Bioavailability Assessment of Metals from the Coastal Sediments of Tropical Estuaries Based on Acid-Volatile Sulfide and Simultaneously Extracted Metals
Abstract
:1. Introduction
2. Material and Methods
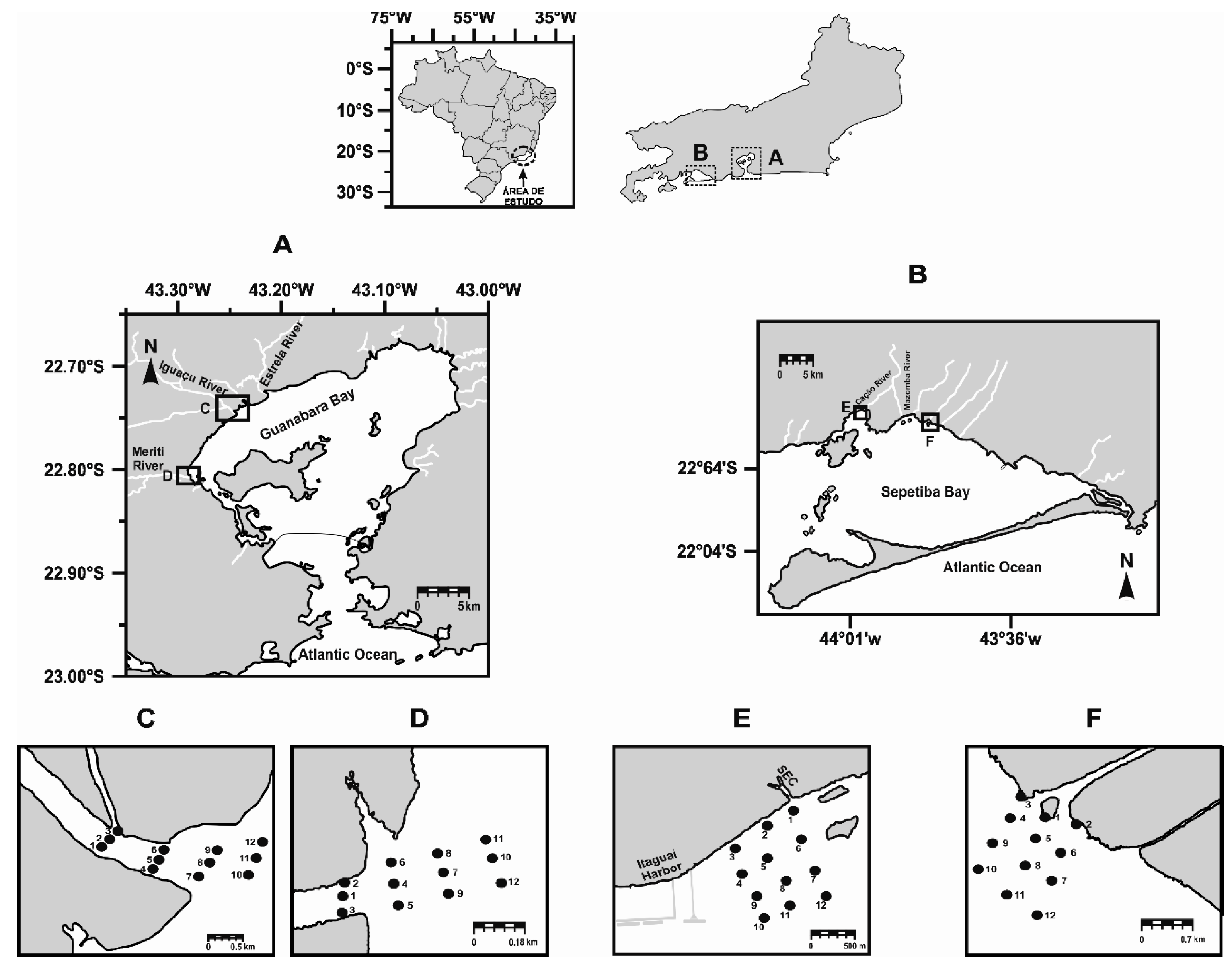
2.1. Study Area
2.2. Sampling
2.3. Acid Volatile Sulfides (AVS)
2.4. Determination of Simultaneously Extracted Metals
2.5. Total Organic Carbon and Granulometry
2.6. Adverse Effect Index
2.7. Data Analysis
3. Results
Correlations between Metals Concentrations and Possible Complexants
4. Discussion
Sediment Quality Guidelines and Adverse Effect Index
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jesus, H.C.; Costa, E.A.; Mendonça, A.S.F.; Zandonade, E. Distribuição de metais pesados em sedimentos do sistema estuarino da Ilha de Vitória-ES. Quím. Nova 2004, 27, 378–386. [Google Scholar] [CrossRef]
- Monte, C.N.; Rodrigues, A.P.C.; Galvão, P.M.A.; Ponte, G.C.; Malm, O.; Wasserman, J.C.; Machado, W. Mercury methylation upon coastal sediment resuspension: A worst-case approach under dark conditions. Environ. Monit. Assess. 2022, 194, 805. [Google Scholar] [CrossRef] [PubMed]
- Arfaeinia, H.; Nabipour, I.; Ostovar, A.; Asadgol, Z.; Abuee, E.; Keshtkar, M.; Dobaradaran, S. Assessment of sediment quality based on acid-volatile sulfide and simultaneously extracted metals in heavily industrialized area of Asaluyeh, Persian Gulf: Concentrations, spatial distributions, and sediment bioavailability/toxicity. Environ. Sci. Pollut. Res. 2016, 23, 9871–9890. