Spectrophotometric and Electrochemical Assessment of the Antioxidant Capacity of Aqueous and Ethanolic Extracts of Citrus Flavedos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Citrus Samples
2.3. Instruments
2.4. Experimental Procedures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution and nutritional importance of citrus fruits. Com. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- European Commission DG Agri G2. Vol 01-Production. The Citrus Market in the EU: Production, Areas and Yields. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/farming/documents/citrus-production_en.pdf (accessed on 18 November 2021).
- AIJN-European Fruit Juice Association. AIJN Liquid Fruit Market Report. Available online: https://aijn.eu/en/publications/market-reports-1/20199 (accessed on 21 February 2022).
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.L.; Yang, Z.H.; Quan, C. Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Helion 2019, 5, e02947. [Google Scholar] [CrossRef] [Green Version]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Alañón, M.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.; Arroyo-Jiménez. M. Bioactive Flavonoids, Antioxidant Behaviour, and Cytoprotective Effects of Dried Grapefruit Peels (Citrus paradisi Macf.). Oxid. Med. Cell Longev. 2016, 1, 8915729. [Google Scholar] [CrossRef] [Green Version]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzou, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Varmie, E.B.; Thakur, M. Utilization of citrus processing waste: A review. Pharm. Inn. J. 2021, 10, 682–697. [Google Scholar]
- Dassoff, E.S.; Guo, J.X.; Liu, Y.; Wang, S.C.; Li, Y.O. Potential development of non-synthetic food additives from orange processing by-products—A review. Food Qual. Saf. 2021, 5, fyaa035. [Google Scholar] [CrossRef]
