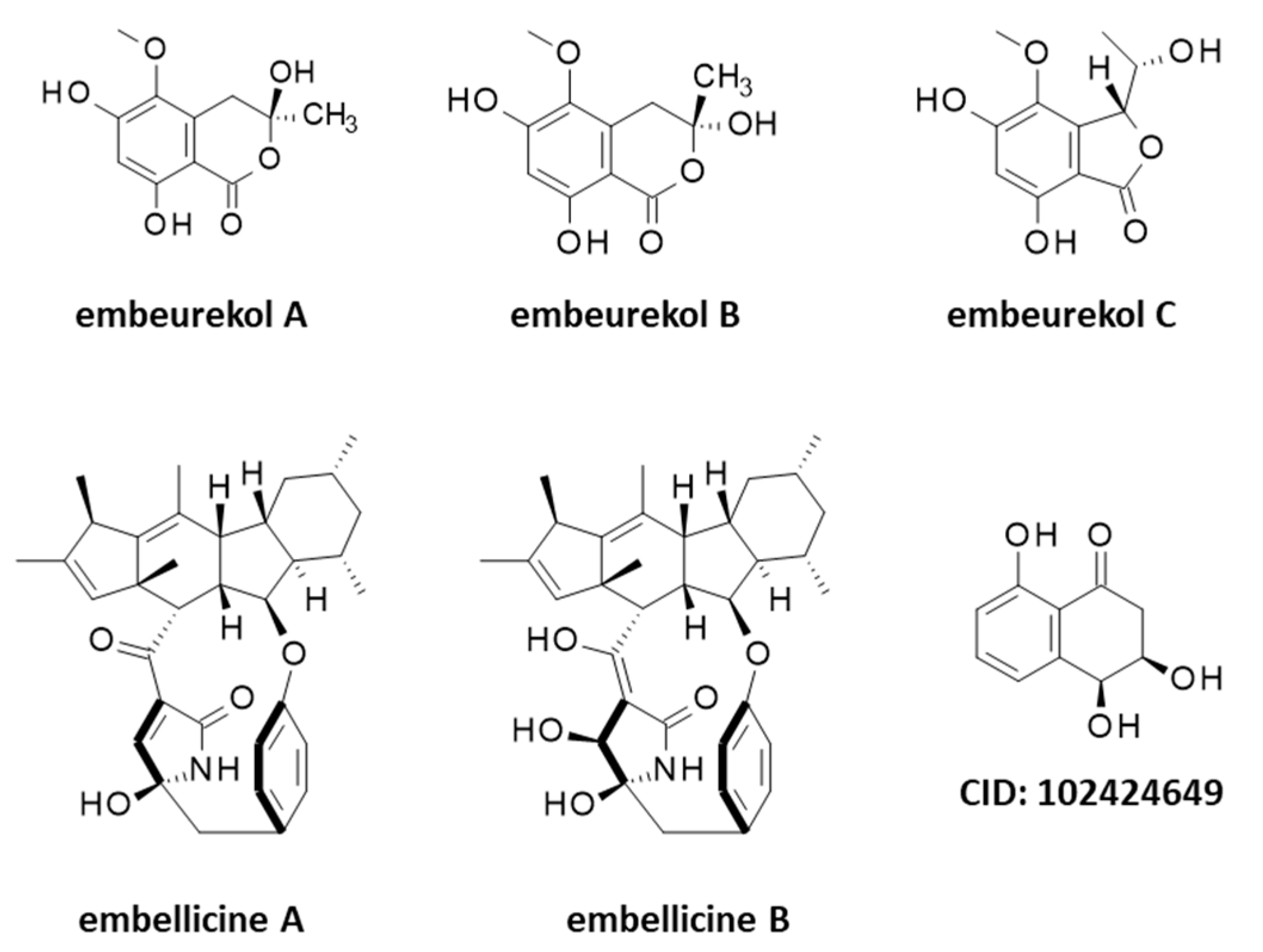
Five other products should be mentioned at this stage, the alkaloids embellicines A and B, and three polyketides named embeurekols A–C (A and B are isomers) but they originate from an endophyte fungus Embellisia eureka (renamed Alternaria eureka) not directly from C. arabicus [69,70] (Figure 7). This fungus grows on the surface of healthy stem tissues of C. arabicus in Morocco. It can be cultivated and used for the biotransformation of natural products, performing oxygenation, oxidation, and epoxidation reactions on sapogenins for example [71,72,73]. Similarly, the small bicyclic molecule 3,4-dihydro-3,4,8-trihydroxy-1[2H]-naphthalenone (Figure 7) has been isolated from an extract of Embellisia eureka collected on a Moroccan sample of C. arabicus [74]. Trihydroxytetralones are bioactive secondary metabolites produced by diverse pathogenic fungi [75,76,77].
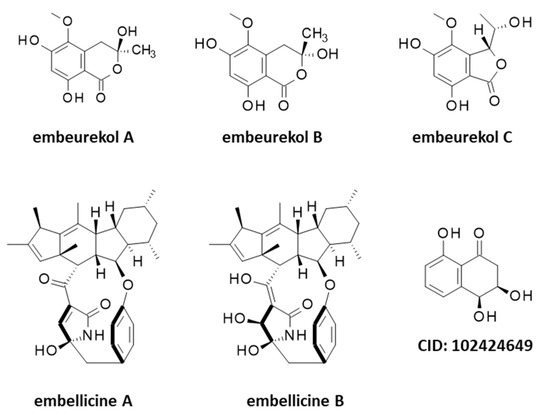
Figure 7.