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kong, M.; Zhang, L.; Chen, K.; Gu, X.; Hao, X. Metal bioavailability during the periodic drying and rewetting process of littoral anoxic sediment. J. Soils Sediments 2020, 20, 2949–2959. [Google Scholar] [CrossRef]
- Di Toro, D.; Mahony, J.D.; Hansen, D.J.; Scott, K.J.; Hicks, M.B.; Mayr, S.M.; Redmond, M.S. Toxicity of cadmium in sediments: The role of acid volatile sulfide. Environ. Toxicol. Chem. 1990, 9, 1487–1502. [Google Scholar] [CrossRef]
- Cappuyns, V.; Swennen, R.; Devivier, A. Dredged river sediments: Potential chemical time bombs? A case study. Water Soil Pollut. 2006, 171, 49–66. [Google Scholar] [CrossRef]
- Monte, C.N.; Rodriguues, A.P.C.; Silva, M.C.; Ferreira, L.J.S.; Monte, G.; Silveira, C.S.; Cordeiro, R.C.; Machado, W. Assessment of eutrophication from phosphorus remobilization after resuspension of coastal sediments from an urban tropical estuary. Environ. Sci. Pollut. Res. 2023, 30, 65500–65511. [Google Scholar] [CrossRef] [PubMed]
- Machado, W.; Villar, L.; Monteiro, F.; Viana, L.C.A.; Santelli, R.E. Relation of acid-volatile sulfides (AVS) with metals in sediments from eutrophicated estuaries: Is it limited by metal-to-AVS ratios? J. Soils Sediments 2010, 10, 1606–1610. [Google Scholar] [CrossRef]
- Allen, H.E.; Gongmin, F.; Boothman, W.; Di Toro, D.; Mahony, J.D. Analytical Method for Determination of Acid-Volatile Sulfide and Selected Simultaneously Extractable Metals in Sediment; United States Environmental Protection Agency Report; USEPA: Washington, DC, USA, 1991.