- Irfan, S.; Ranjha, M.; Mahmood, S.; Saeed, W.; Alam, M. Lemon Peel: A Natural Medicine. Int. J. Biotech. Allied Fields 2019, 7, 185–194. [Google Scholar]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.W.; Hill, R.A.; Klaassen, C.D.; Liebler, C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Citrus Peel-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2021, 40 (Suppl. S3), 77S–99S. [Google Scholar] [CrossRef]
- Khang, D.T.; Tien, L.T.T.; Men, T.T.; Thuy, N.P. Potential of Fermented Fruit Peel Liquid in Cosmetics as a Skin Care Agent. Cosmetics 2021, 8, 33. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, M.; Park, E. Antioxidant Activity of Orange Flesh and Peel Extracted with Various Solvents. Prev. Nutr. Food Sci. 2014, 19, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehmood, T.; Khan, M.R.; Shabbir, M.A.; Zia, M.A. Phytochemical profiling and HPLC quantification of citrus peel from different varieties. Progr. Nutr. 2018, 20, 279–288. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef]
- Imran, M.; Basharat, S.; Khalid, S.; Aslam, M.; Syed, F.; Jabeen, S.; Kamran, H.; Shahid, M.Z.; Tufail, T.; Shah, F.; et al. Citrus peel polyphenols: Recent updates and perspectives. Int. J. Biosci. 2020, 16, 53–70. [Google Scholar] [CrossRef]
- Gao, X.; Xu, D.; Zhang, X.; Zhao, H. Protective Effect of Lemon Peel Polyphenols on Oxidative Stress-Induced Damage to Human Keratinocyte HaCaT Cells Through Activation of the Nrf2/HO-1 Signaling Pathway. Front. Nutr. 2021, 7, 606776. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Ibrahim, E.A.; Salama, Z. Citrus Peels as a Source of Bioactive Compounds with Industrial and Therapeutic Applications. In Phenolic Compounds—Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; Badria, F.A., Ed.; IntechOpen: London, UK, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Chen, X.