Structures of several guaianolides related to cladantholide and germacranolides related to sintenin.
Most phytochemical studies of C. arabicus are concerned with the terpenes and alkaloids found in the plant. But, as mentioned above, diverse flavonoids have been identified in plant extracts, such as apigenin and its glycosyl derivative apigenin-7-glucoside [36,44]. Another flavonoid of medicinal interest found in C. arabicus is diosmetin [43] (Figure 3). This common flavonoid, found in many plants, displays anti-inflammatory, antioxidant, antimicrobial, antilipolytic, and analgesic activities [78]. Diosmetin might well contribute to the antidiabetic action of C. arabicus extracts because it has been shown to ameliorate glucose metabolism in diabetic mice via the regulation of the PI3K/AKT signaling pathway and a restoration of the unbalanced gut microbiota [79]. It is an inhibitor of aldose reductase (IC50 = 15.7 µM), an enzyme of the polyol pathway implicated in hyperglycemia, and a useful compound to combat type 2 diabetes mellitus [80,81].
5. Discussion
C. arabicus is endemic to the West Mediterranean area, in particular to Morocco where the plant is abundant and underused [82]. Large fields of this wild sunflower plant can be found, notably in the Aouganz region in the High Atlas [83]. It is one of the many Moroccan medicinal plants traditionally used to treat painful digestive symptoms and to combat certain microbial infections [84]. C. arabicus is considered to be an antidiabetic plant, as it is the case for C. mixtus and many other Moroccan plants [25,29,85]. Extracts of C. arabicus are of interest to treat type 2 diabetes, owing to the presence of compounds like diosmetin and luteolin. This later flavonoid is well-known for its anti-inflammatory and antidiabetic properties [86,87]. But it is a common antioxidant compound found in many plants and is not specific to C. arabicus. Diosmetin can be readily isolated from in citrus fruits for examples [88].
Despite the natural abundance of the plant and its multiple medicinal usages, C. arabicus is little exploited at present. The plant is consumed by some herbivores [9,10] and by local monkeys (the Barbary macaques, also known as the Barbary ape or magot (Macaca sylvanus) [89]. Therefore, the plant may be used more broadly to feed animals, at least when combined with other grass species. The shoot tissue of C. arabicus may accumulate heavy metals, notably chromium [90]. Therefore, the potential use of the plant as a food source for animal feeding must be monitored and adapted. The wild plant is abundant, at least in some parts of Morocco. In addition, the cultivation of the plant could be developed. This is already the case for the related species C. mixtus and C. scariosus for which procedures for seed germination and the growth of plantlets have been developed [91,92]. Similar processes could be adapted for C. arabicus.
One of the main reasons for interest in C. arabicus, at least from a phytochemical perspective, is the presence of the two rare sesquiterpenes sintenin and cladantholide, which are both relatively abundant in the plant and readily extractable. These two original products have been little studied in recent years and largely neglected in general. It is time to underline the potential uses of these molecules, if not for their pharmacological properties (largely unknown at present) but at least as starting materials for the design of other molecules. The synthesis of guaianolide sesquiterpene lactones is a current topic of interest to design novel anticancer and/or anti-inflammatory agents [93,94,95]. Novel guaianolide lactones capable of inhibiting the release of pro-inflammatory cytokines are regularly reported, such as the millefoliumines which inhibit the release of nitric oxide and the expression of TNF-α and IL-6 [96]. Cladantholide could be used as a model 6,12-guaianolide to modulate or to substitute the lactone A-ring, and/or to modify the cyclopentenone C-ring. It may serve as a model to access diverse analogues, as has been the cases with the prototypic 6,12-guaianolides arglabin and thapsigargin [97,98]. Arglabin (Figure 5) has recently been characterized as an anticancer inhibitor of EGFR tyrosine kinase [99,100] and a regulator of neuroinflammation [101]. Hemi-synthetic derivatives could be designed starting from cladantholide. Thapsigargin is also a leading 6,12-guaianolide from which analogues of pharmacological interest have been designed, such as the prodrug mipsagargin (G-202) which is currently in phase II clinical testing for the treatment of advanced hepatocellular carcinoma [102,103,104]. It is, therefore, of interest to consider cladantholide as a source compound for the design of novel 6,12-guaianolides. By the same token, sintenin could be used as a starting material for the design of germacradienolide-type derivatives. Sintenin is a diacetylated derivative of costunolide which is a potent anti-inflammatory agent and a blood–brain barrier permeable compound of interest to address certain brain diseases [105,106,107]. Costunolide derivatives targeting pyruvate kinase M2 (PKM2) are being designed for the treatment of ulcerative colitis [108]. Other derivatives could be designed based on the sintenin scaffold.
The presence of the sesquiterpenes cladantholide and sintenin make C. arabicus a plant of prime interest. Thus far, different studies have been focused in other Cladanthus species, notably C. mixtus which is abundant in the northern part of Morocco [4]. This species contains many useful bioactive products (polyphenols, flavonoids, terpenoids, sterols, etc.) and displays anticancer properties [5,109]. But its phytochemical profile is distinct from that of C. arabicus, which is the only species that contains cladantholide and sintenin. This plant produces an unusual amount and variety of terpenoids. The unique terpenoid composition of C. arabicus likely arises from the involvement of terpene synthases with different specificities, but they have not been identified thus far.