- Gao, X.; Song, J.; Li, X.; Yuan, H.; Zhao, J.; Xing, Q.; Peimiao, L. Sediment quality of the Bohai Sea and the northern Yellow Sea indicated by the results of acid-volatile sulfide and simultaneously extracted metals determinations. Mar. Pollut. Bull. 2020, 155, 111147. [Google Scholar] [CrossRef]
- Chapman, P.M.; Wang, F.; Janssen, C.; Persoone, G.; Allen, H.E. Ecotoxicology of metals in aquatic sediments: Binding and release, bioavilability, risk assessment, and remediation. Can. J. Fish. Aquat. Sci. 1998, 55, 2221–2243. [Google Scholar] [CrossRef]
- Morse, J.W. Interactions of trace metals with authigenic sulfide minerals: Implications for their bioavailability. Mar. Chem. 1994, 46, 1–6. [Google Scholar] [CrossRef]
- Matamoros-Veloza, A.; Cespedes, O.; Johnson, B.R.G.; Stawski, T.M.; Terranova, U.; Leeuw, N.H.; Benning, L.G. A highly reactive precursor in the iron sulfide system. Nat. Commun. 2018, 9, 3125. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.M.; El-Zokm, G.M.; Okbah, M.A. Spatial variation of acid-volatile sulfide and simultaneously extracted metals in Egyptian Mediterranean Sea lagoon sediments. Environ. Monit. Assess. 2014, 186, 3567–3579. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Nóbrega, G.N.; Otero, X.L.; Ferreira, T.O. Are acid volatile sulfides (AVS) important trace metals sinks in semi-arid mangroves? Mar. Pollut. Bull. 2018, 126, 318–322. [Google Scholar] [CrossRef]
- Barcellos, C.; Rezende, C.E.; Pfeiffer, W.C. Zn e Cd prodution and polluition in a Brazilian Coastal Region. Mar. Pollut. Bull. 1991, 11, 58–561. [Google Scholar] [CrossRef]
- Gomes, F.C.; Godoy, J.M.; Godoy, M.L.D.P.; Carvalho, Z.L.; Lopes, R.T.; Sanchez-Cabeza, J.A.; Lacerda, L.D.; Wasserman, J.C. Metal concentrations, fluxes, inventories and chronologies in sediments from Sepetiba and Ribeira Bays: A comparative study. Mar. Pollut.Bull. 2009, 59, 123–133. [Google Scholar] [CrossRef]
- Monte, C.N.; Rodrigues, A.P.C.; Cordeiro, R.C.; Freire, A.S.; Santelli, R.E.; Machado, W. Changes in Cd and Zn bioavailability upon an experimental resuspension of highly contaminated coastal sediments from a tropical estuary. Sustain. Water Resour. Manag. 2015, 1, 332–335. [Google Scholar] [CrossRef]
- Abreu, I.M.; Cordeiro, R.C.; Soares-Gomes, A.; Abessa, D.M.S.; Maranho, L.A.; Santelli, R.E. Ecological risk evaluation of sediment metals in a tropical Eutrophic Bay, Guanabara Bay, Southeast Atlantic. Mar. Pollut. Bull. 2016, 109, 435–455. [Google Scholar] [CrossRef]
- Monte, C.N.; Rodrigues, A.P.C.; Freitas, A.R.; Braz, B.F.; Freire, A.S.; Cordeiro, R.C.; Santelli, R.E.; Machado, W. Ecological risks associated to trace metals of contaminated sediments from a densely urbanized tropical eutrophic estuary. Environ. Monit. Assess. 2021, 193, 12. [Google Scholar] [CrossRef]