-N.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Recovery of Added-Value Compounds from Orange and Spinach Processing Residues: Green Extraction of Phenolic Compounds and Evaluation of Antioxidant Activity. Antioxidants 2021, 10, 1800. [Google Scholar] [CrossRef] [PubMed]
- Olfa, T.; Gargouri, M.; Akrouti, A.; Brits, A.; Gargouri, M.; Ben Ameur, R.; Pieters, L.; Fourbet, K.; Magné, C.; Soussi, A.; et al. A comparative study of physichochemical investigation and antioxidative activities of six citrus peel species. Flavour. Frag. J. 2021, 36, 564–575. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Anesi, A. Flavedo and albedo of five citrus fruits from Southern Italy: Physicochemical characteristics and enzyme-assisted extraction of phenolic compounds. J. Food Meas. Charact. 2021, 15, 1754–1762. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovac, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Danet, A.F. Recent Advances in Antioxidant Capacity Assays. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2021; pp. 1–17. [Google Scholar] [CrossRef]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity-Relevance, methods and clinical implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety-Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Ozyurek, M.; Guclu, K.; Çapanoglu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Capanoglu, E.; Shahidi, F. (Eds.) Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; Wiley: Chichester, UK, 2018. [Google Scholar]
- Bayarsaikhan, G.; Bayarsaikhan, D.; Oh, P.C.; Kang, W.C.; Lee, B. CUPRAC-Reactive Advanced Glycation End Products as Prognostic Markers of Human Acute Myocardial Infarction. Antioxidants 2021, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.T.; Rodríguez Mellado, J.M.; Medina, A. Rapid electrochemical determination of antioxidant capacity using glassy carbon electrodes modified with copper and polyaniline. Application to ascorbic and gallic acids. Biointerface Res. Appl. Chem. 2023, 13, 23. [Google Scholar] [CrossRef]
- Moreno, M.T.; Estévez Brito, R.; Córdoba, M.; Rodríguez Mellado, J.M. A simple and rapid electrochemical determination of pungency: Application to aqueous and ethanolic extracts of Capsicum annuum cubana red. J. Chem. Res. 2021, 45, 111–115. [Google Scholar] [CrossRef]
- Cortez, J.D.; Faicán, M.A.; Pirovani, M.A.; Piagentini, A.M. Determination of polyphenols in fruits with added vitamin C: Methodology to improve the specificity of the FolinCiocalteu test. Rev. Iberoam. Tecnol. Postcosecha 2018, 19, 148–158. [Google Scholar]
- Liu, Y.; Liu, C.; Li, J. Comparison of Vitamin C and Its Derivative Antioxidant Activity: Evaluated by Using Density Functional Theory. ACS Omega 2020, 5, 25467–25475. [Google Scholar] [CrossRef]
- Pandithavidana, D.R.; Jayawardana, S.B. Comparative Study of Antioxidant Potential of Selected Dietary Vitamins; Computational Insights. Molecules 2019, 24, 1646. [Google Scholar] [CrossRef] [Green Version]
- Escobedo-Avellaneda, Z.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; Torres, J.A.; Welti-Chanes, J. Phytochemicals and antioxidant activity of juice, flayed, albedo and comminuted orange. J. Functional Foods. 2014, 6, 470–481. [Google Scholar] [CrossRef]
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Klimowicz, A. The antioxidant potential of flesh, albedo and flavedo extracts from different varieties of grapefruits. Acta Sci. Pol. Technol. Aliment. 2019, 18, 453–462. [Google Scholar] [CrossRef]