In conclusion, the plant Cladanthus arabicus which grows well and is abundant in Morocco has received little attention thus far, apart from its ornamental characteristics and the occasional use in traditional medicine to treat diabetes and other mild affections. The plant has been largely neglected thus far as a possible source of bioactive compounds, despite the presence in the stems and leaves of original natural products, notably the germacranolide sintenin and the 6,12-guaianolide cladantholide. These two interesting products shall be better considered as precursors for the design of bioactive derivatives. The time has come today to have a new look at this abundant but neglected plant for the identification of novel phytochemicals and the development of new remedies against human diseases.
Author Contributions
L.B.: Investigation; Writing—and review and editing. C.B.: Conceptualization; Investigation; Visualization; Writing—original draft and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Author Christian Bailly is the owner of OncoWitan, a private consulting office. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bellioua, S.; Amari, S.; Warda, K.; Aghraz, A.; Dilagui, I.; Ouhaddou, S.; Sissi, S.; Bekkouche, K.; Larhsini, M.; Markouk, M. Chemical profile, antioxidant and antimicrobial effects of essential oil from the Moroccan endemic plant Cladanthus scariosus (L.). J. Essent. Oil Res. 2022, 34, 394–404. [Google Scholar] [CrossRef]
- El Hafidi, S.; Bakhy, K.; Ouhssine, M.; Benzakour, A.; Khamar, H.; Casanova, J.; Paoli, M.; Tomi, F. Composition and Chemical Variability of the Essential Oil from Aerial Parts of Cladanthus scariosus, an Endemic Species to the Moroccan High Atlas. Chem. Biodivers. 2023, 20, e202201022. [Google Scholar] [CrossRef]
- Elouaddari, A.; El Amrani, A.; JamalEddine, J. Intraspecific variability of the essential oil of Cladanthus mixtus from Morocco. Nat. Prod. Commun. 2014, 9, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Benmerache, A.; Alabdul Magid, A.; Kabouche, A.; Harakat, D.; Voutquenne-Nazabadioko, L.; Kabouche, Z. 6‴-O-acetylisospinosin, a new C-glycosylflavone and known compounds from the aerial parts of Cladanthus mixtus. Nat. Prod. Res. 2020, 34, 2887–2893. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Erbiai, E.H.; Charfi, S.; Pinto, E.; Castillo, M.E.C.; Hernández-Ruiz, J.; Cano, A.; Badoc, A.; Lamarti, A.; Esteves da Silva, J.C.G.; et al. Chemical Characterization and Several Bioactivities of Cladanthus mixtus from Morocco. Molecules 2023, 28, 3196. [Google Scholar] [CrossRef] [PubMed]
- Elouaddari, A.; El Amrani, A.; Eddine, J.; Correia, A.I.D.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C. Yield and chemical composition of the essential oil of Moroccan chamomile [Cladanthus mixtus (L.) Chevall.] growing wild at different sites in Morocco. Flavour Fragance J. 2013, 28, 360–366. [Google Scholar] [CrossRef]
- Ghanimi, R.; Ouhammou, A.; Babahmad, R.A.; Cherkaoui, M. A Quantitative Study on the Ethnobotanical Knowledge about Wild Edible Plants among the Population of Messiwa. Ethiop. J. Health Sci. 2022, 32, 1237–1244. [Google Scholar] [PubMed]
- Ghanimi, R.; Ouhammou, A.; Ahouach, A.; Cherkaoui, M. Ethnobotanical study on wild edible plants traditionally used by Messiwa people, Morocco. J. Ethnobiol. Ethnomed. 2022, 18, 16. [Google Scholar] [CrossRef]
- Ait Baamrane, M.A.; Shehzad, W.; Ouhammou, A.; Abbad, A.; Naimi, M.; Coissac, E.; Taberlet, P.; Znari, M. Assessment of the food habits of the Moroccan dorcas gazelle in M’Sabih Talaa, west central Morocco, using the trnL approach. PLoS ONE 2012, 7, e35643. [Google Scholar] [CrossRef]
- Blajan, L.; Lasnami, K. Nutrition et pathologie du dromadaire. Options Méditerr. 1989, 2, 131–139. [Google Scholar]
- Kumar, P.; Kumar, R. Rural Health Scenario—Role of family medicine: Academy of Family Physicians of India Position Paper. J. Fam. Med. Prim. Care 2018, 7, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Kolié, D.; Van De Pas, R.; Codjia, L.; Zurn, P. Increasing the availability of health workers in rural sub-Saharan Africa: A scoping review of rural pipeline programmes. Hum. Resour. Health 2023, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- WHO Established the Global Center for Traditional Medicine in India. Available online: https://www.who.int/news/item/25-03-2022-who-establishes-the-global-centre-for-traditional-medicine-in-india (accessed on 30 October 2023).