- Machado, W.; Moscatelli, M.; Rezende, L.G.; Lacerda, L.D. Mercury, Zinc and Copper accumulation in mangrove sediments surrounding a large landfill in southeast Brazil. Environ. Pollut. 2002, 1201, 455–461. [Google Scholar] [CrossRef]
- Cordeiro, R.C.; Santelli, R.E.; Machado, W.; Moreira, L.S.; Freire, A.S.; Braz, B.F.; Rizzini-Ansari, N.; Bidone, E.D.; Meniconi, M.F.G. Biogeochemical factors controlling arsenic distribution in a densely populated tropical estuary (Guanabara Bay, RJ, Brazil). Environ. Earth Sci. 2017, 76, 561. [Google Scholar] [CrossRef]
- Molisani, M.M.; Marins, R.V.; Machado, W.; Paraquetti, H.H.M.; Bidone, E.D.; Lacerda, L.D. Environmental Changes in Sepetiba Bay, SE, Brazil. Reg. Environ. Change 2004, 4, 17–27. [Google Scholar] [CrossRef]
- Moreira, V.A.; Carvalho, A.C.B.; Fontana, L.F.; Bidone, E.D.; Sabadini-Santos, E. Applying enzymatic biomarkers of the in situ microbial community to assess the sediment risk from Sepetiba Bay (Brazil). Mar. Pollut. Bull. 2021, 169, 112547. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.R.; Rodrigues, A.P.C.; Monte, C.N.; Soares, A.F.; Santelli, R.E.; Machado, W.; Sabadini-Santos, E. Increase in the bioavailability of trace metals after sediment resuspension. SN Appl. Sci. 2019, 1, 1288. [Google Scholar] [CrossRef]
- Aguiar, V.M.C.; Baptista Neto, A.A.; Rangel, C.M. Eutrophication and hypoxia in four streams discharging in Guanabara Bay, RJ, Brazil, a case study. Mar. Pollut. Bull. 2011, 65, 1915–1919. [Google Scholar] [CrossRef]
- Machado, W.; Rodrigues, A.P.C.; Bidone, E.D.; Sella, S.M.; Santelli, R.E. Evaluation of Cu potential bioavailability changes upon coastal sediment resuspension: An exampleon how to improve the assessment of sediment dredging environmental risks. Environ. Sci. Pollut. Res. 2011, 18, 1033–1036. [Google Scholar] [CrossRef]
- Silveira, R.P.; Rodrigues, A.P.C.; Santelli, R.E.; Cordeiro, R.C.; Bidone, E.D. Mass balance in the monitoring of pollutants in tidal Rivers of the Guanabara Bay, Rio de Janeiro, Brazil. Environ. Monit. Assess. 2011, 181, 165–173. [Google Scholar] [CrossRef]
- Machado, W.; Santelli, R.E.; Loureiro, D.D.; Oliveira, E.L.; Borges, A.C.; Ma, V.K.; Lacerda, L.D. Mercury accumulation in sediments along an eutrophication gradient in Guanabara Bay, Southeast Brazil. J. Braz. Chem. Soc. 2008, 19, 569–575. [Google Scholar] [CrossRef]
- Cassella, R.J.; de Oliveira, L.G.; Santelli, R.E. On line dissolution of ZnS for sulfide determination in stabilized water samples with zinc acetate using spectrophotometry by methylene blue method. Spectrosc. Lett. 1999, 32, 469–484. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Monte, C.N.; Rodrigues, A.P.C.; Freitas, A.R.; Freire, A.S.; Santelli, R.E.; Braz, B.F.; Machado, W. Dredging impact on trace metal behavior in a polluted estuary: A discussion about sampling design. Braz. J Oceanogr. 2019, 67, 19227. [Google Scholar] [CrossRef]