- Šantić, Ž.; Pravdić, N.; Bevanda, M.; Galić, K. The historical use of medicinal plants in traditional and scientific medicine. Psychiatr. Danub. 2017, 29, 787–792. [Google Scholar] [PubMed]
- Cingi, C.; Bayar Muluk, N.; Tezol, A.; Çukurova, I. Efficacy of traditional herbal formulas on human immunity. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 27–40. [Google Scholar] [PubMed]
- Kurek, M.; Benaida-Debbache, N.; Elez Garofulić, I.; Galić, K.; Avallone, S.; Voilley, A.; Waché, Y. Antioxidants and Bioactive Compounds in Food: Critical Review of Issues and Prospects. Antioxidants 2022, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Liu, Y.; Li, S.H. The untapped potential of plant sesterterpenoids: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2293–2314. [Google Scholar] [CrossRef]
- Calixto, J.B.; Beirith, A.; Ferreira, J.; Santos, A.R.; Filho, V.C.; Yunes, R.A. Naturally occurring antinociceptive substances from plants. Phytother. Res. 2000, 14, 401–418. [Google Scholar] [CrossRef]
- Dewanjee, S.; Sohel, M.; Hossain, M.S.; Ansari, F.; Islam, M.T.; Sultana, F.; Al Mamun, A.; Islam, M.M.; Amin, M.N. A comprehensive review on clinically proven natural products in the management of nerve pain, with mechanistic insights. Heliyon 2023, 9, e15346. [Google Scholar] [CrossRef]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Zare Marzouni, H. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid. Based Complement. Altern. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal Plants in the Prevention and Treatment of Colon Cancer. Oxid. Med. Cell Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef]
- Menezes, R.; Foito, A.; Jardim, C.; Costa, I.; Garcia, G.; Rosado-Ramos, R.; Freitag, S.; Alexander, C.J.; Outeiro, T.F.; Stewart, D.; et al. Bioprospection of Natural Sources of Polyphenols with Therapeutic Potential for Redox-Related Diseases. Antioxidants 2020, 9, 789. [Google Scholar] [CrossRef]
- Rosa, M.N.; E Silva, L.R.V.; Longato, G.B.; Evangelista, A.F.; Gomes, I.N.F.; Alves, A.L.V.; de Oliveira, B.G.; Pinto, F.E.; Romão, W.; de Rezende, A.R.; et al. Bioprospecting of Natural Compounds from Brazilian Cerrado Biome Plants in Human Cervical Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 3383. [Google Scholar] [CrossRef]
- Rani, D.M.; Wongso, H.; Purwoko, R.Y.; Winarto, N.B.; Shalas, A.F.; Triatmoko, B.; Pratama, A.N.W.; Keller, P.A.; Nugraha, A.S. Anti-cancer bioprospecting on medicinal plants from Indonesia: A review. Phytochemistry 2023, 216, 113881. [Google Scholar] [CrossRef]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin. Phytosci. 2020, 6, 18. [Google Scholar] [CrossRef]
- Benkhnigue, O.; Ben Akka, F.; Salhi, S.; Fadli, M.; Douira, A.; Zidane, L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc). J. Anim. Plant Sci. 2014, 23, 3539–3568. [Google Scholar]
- Belhaj, S.; Chaachouay, N.; Zidane, L. Ethnobotanical and toxicology study of medicinal plants used for the treatment of diabetes in the High Atlas Central of Morocco. J. Pharm. Pharmacog. Res. 2021, 9, 619–662. [Google Scholar] [CrossRef]
- Benkhnigue, O.; Chaachouay, N.; Khamar, H.; El Azzouzi, F.; Douira, A.; Zidane, L. Ethnobotanical and ethnopharmacological study of medicinal plants used in the treatment of anemia in the region of Haouz-Rehamna (Morocco). J. Pharm. Pharmacogn. Res. 2022, 10, 279–302. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Alaoui, A.; Laaribya, S.; Ayan, S. Ethnobotanical survey of medicinal plants used for treating diabetes in Agadir Ida Outanane region, Southwestern Morocco. Arab. J. Med. Aromat. Plants 2020, 6, 72–86. [Google Scholar]
- Katiri, A.; Karkaoui, M.; Msanda, F.; Boubaker, H. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes in the Tizi n’ Test Region (Taroudant Province, Morocco). J. Pharmacogn. Nat. Prod. 2017, 3, 1000130. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Fadli, M.; El Ibaoui, H.; Zidane, L. Ethnobotanical and ethnopharmacological studies of medicinal and aromatic plants used in the treatment of metabolic diseases in the Moroccan Rif. Heliyon 2019, 5, e02191. [Google Scholar] [CrossRef] [PubMed]
- Naceiri Mrabti, H.; Bouyahya, A.; Naceiri Mrabti, N.; Jaradat, N.; Doudach, L.; Faouzi, M.E.A. Ethnobotanical Survey of Medicinal Plants Used by Traditional Healers to Treat Diabetes in the Taza Region of Morocco. eCAM 2021, 2021, 5515634. [Google Scholar] [CrossRef]
- Daoudi, A.; Bammou, M.; Zarkani, S.; Slimani, I.; Ibijbijen, J.; Nassiri, L. Ethnobotanical study of medicinal flora in rural municipality of Aguelmouss—Khenifra province—(Morocco). Phytothérapie 2016, 14, 220–228. [Google Scholar] [CrossRef]