- Muñoz-Barbosa, A.; Gutiérrez-Galindo, E.A.; Daesslé, L.W.; Orozco-Borbón, M.V.; Segovia-Zaval, J.A. Relationship between metal enrichments and a biological adverse effects index in sediments from Todos Santos Bay, northwest coast of Baja California, México. Mar. Pollut. Bull. 2012, 64, 405–409. [Google Scholar] [CrossRef]
- Rodrigues, S.K.; Abessa, D.M.S.; Rodrigues, A.P.C.; Soares-Gomes, A.; Freitas, C.B.; Santelli, R.E.; Freire, A.S.; Machado, W. Sediment quality in a metal-contaminated tropical bay assessed with a multiple lines of evidence approach. Environ. Pollut. 2017, 228, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Tonhá, M.S.; Garnier, J.; Araújo, D.F.; Cunha, B.C.A.; Machado, W.; Dantas, E.; Araújo, R.; Kutter, V.T.; Bonnet, M.; Seyler, P. Behavior of metallurgical zinc contamination in coastal environments: A survey of Zn from electroplating wastes and partitioning in sediments. Sci. Total Environ. 2020, 743, 140610. [Google Scholar] [CrossRef] [PubMed]
- Castelo, W.F.L.; Martins, M.V.A.; de Lima Ferreira, P.A.; Figueira, R.; Costa, C.F.; Fonseca, L.B.; Bergamashi, S.; Pereira, E.; Terroso, D.; Pinto, A.F.S.; et al. Long-term eutrophication and contamination of the central area of Sepetiba Bay (SW Brazil). Environ. Monit. Assess. 2021, 193, 100. [Google Scholar] [CrossRef]
- Carvalho, C.E.V.; Lacerda, L.D.; Gomes, M.P. Heavy metal contamination of the marine biota along the Rio de Janeiro Coast, SE Brazil. Water Air Soil Pollut. 1991, 57, 645–653. [Google Scholar] [CrossRef]
- Kjerfve, B.; Ribeiro, C.H.A.; Dias, G.T.M.; Filippo, A.M.; Quaresma, V.S. Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Cont. Shelf Res. 1997, 17, 1609–1643. [Google Scholar] [CrossRef]
- Zhang, C.; Zhi-Gang, Y.U.; Zeng, Z.; Jiang, G.; Yang, M.; Cui, F.; Zhu, M.; Shen, L.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Oliveira, D.F.; Rezende, C.E.; Almeida, P.; Lacerda, L.D.; Gama, B.A.P.; Godoy, J.M. Spatial and temporal effects of decommissioning a zinc smelter on the sediment quality of an estuary system: Sepetiba Bay, Rio de Janeiro, Brazil. J. Braz. Chem. Soc. 2020, 31, 683–693. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Figueiredo, A.M.G.; Santos, J.O.; Dantas, E.; Cotrim, M.E.B.; Figueira, R.C.L.; Silva-Filho, E.V.; Wasserman, J.C. Combined SEM/AVS and attenuation of concentrations models for the assessment of bioavailability and mobility in sediments of Sepetiba Bay (SE Brazil). Mar. Pollut. Bull. 2013, 68, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.F.; Baptista Neto, J.A.; Silva, C.G. Heavy metal accumulation in mangrove sediments surrounding a large waste reservoir of a local metallurgical plant, Sepetiba Bay, SE, Brazil. Environ. Earth Sci. 2013, 70, 643–650. [Google Scholar] [CrossRef]
- Maddock, J.E.L.; Carvalho, M.F.; Santelli, R.E.; Machado, W. Contaminant Metal Behavior During Re-suspension of Sulphidic Estuarine Sediments. Water Air Soil Pollut. 2007, 181, 193–200. [Google Scholar] [CrossRef]
- Brezonik, P.L.; King, O.S.; Mach, C.E. The influence of water chemistry on trace metal bioavailability and toxicity to aquatic organisms. In Metal Ecotoxicology: Concepts and Application; Newman, M.C., McIntash, A.W., Eds.; Lewis Publishers: New York, NY, USA, 1991; pp. 2–31. [Google Scholar]
- Moiseenko, T.I. Bioavailability and Ecotoxicity of Metals in Aquatic Systems: Critical Contamination Levels. Geochem. Int. 2019, 57, 737–750. [Google Scholar] [CrossRef]
- Reimann, C.; De Caritat, P. Chemical Elements in the Environment: Factsheets for the Geochemist and Environmental Scientist; Springer: Berlin/Heidelberg, Germany, 1998; p. 398. [Google Scholar]
- Jiménez-Ballesta, R.; García-Navarro, F.J.; Bravo, S.; Amoros, J.Á.; Pérez de los Reyes, C.; Mejias, M. Environmental assessment of potential toxic elements contents in the inundated floodplain área of Tablas de Daimiel wetland (Spain). Environ. Geochem. Health 2017, 39, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- Baptista Neto, J.A.; Peixoto, T.C.S.; Smith, B.J.; MCalister, J.J.; Patchineelam, S.M.; Patchineelam, S.R.; Fonseca, E.M. Geochronology and heavy metal flux to Guanabara Bay, Rio de Janeiro state: A preliminary Study. An. Acad. Bras. Ciências 2013, 85, 1317–1327. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Figueiredo, A.M.G.; Santos, J.O.; Ferreira, P.A.L.; Graudenz, G.S.; Ruiz, M.S.; Mahiques, M.M.; Figueira, R.C.L.; Wasserman, J.C.F. Effects of contamination with toxic metals on the environmental quality of Sepetiba Bay (SE Brazil). Manag. Environ. Qual. 2015, 26, 538–551. [Google Scholar] [CrossRef]

| Madeira Island | |||||
|---|---|---|---|---|---|
| Sample | pH | OD (mg L−1) | OD (%) | Temperature (°C) | Conductivity (mS cm−1) |
| 1 | 7.2 | 4.7 | 67.9 | 23.5 | 47.9 |
| 2 | 7.6 | 4.5 | 59.8 | 23.2 | 48.0 |
| 3 | 7.7 | 4.3 | 60.4 | 23.2 | 48.2 |
| 4 | 7.2 | 4.5 | 63.0 | 23.3 | 48.2 |
| 5 | 6.2 | 4.4 | 62.4 | 23.2 | 50.0 |
| 6 | 7.7 | 4.3 | 61.0 | 23.2 | 48.3 |
| 7 | 7.8 | 4.4 | 62.6 | 22.9 | 48.0 |
| 8 | 7.9 | 4.5 | 63.5 | 23.3 | 48.3 |
| 9 | 7.9 | 4.7 | 67.4 | 23.3 | 48.3 |
| 10 | 7.9 | 4.9 | 68.9 | 23.5 | 48.6 |
| 11 | 7.9 | 4.8 | 66.7 | 23.4 | 48.5 |
| 12 | 7.9 | 4.6 | 65.0 | 23.4 | 48.5 |
| São Francisco Chanel | |||||
| Sample | pH | OD (mg L−1) | OD (%) | Temperature (°C) | Conductivity (mS cm−1) |
| 1 | 6.8 | 10.8 | 126.2 | 22.8 | 3.0 |
| 2 | 6.8 | 8.9 | 110.2 | 23.6 | 12.2 |
| 3 | 6.8 | 8.9 | 106.5 | 23.1 | 28.8 |
| 4 | 6.8 | 7.9 | 103.0 | 23.4 | 23.9 |