- El Hanbali, F.; Mellouki, F.; Akssira, M.; El hassani, B.; Blázquez, M.A.; Boira, H. Composition and Antibacterial Activity of Essential Oils of Cladanthus arabicus Cass. (Asteraceae). J. Essent. Oil Bear. Plants 2005, 8, 213–217. [Google Scholar] [CrossRef]
- Aghraz, A.; Wanner, J.; Schmidt, E.; Aitdra, L.; Aitsidibrahim, M.; Tabanca, N.; Ali, A.; Nafis, A.; Hassani, L.; Markouk, M.; et al. Chemical Composition, in vitro Antioxidant, Antimicrobial and Insecticidal Activities of Essential Oil from Cladanthus arabicus. J. Essent. Oil Bear. Plants 2017, 20, 601–609. [Google Scholar] [CrossRef]
- Aghraz, A.; Benameur, Q.; Gervasi, T.; Ait Dra, L.; Ben-Mahdi, M.H.; Larhsini, M.; Markouk, M.; Cicero, N. Antibacterial activity of Cladanthus arabicus and Bubonium imbricatum essential oils alone and in combination with conventional antibiotics against Enterobacteriaceae isolates. Lett. Appl. Microbiol. 2018, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mziouid, A.; Chebli, B.; Berrabah, M.; Chebli, H.; Heimeur, N.; Bounimi, S.; Mayad, E.H. Phytochemical screening and antioxidant activity of four Moroccan aromatic plant methanolic extracts and essential oils. Arab. J. Med. Aromat. Plants 2022, 8, 117–132. [Google Scholar]
- Dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (-)-T-Cadinol-a Sesquiterpene Isolated From Casearia sylvestris (Salicaceae)-Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi. Front. Pharmacol. 2021, 12, 734127, Corrigendum in Front. Pharmacol. 2022, 13, 865432. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, R.; Zhou, Z.; Liu, R.; Wen, J. Efficacy and safety of caffeic acid tablets in the treatment of thrombocytopenia: A systematic review and meta-analysis. Medicine 2023, 102, 35353. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Singh, S.V.; Jaiswal, K.; Kumar, R.; Pandey, A.K. Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Okpara, E.S.; Adedara, I.A.; Guo, X.; Klos, M.L.; Farombi, E.O.; Han, S. Molecular mechanisms associated with the chemoprotective role of protocatechuic acid and its potential benefits in the amelioration of doxorubicin-induced cardiotoxicity: A review. Toxicol. Rep. 2022, 9, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef]
- Aghraz, A.; Albergamo, A.; Benameur, Q.; Salvo, A.; Larhsini, M.; Markouk, M.; Gervasi, T.; Cicero, N. Polyphenols contents, heavy metals analysis and in vitro antibacterial activity of extracts from Cladanthus arabicus and Bubonium imbricatum of Moroccan Origin. Nat. Prod. Res. 2020, 34, 63–70. [Google Scholar] [CrossRef]
- Aghraz, A.; Gonçalves, S.; Rodríguez-Solana, R.; Ait Dra, L.; Di Stefano, V.; Dugo, G.; Cicero, N.; Larhsini, M.; Markouk, M.; Romano, A. Antioxidant activity and enzymes inhibitory properties of several extracts from two Moroccan Asteraceae species. S. Afr. J. Bot. 2018, 118, 58–64. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Danikiewicz, W.; Gumulka, M.; Pankowska, E.; Krajewski, J.; Grabarczyk, H.; Wichlacz, M. Sesquiterpenes of Cladanthus arabicus. Phytochemistry 1993, 34, 1639–1641. [Google Scholar] [CrossRef]
- Monde, K.; Oya, T.; Takasugi, M.; Shirata, A. A guaianolide phytoalexin, cichoralexin, from Cichorium intybus. Phytochemistry 1990, 29, 3449–3451. [Google Scholar] [CrossRef]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Chávez-Silva, F.; Navarrete-Vázquez, G.; León-Rivera, I.; Perea-Arango, I.; Villalobos-Molina, R.; Ibarra-Barajas, M. Tracheal relaxation through calcium channel blockade of Achillea millefolium hexanic extract and its main bioactive compounds. J. Ethnopharmacol. 2020, 253, 112643. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Frattaruolo, L.; Haworth, I.; Brindisi, M.; El-magboub, A.; Ferrario, A.; Gomer, C.; Aiello, F.; Adams, J.D. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, e01366, Corrigendum in Heliyon 2019, 5, e01513. [Google Scholar] [CrossRef] [PubMed]
- Khazneh, E.; Hřibová, P.; Hošek, J.; Suchý, P.; Kollár, P.; Pražanová, G.; Muselík, J.; Hanaková, Z.; Václavík, J.; Miłek, M.; et al. The Chemical Composition of Achillea wilhelmsii C. Koch and Its Desirable Effects on Hyperglycemia, Inflammatory Mediators and Hypercholesterolemia as Risk Factors for Cardiometabolic Disease. Molecules 2016, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, X.F.; Jin, M.J.; Li, Y.; Wu, Y.L.; Jin, Q.; Zhang, Y.; Li, X.; Jiang, M.; Cui, B.W.; et al. Leucodin attenuates inflammatory response in macrophages and lipid accumulation in steatotic hepatocytes via P2x7 receptor pathway: A potential role in alcoholic liver disease. Biomed. Pharmacother. 2018, 107, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Millán-Pacheco, C.; Navarrete-Vázquez, G.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Almanza-Pérez, J.C. Antihypertensive and vasorelaxant effect of leucodin and achillin isolated from Achillea millefolium through calcium channel blockade and NO production: In vivo, functional ex vivo and in silico studies. J. Ethnopharmacol. 2021, 273, 113948. [Google Scholar] [CrossRef] [PubMed]