| 5 | 6.8 | 6.4 | 106.7 | 23.2 | 47.0 |
| 6 | 6.8 | 7.8 | 97.9 | 23.0 | 18.3 |
| 7 | 6.8 | 7.3 | 92.9 | 23.3 | 24.1 |
| 8 | 6.8 | 7.3 | 102.2 | 23.6 | 48.7 |
| 9 | 6.8 | 7.3 | 107.8 | 23.6 | 49.8 |
| 10 | - | 7.3 | 97.8 | 23.0 | 48.8 |
| 11 | 6.8 | 7.0 | 97.8 | 23.9 | 44.9 |
| 12 | - | 7.2 | 103.4 | 23.3 | 48.4 |
| Iguaçu River | |||||
|---|---|---|---|---|---|
| Sample | pH | OD (mg L−1) | OD (%) | Temperature (°C) | Conductivity (mS cm−1) |
| 1 | 6.8 | 2.4 | 30.9 | 27.0 | 2.0 |
| 2 | 6.9 | 2.7 | 34.4 | 27.3 | 2.8 |
| 3 | 6.6 | 1.8 | 23.0 | 27.5 | 0.6 |
| 4 | 7.5 | 4.8 | 61.3 | 27.8 | 7.9 |
| 5 | 7.2 | 3.0 | 38.5 | 27.6 | 5.4 |
| 6 | 7.3 | 5.3 | 68.4 | 28.4 | 5.5 |
| 7 | 7.4 | 4.8 | 61.1 | 27.6 | 6.6 |
| 8 | 7.5 | 5.7 | 71.6 | 27.4 | 7.4 |
| 9 | 7.9 | 7.9 | 100.0 | 27.5 | 9.0 |
| 10 | 7.9 | 8.2 | 104.2 | 27.7 | 9.0 |
| 11 | 7.9 | 7.8 | 98.6 | 27.6 | 9.2 |
| 12 | 8.7 | 11.5 | 147.0 | 27.8 | 11.5 |
| Meriti River | |||||
| Sample | pH | OD (mg L−1) | OD (%) | Temperature (°C) | Conductivity (mS cm−1) |
| 1 | 7.2 | 0.00 | 0.0 | 30.3 | 8.1 |
| 2 | 7.2 | 0.00 | 0.0 | 30.8 | 10.7 |
| 3 | 7.4 | 0.00 | 0.0 | 28.8 | 29.1 |
| 4 | 7.5 | 0.40 | 0.1 | 30.8 | 23.7 |
| 5 | 7.7 | 1.6 | 0.2 | 33.1 | 23.0 |
| 6 | 8.3 | 3.8 | 0.6 | 28.7 | 42.1 |
| 7 | 8.5 | 4.5 | 0.7 | 28.2 | 44.0 |
| 8 | 8.6 | 5.0 | 0.7 | 27.7 | 46.5 |
| 9 | 8.4 | 3.8 | 0.5 | 27.6 | 45.3 |
| 10 | 8.4 | 3.6 | 0.5 | 27.2 | 45.8 |
| 11 | 8.7 | 6.0 | 0.9 | 27.7 | 45.4 |
| 12 | 8.7 | 5.6 | 0.8 | 27.6 | 45.8 |
| Metal | Content | MID | SFC | IR | MR | ERL | ERM |
|---|---|---|---|---|---|---|---|
| Cd | Max | 22.4 | 1.3 | 0.6 | 3.2 | 1.2 | 9.6 |
| Min | 2.2 | 0 | 0.1 | 1.1 | |||
| Average | 7.4 | 0.6 | 0.4 | 1.8 | |||
| Cu | Max | 25.4 | 22.3 | 43.5 | 171.6 | 34 | 270 |
| Min | 3.8 | 1.2 | 2.2 | 34.3 | |||
| Average | 16.4 | 8.9 | 15.1 | 107.8 | |||
| Ni | Max | 28.6 | 22 | 2.9 | 52.2 | 20.9 | 51.6 |
| Min | 0.8 | 1.3 | 0.5 | 11.2 | |||
| Average | 10.6 | 7.7 | 1.4 | 27 | |||
| Pb | Max | 38.2 | 23.7 | 50.4 | 238.3 | 46.7 | 218 |
| Min | 5.3 | 1.6 | 6.8 | 35.2 | |||
| Average | 25.8 | 11.2 | 31.7 | 86.7 | |||
| Zn | Max | 3646.3 | 518.6 | 309.4 | 1634.5 | 150 | 410 |
| Min | 440 | 47 | 41.8 | 379.2 | |||
| Average | 1360.1 | 231 | 229.6 | 1106.0 |
| Sample | Cd mg/kg | Cu mg/Kg | Ni mg/Kg | Pb mg/Kg | Zn mg/Kg |
|---|---|---|---|---|---|
| MID | 18.6 | 0.7 | 0.5 | 1.1 | 24.3 |
| 8.8 | 0.5 | 0.2 | 0.7 | 12.9 | |
| 7.0 | 0.7 | 0.9 | 0.6 | 10.2 | |
| 7.6 | 0.7 | 0.2 | 0.8 | 12.6 | |
| 2.6 | 0.7 | 1.2 | 0.4 | 3.9 | |
| 1.9 | 0.4 | 0.3 | 0.4 | 2.9 | |
| 2.1 | 0.5 | 1.4 | 0.4 | 3.0 | |
| 5.0 | 0.2 | 0.1 | 0.4 | 6.9 | |
| 2.4 | 0.1 | 0.3 | 0.1 | 3.9 | |
| 3.1 | 0.1 | 0.0 | 0.2 | 4.7 | |
| 10.6 | 0.6 | 0.1 | 0.8 | 16.5 | |
| 4.8 | 0.5 | 0.8 | 0.6 | 6.8 | |
| SFC | 0.3 | 0.7 | 0.8 | 0.3 | 1.6 |
| 0.1 | 0.0 | 0.1 | 0.0 | 0.3 | |