- Tschiggerl, C.; Bucar, F. Guaianolides and volatile compounds in chamomile tea. Plant Foods Human Nutr. 2012, 67, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Réthy, B.; Falkay, G.; Forgo, P.; Hohmann, J. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother. Res. 2009, 23, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, S.; Isaković, A.M.; Isaković, A.; Vučković, I.; Mandić, B.; Novaković, M.; Vajs, V.; Milosavljević, S.; Trajković, V. Isolation, characterization, and in vitro cytotoxicity of new sesquiterpenoids from Achillea clavennae. Planta Med. 2014, 80, 297–305. [Google Scholar] [CrossRef]
- Gören, N.; Oksüz, S.; Ulubelen, A. A sesquiterpene lactone, sintenin, from Achillea sintenisii. Phytochemistry 1988, 27, 2346–2347. [Google Scholar] [CrossRef]
- Bruno, M.; Rosselli, S.; Raccuglia, R.A.; Maggio, A.; Senatore, F.; Arnold, N.A.; Griffin, C.A.; Herz, W. Terpenoids and Flavones from Achillea falcata (Asteraceae). Rev. Soc. Quim. México 2003, 47, 130–131. [Google Scholar]
- Hatam, N.A.R.; Yousif, N.J.; Porzel, A.; Seifert, K. Sesquiterpene lactones from Achillea micrantha. Phytochemistry 1992, 31, 2160–2162. [Google Scholar] [CrossRef]
- Korkmaz, B.; Renda, G.; Erik, İ.; Kılıç, G.; Coşkunçelebi, K.; Yaylı, N. Two new dihydroisocoumarins and terpenoids from Scorzonera longiana Sümbül an endemic species to Turkey and their antimicrobial activity. Nat. Prod. Res. 2023, 37, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yoon, C.H.; Sung, Y.S.; Kim, Y.K.; Yun, M.; Kim, S. Total Synthesis of (+)-Cladantholide and (−)-Estafiatin: 5-Exo,7-Endo Radical Cyclization Strategy for the Construction of Guaianolide Skeleton. J. Am. Chem. Soc. 1997, 119, 8391–8392. [Google Scholar] [CrossRef]
- Schall, A.; Reiser, O. Synthesis of Biologically Active Guaianolides with a trans-Annulated Lactone Moiety. Eur. J. Org. Chem. 2008, 14, 2353–2364. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Moharana, S.; Khatun, G.N. Recent advances in the syntheses of guaianolides. Org. Biomol. Chem. 2023, 21, 6652–6670. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Shalaby, N.M.; Hamed, M.A.; El-Rigal, N.S.; Al-Ghamdi, S.N.; Bouajila, J. Phytochemical composition, protective and therapeutic effect on gastric ulcer and α-amylase inhibitory activity of Achillea biebersteinii Afan. Arch. Pharmacal Res. 2016, 39, 10–20. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Japonicone A and related dimeric sesquiterpene lactones: Molecular targets and mechanisms of anticancer activity. Inflamm. Res. 2022, 71, 267–276. [Google Scholar] [CrossRef]
- Migheli, R.; Virdis, P.; Galleri, G.; Arru, C.; Lostia, G.; Coradduzza, D.; Muroni, M.R.; Pintore, G.; Podda, L.; Fozza, C.; et al. Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines 2022, 10, 2739. [Google Scholar] [CrossRef] [PubMed]
- Fadul, E.; Nizamani, A.; Rasheed, S.; Adhikari, A.; Yousuf, S.; Parveen, S.; Gören, N.; Alhazmi, H.A.; Choudhary, M.I.; Khalid, A. Anti-glycating and anti-oxidant compounds from traditionally used anti-diabetic plant Geigeria alata (DC) Oliv. & Hiern. Nat. Prod. Res. 2020, 34, 2456–2464. [Google Scholar] [PubMed]
- Hu, L.H.; Zou, H.B.; Gong, J.X.; Li, H.B.; Yang, L.X.; Cheng, W.; Zhou, C.X.; Bai, H.; Guéritte, F.; Zhao, Y. Synthesis and biological evaluation of a natural ester sintenin and its synthetic analogues. J. Nat. Prod. 2005, 68, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Zhang, L.J.; Huang, K.X.; Li, X.K.; Hu, L.H.; Wang, X.Y.; Stockigt, J.; Zhao, Y. Antioxidant and neuroprotective effects of synthesized sintenin derivatives. J. Enzym. Inhib. Med. Chem. 2009, 24, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wichlacz, M.; Daniewski, W.M.; Danikiewicz, W.; Gumulka, M.; Drozdz, B.; Grabarczyk, H. Constituents of Cladanthus Arabicus. Pol. J. Chem. 1994, 68, 2147–2152. [Google Scholar]
- Ebrahim, W.; Aly, A.H.; Mándi, A.; Wray, V.; Essassi el, M.; Ouchbani, T.; Bouhfid, R.; Lin, W.; Proksch, P.; Kurtán, T.; et al. O-heterocyclic embeurekols from Embellisia eureka, an endophyte of Cladanthus arabicus. Chirality 2013, 25, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, W.; Aly, A.H.; Wray, V.; Mándi, A.; Teiten, M.H.; Gaascht, F.; Orlikova, B.; Kassack, M.U.; Lin, W.; Diederich, M.; et al. Embellicines A and B: Absolute configuration and NF-κB transcriptional inhibitory activity. J. Med. Chem. 2013, 56, 2991–2999. [Google Scholar] [CrossRef]
- Özçınar, Ö.; Özgür, T.; Yusufoglu, H.; Kivçak, B.; Bedir, E. Biotransformation of Neoruscogenin by the Endophytic Fungus Alternaria eureka. J. Nat. Prod. 2018, 81, 1357–1367. [Google Scholar] [CrossRef]
- Duman, S.; Ekiz, G.; Yılmaz, S.; Yusufoglu, H.; Ballar Kırmızıbayrak, P.; Bedir, E. Telomerase activators from 20(27)-octanor-cycloastragenol via biotransformation by the fungal endophytes. Bioorg. Chem. 2021, 109, 104708. [Google Scholar] [CrossRef]
- Küçüksolak, M.; Üner, G.; Ballar Kırmızıbayrak, P.; Bedir, E. Neuroprotective metabolites via fungal biotransformation of a novel sapogenin, cyclocephagenol. Sci. Rep. 2022, 12, 18481. [Google Scholar] [CrossRef]
- Ouchbani, T.; Janati Idriss, F.E.; Bouhfid, R.; Proksch, P.; Essassi, E.M. Neonectria Macrodidyma and Embellisia eureka, two novel producers of brefeldin A and 3,4-dihydro-3,4-8-trihydroxy’1[2H]-naphthalenone. J. Maroc. Chim. Hétérocycl. 2012, 11, 23–35. [Google Scholar]
- Gremaud, G.; Tabacchi, R. Relationship between the fungus Ceratocystis fimbriata coffea and the canker disease of the coffee tree. Phytochemistry 1996, 42, 1547–1549. [Google Scholar] [CrossRef]
- Okole, B.N.; Schulz, F.A. Selection of Mycosphaerella fijiensis-resistant cell lines from micro-cross sections of banana and plantain. Plant Cell Rep. 1997, 16, 339–343. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef]
- Garg, M.; Chaudhary, S.K.; Goyal, A.; Sarup, P.; Kumari, S.; Garg, N.; Vaid, L.; Shiveena, B. Comprehensive review on therapeutic and phytochemical exploration of diosmetin: A promising moiety. Phytomed. Plus 2022, 2, 100179. [Google Scholar] [CrossRef]
- Gong, X.; Xiong, L.; Caihong, B.; Zhang, B. Diosmetin ameliorate type 2 diabetic mellitus by up-regulating Corynebacterium glutamicum to regulate IRS/PI3K/AKT-mediated glucose metabolism disorder in KK-Ay mice. Phytomedicine 2021, 87, 153582. [Google Scholar] [CrossRef]
- Angamuthu, H.; Ramachandrane, M. Investigations on the structural, vibrational, computational, and molecular docking studies on potential antidiabetic chemical agent Diosmetin. J. Mol. Recogn. 2020, 33, e2819. [Google Scholar] [CrossRef]
- Comakli, V.; Adem, S.; Oztekin, A.; Demirdag, R. Screening inhibitory effects of selected flavonoids on human recombinant aldose reductase enzyme: In vitro and in silico study. Arch. Physiol. Biochem. 2022, 128, 1368–1374. [Google Scholar] [CrossRef]
- Valdés, B. Early botanical exploration of the Maghreb. Flora Mediterr. 2021, 31, 5–18. [Google Scholar]
- Tanji, A.; Ait Lhaj, A. Weeds of barley and wheat in Souss-Massa region. Rev. Maroc. Protect Plants 2010, 1, 11–23. [Google Scholar]
- Ait-Sidi-Brahim, M.; Markouk, M.; Larhsini, M. Chapter 5. Moroccan Medicinal Plants as Anti-infective and Antioxidant Agents. In New Look to Phytomedicine. Advancements in Herbal Products as Novel Drug Leads; Academic Press: Cambridge, MA, USA, 2019; pp. 91–142. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Assaggaf, H.; Qasem, A.; El-Shemi, A.G.; Abdallah, E.M.; Mrabti, H.N.; Bouyahya, A. Antioxidant, Antidiabetic, and Antibacterial Potentials and Chemical Composition of Salvia officinalis and Mentha suaveolens Grown Wild in Morocco. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 2844880. [Google Scholar] [CrossRef]
- Han, M.; Lu, Y.; Tao, Y.; Zhang, X.; Dai, C.; Zhang, B.; Xu, H.; Li, J. Luteolin Protects Pancreatic β Cells against Apoptosis through Regulation of Autophagy and ROS Clearance. Pharmaceuticals 2023, 16, 975. [Google Scholar] [CrossRef]
- Shehnaz, S.I.; Roy, A.; Vijayaraghavan, R.; Sivanesan, S.; Pazhanivel, N. Modulation of PPAR-γ, SREBP-1c and inflammatory mediators by luteolin ameliorates β-cell dysfunction and renal damage in a rat model of type-2 diabetes mellitus. Mol. Biol. Rep. 2023, 50, 9129–9142. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- El Alami, A.; Chait, A. Etude de l’alimentation du magot Macaca sylvanus dans le site touristique des cascades d’Ouzoud (Maroc). Rev. Primatol. 2016, 7, 2748. [Google Scholar] [CrossRef]
- Nouri, M.; Gonçalves, F.; Sousa, J.P.; Römbke, J.; Ksibi, M.; Pereira, R.; Haddioui, A. Metal and Phosphorus Uptake by Spontaneous Vegetation in an abandoned iron mine from a Semiarid Area in Center Morocco: Implications for Phytoextraction. Environ. Res. Eng. Manag. 2013, 2, 59–71. [Google Scholar] [CrossRef]
- Harras, N.; Lamarti, A. In Vitro Germination and Plantlet Establishment of Wild Chamomile of Morocco Cladanthus mixtus (L.) Oberpr. and Vogt. Am. J. Plant Sci. 2014, 5, 2623–2632. [Google Scholar] [CrossRef]