| 0.0 | 0.0 | 0.7 | 0.1 | 0.3 | |
| 0.7 | 0.2 | 0.1 | 0.2 | 1.4 | |
| 0.2 | 0.1 | 1.1 | 0.2 | 0.7 | |
| 0.5 | 0.3 | 0.1 | 0.3 | 1.1 | |
| 0.8 | 0.3 | 0.2 | 0.3 | 3.5 | |
| 0.7 | 0.2 | 0.4 | 0.2 | 3.1 | |
| 0.4 | 0.2 | 0.7 | 0.2 | 1.2 | |
| 1.1 | 0.5 | 0.2 | 0.5 | 2.4 | |
| 0.6 | 0.2 | 0.1 | 0.2 | 1.4 | |
| 0.7 | 0.5 | 0.2 | 0.4 | 1.5 | |
| IR | 0.3 | 1.3 | 0.1 | 1.1 | 2.1 |
| 0.4 | 0.5 | 0.1 | 0.8 | 1.8 | |
| 0.3 | 0.3 | 0.1 | 0.7 | 1.5 | |
| 0.3 | 0.3 | 0.1 | 0.4 | 0.9 | |
| 0.3 | 0.3 | 0.1 | 0.8 | 1.7 | |
| 0.4 | 0.3 | 0.0 | 0.7 | 1.6 | |
| 0.4 | 0.2 | 0.1 | 0.8 | 1.9 | |
| 0.1 | 0.0 | 0.0 | 0.1 | 0.3 | |
| 0.4 | 0.5 | 0.0 | 0.7 | 2.0 | |
| 0.4 | 0.6 | 0.1 | 0.9 | 1.8 | |
| 0.5 | 0.6 | 0.0 | 0.8 | 2.0 | |
| 0.2 | 0.1 | 0.0 | 0.2 | 0.6 | |
| MR | 1.4 | 3.8 | 1.7 | 1.8 | 9.5 |
| 1.3 | 3.1 | 1.2 | 1.4 | 7.4 | |
| 1.2 | 3.3 | 1.5 | 1.7 | 8.6 | |
| 1.5 | 3.8 | 1.7 | 1.5 | 7.4 | |
| 1.3 | 5.0 | 2.5 | 5.1 | 5.8 | |
| 1.1 | 2.9 | 0.7 | 1.6 | 7.2 | |
| 1.2 | 4.5 | 1.5 | 2.2 | 10.9 | |
| 1.9 | 1.0 | 0.6 | 0.8 | 2.5 | |
| 2.7 | 3.0 | 0.8 | 1.8 | 10.0 | |
| 1.8 | 3.2 | 1.5 | 1.9 | 9.9 | |
| 1.0 | 2.1 | 1.3 | 1.2 | 3.7 | |
| 1.4 | 2.3 | 0.5 | 1.4 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.P.d.C.; Pereira, M.M.; Campos, A.; da Silva Quaresma, T.L.; Pova, R.; Vieira, T.C.; Diaz, R.A.; Moreira, M.; Araripe, D.; Monte, C.d.N.; et al. Bioavailability Assessment of Metals from the Coastal Sediments of Tropical Estuaries Based on Acid-Volatile Sulfide and Simultaneously Extracted Metals. Coasts 2023, 3, 313-327. https://doi.org/10.3390/coasts3040019
Rodrigues APdC, Pereira MM, Campos A, da Silva Quaresma TL, Pova R, Vieira TC, Diaz RA, Moreira M, Araripe D, Monte CdN, et al. Bioavailability Assessment of Metals from the Coastal Sediments of Tropical Estuaries Based on Acid-Volatile Sulfide and Simultaneously Extracted Metals. Coasts. 2023; 3(4):313-327. https://doi.org/10.3390/coasts3040019
Chicago/Turabian StyleRodrigues, Ana Paula de Castro, Matheus Marinho Pereira, Aline Campos, Tássia Lins da Silva Quaresma, Rodrigo Pova, Thatianne Castro Vieira, Rút Amélia Diaz, Manuel Moreira, Denise Araripe, Christiane do Nascimento Monte, and et al. 2023. "Bioavailability Assessment of Metals from the Coastal Sediments of Tropical Estuaries Based on Acid-Volatile Sulfide and Simultaneously Extracted Metals" Coasts 3, no. 4: 313-327. https://doi.org/10.3390/coasts3040019
APA StyleRodrigues, A. P. d. C., Pereira, M. M., Campos, A., da Silva Quaresma, T. L., Pova, R., Vieira, T. C., Diaz, R. A., Moreira, M., Araripe, D., Monte, C. d. N., & Machado, W. (2023). Bioavailability Assessment of Metals from the Coastal Sediments of Tropical Estuaries Based on Acid-Volatile Sulfide and Simultaneously Extracted Metals. Coasts, 3(4), 313-327. https://doi.org/10.3390/coasts3040019