- El Hafidi, S.; Ouhssine, M.; Benzakour, A.; Gaboun, F.; Khamar, H.; Bakhy, K.; Homrani Bakali, A. Site effect on seed germination of two species of Cladanthus in Morocco. Afr. Mediterr. Agric. J. 2022, 137, 103–121. [Google Scholar]
- Tambewagh, U.U.; Kandhare, A.D.; Honmore, V.S.; Kadam, P.P.; Khedkar, V.M.; Bodhankar, S.L.; Rojatkar, S.R. Anti-inflammatory and antioxidant potential of Guaianolide isolated from Cyathocline purpurea: Role of COX-2 inhibition. Int. Immunopharmacol. 2017, 52, 110–118. [Google Scholar] [CrossRef]
- Adekenov, S. Syntheses Based on 3,4α-Epoxy-1,5,7α,6β(H)-guai-10(14),11(13)-dien-6,12-olide. Molecules 2022, 27, 1862. [Google Scholar] [CrossRef]
- Hu, Y.; Saito, Y.; Okamoto, Y.; Matsuo, Y.; Gong, X.; Tanaka, T. Chemical Compositions of Eupatorium heterophyllum Leaf Samples from Yunnan and Sichuan Provinces of China-Isolation of 13 New Sesquiterpene Lactones. Molecules 2023, 28, 5107. [Google Scholar] [CrossRef]
- Li, H.; Xu, N.; Li, J.; Aisa, H.A. Guaianolide-type sesquiterpene lactones from Achillea millefolium L. and their anti-inflammatory activity. Phytochemistry 2023, 216, 113894. [Google Scholar] [CrossRef]
- Wen, B.; Hexum, J.K.; Widen, J.C.; Harki, D.A.; Brummond, K.M. A redox economical synthesis of bioactive 6,12-guaianolides. Org. Lett. 2013, 15, 2644–2647. [Google Scholar] [CrossRef]
- Wells, S.M.; Brummond, K.M. Conditions for a Rh(I)-catalyzed [2 + 2 + 1] cycloaddition reaction with methyl substituted allenes and alkynes. Tetrahedron Lett. 2015, 56, 3546–3549. [Google Scholar] [CrossRef]
- He, W.; Lai, R.; Lin, Q.; Huang, Y.; Wang, L. Arglabin is a plant sesquiterpene lactone that exerts potent anticancer effects on human oral squamous cancer cells via mitochondrial apoptosis and downregulation of the mTOR/PI3K/Akt signaling pathway to inhibit tumor growth in vivo. J. BUON 2018, 23, 1679–1685. [Google Scholar]
- El Gaafary, M.; Morad, S.A.F.; Schmiech, M.; Syrovets, T.; Simmet, T. Arglabin, an EGFR receptor tyrosine kinase inhibitor, suppresses proliferation and induces apoptosis in prostate cancer cells. Biomed. Pharmacother. 2022, 156, 113873. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Wang, J.; Li, W.; Zhou, X.; Zhang, C.; Han, C. Arglabin regulates microglia polarization to relieve neuroinflammation in Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2022, 36, e23045. [Google Scholar] [CrossRef]
- Mahalingam, D.; Peguero, J.; Cen, P.; Arora, S.P.; Sarantopoulos, J.; Rowe, J.; Allgood, V.; Tubb, B.; Campos, L. A Phase II, Multicenter, Single-Arm Study of Mipsagargin (G-202) as a Second-Line Therapy Following Sorafenib for Adult Patients with Progressive Advanced Hepatocellular Carcinoma. Cancers 2019, 11, 833. [Google Scholar] [CrossRef]
- Isaacs, J.T.; Brennen, W.N.; Christensen, S.B.; Denmeade, S.R. Mipsagargin: The Beginning-Not the End-of Thapsigargin Prodrug-Based Cancer Therapeutics. Molecules 2021, 26, 7469. [Google Scholar] [CrossRef]
- Christensen, S.B.; Simonsen, H.T.; Engedal, N.; Nissen, P.; Møller, J.V.; Denmeade, S.R.; Isaacs, J.T. From Plant to Patient: Thapsigargin, a Tool for Understanding Natural Product Chemistry, Total Syntheses, Biosynthesis, Taxonomy, ATPases, Cell Death, and Drug Development. Prog. Chem. Org. Nat. Prod. 2021, 115, 59–114. [Google Scholar]
- Kim, D.Y.; Choi, B.Y. Costunolide-A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Marques, D.; Figueira, I.; Cankar, K.; Bosch, D.; Brito, M.A.; Dos Santos, C.N. Costunolide and parthenolide: Novel blood-brain barrier permeable sesquiterpene lactones to improve barrier tightness. Biomed. Pharmacother. 2023, 167, 115413. [Google Scholar] [CrossRef]
- Zhan, Z.Y.; Zhang, Z.H.; Yang, H.X.; Wu, Y.L.; Nan, J.X.; Lian, L.H. Potential skin health promoting benefits of costunolide: A therapeutic strategy to improve skin inflammation in imiquimod-induced psoriasis. Food Funct. 2023, 14, 2392–2403. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.; Lin, W.; Zhou, J.; Liu, Y.; Ma, L.; Li, M.; Hu, Y.; Yu, C.; Zhang, Y.; et al. Discovery of Novel Sesquiterpene Lactone Derivatives as Potent PKM2 Activators for the Treatment of Ulcerative Colitis. J. Med. Chem. 2023, 66, 5500–5523. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Charfi, S.; Erbiai, E.H.; Pereira, M.; Duarte, D.; Vale, N.; Candela Castillo, M.E.; Badoc, A.; Lamarti, A.; Esteves da Silva, J.C.G.; et al. Phytochemical Compounds and Anticancer Activity of Cladanthus mixtus Extracts from Northern Morocco. Cancers 2022, 15, 152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).