- Moreno, M.T.; Estévez Brito, R.; Rodríguez Mellado, J.M. Modified CUPRAC method with electrochemical detection for the determination of antioxidant capacity of gallic acid. C. R. Chim. 2020, 23, 395–401. [Google Scholar] [CrossRef]
- Irkin, R.; Dogan, S.; Degirmenioglu, N.; Diken, M.E.; Guldas, M. Phenolic content, antioxidant activities and stimulatory roles of citrus fruits on some lactic acid bacteria. Arch. Biol. Sci. 2015, 67, 1313–1321. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Danilewicz, J.C. The Folin-Ciocalteu, FRAP, and DPPH• Assays for Measuring Polyphenol Concentration in White Wine. Am. J. Enol. Vitic. 2015, 66, 463–471. [Google Scholar] [CrossRef]
- Ordóñez-Gómez, E.S.; Reátegui-Díaz, D.; Villanueva-Tiburcio, J.E. Total polyphenols and antioxidant capacity of peel and leaves in twelve citrus. Sci. Agropecu. 2018, 9, 123–131. [Google Scholar] [CrossRef] [Green Version]
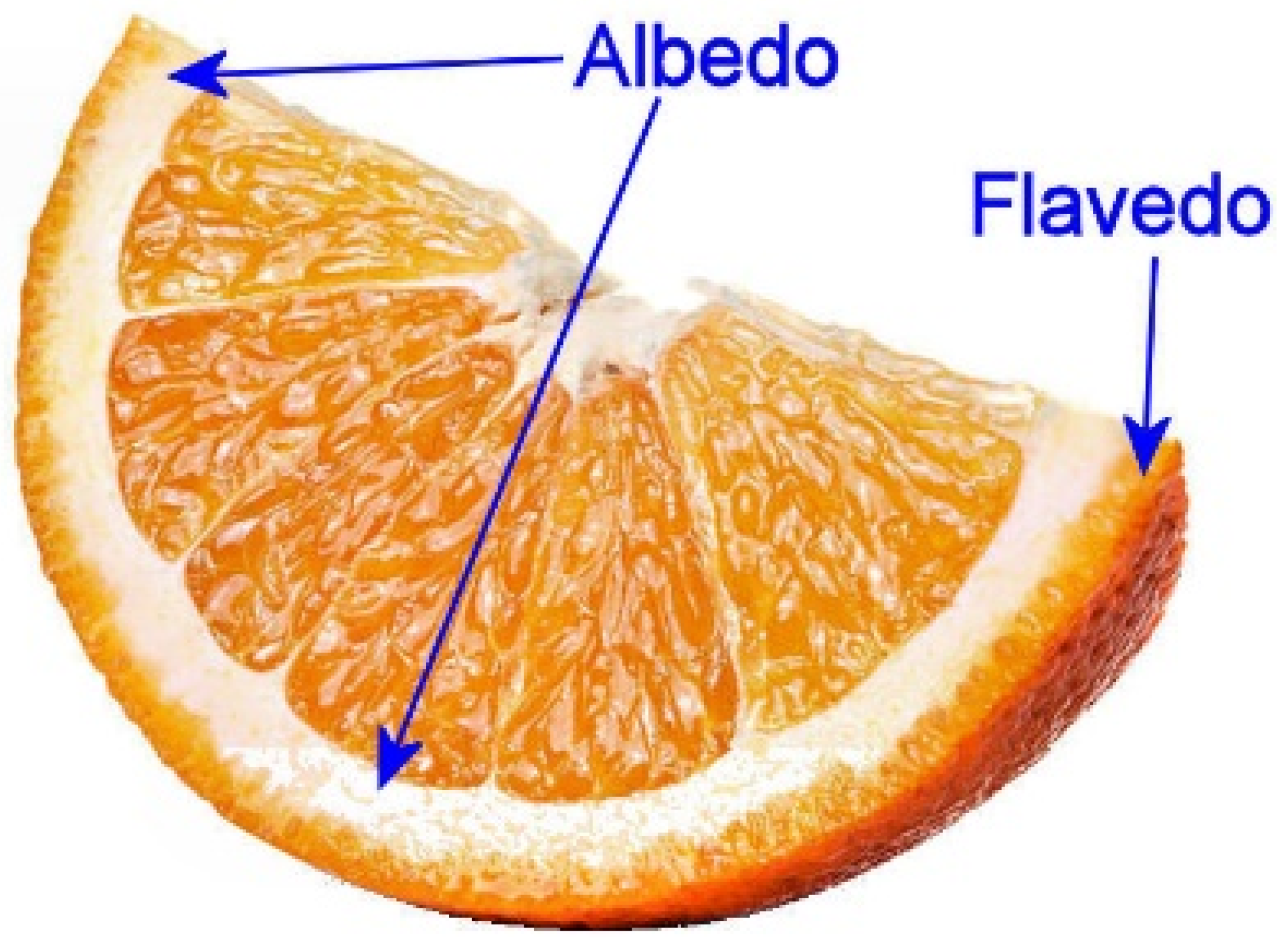
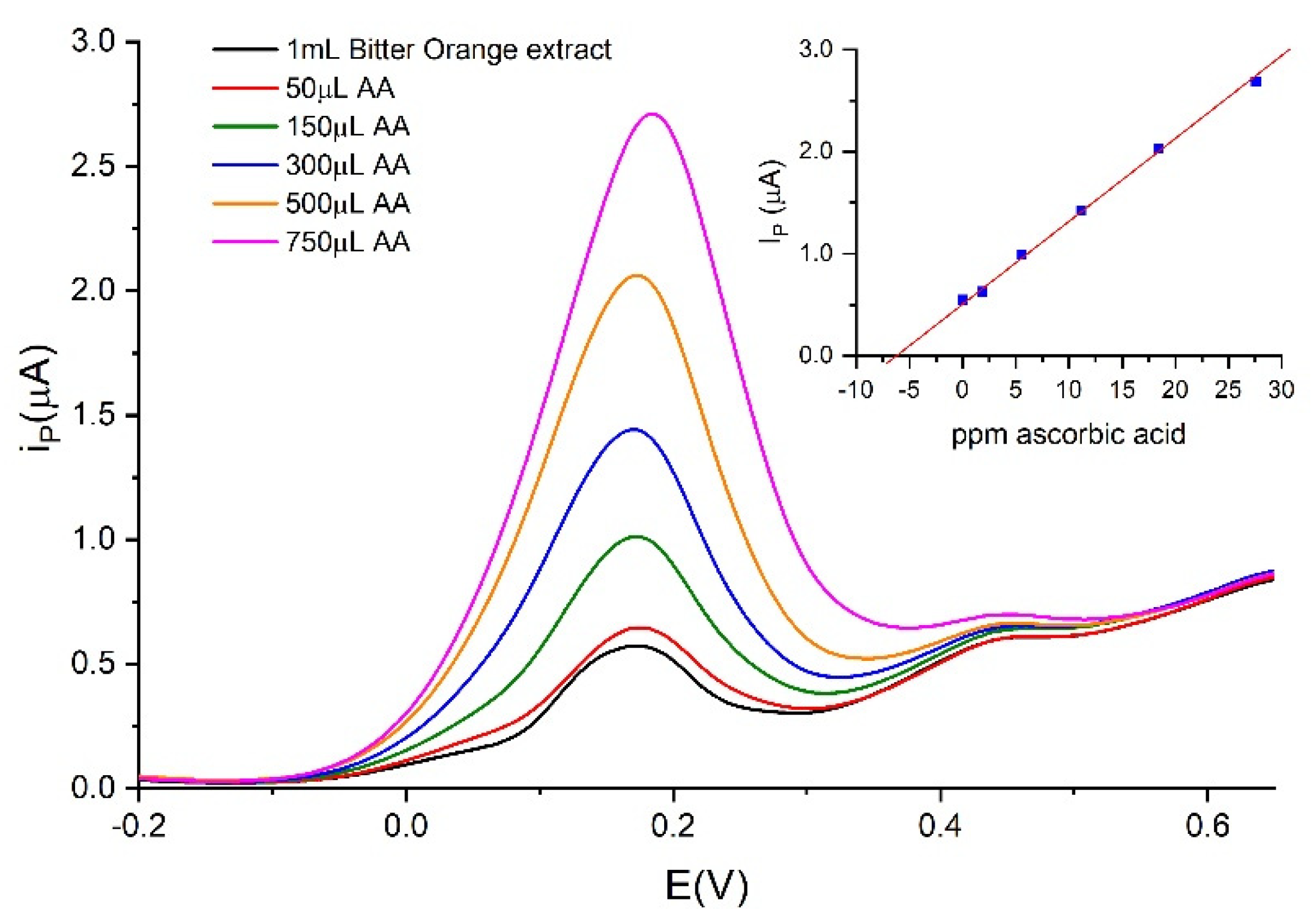

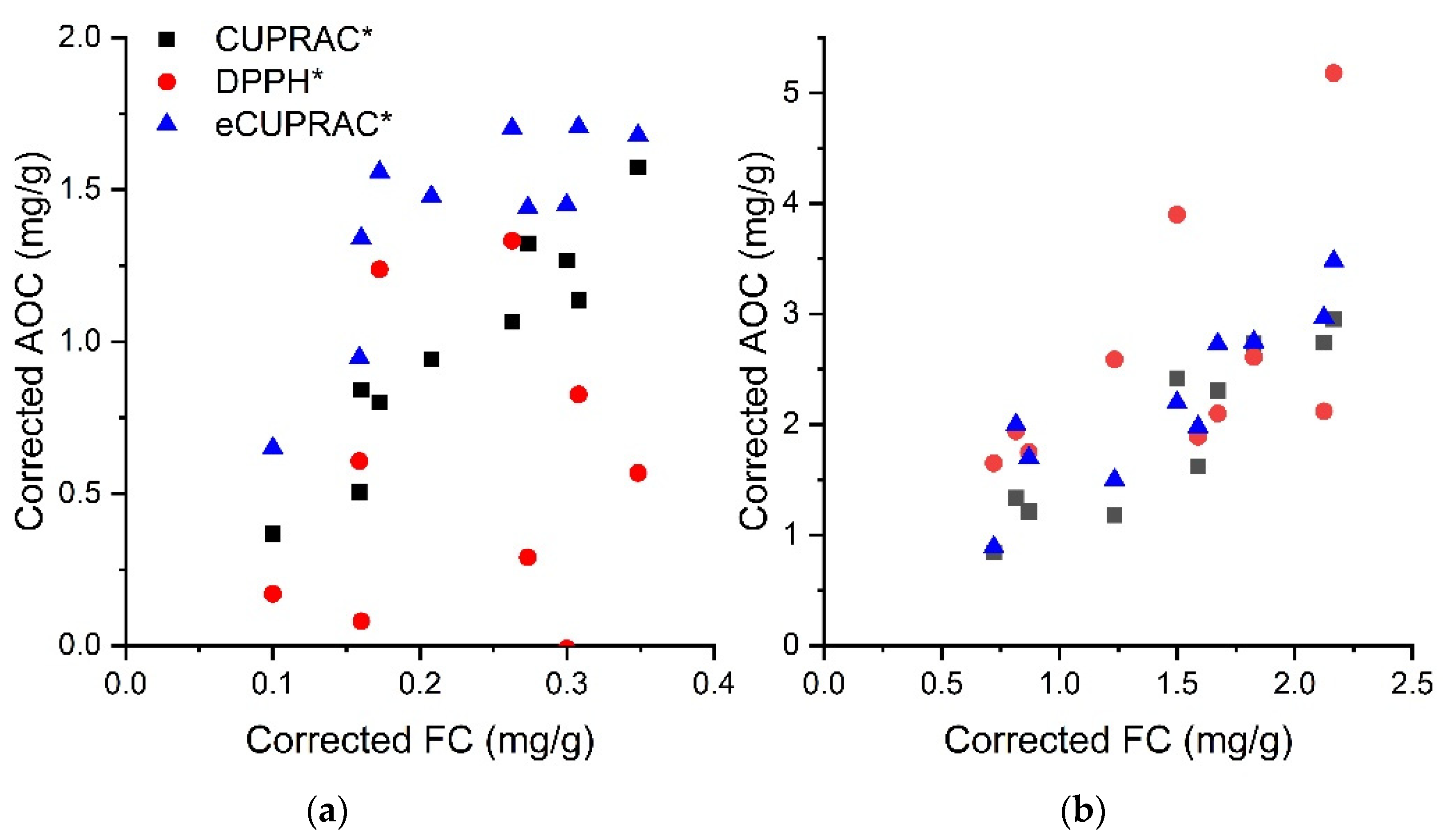

| Sample | FC 1 | Ascorbic Acid 2 | CUPRAC 1 | DPPH 1 | eCUPRAC 1 |
|---|---|---|---|---|---|
| Bergamot | 0.801 | 988 | 1.33 ± 0.12 | 1.74 ± 0.23 | 2.06 ± 0.18 |
| Faustrime lime | 0.885 | 963 | 1.84 ± 0.19 | 0.78 ± 0.20 | 1.93 ± 0.19 |
| Kumquat | 0.375 | 433 | 0.6 ± 0.11 | 0.39 ± 0.19 | 0.87 ± 0.15 |
| Lima | 1.085 | 1295 | 1.76 ± 0.17 | 1.99 ± 0.27 | 2.36 ± 0.17 |
| Tahitian lime | 0.825 | 827 | 1.71 ± 0.18 | 0.41 ± 0.22 | 1.87 ± 0.18 |
| Lemon | 0.625 | 734 | 0.91 ± 0.14 | 0.98 ± 0.25 | 1.32 ± 0.16 |
| Mandarin | 0.785 | 909 | 1.43 ± 0.16 | 0.40 ± 0.20 | 1.94 ± 0.19 |
| Bitter orange | 1.203 | 1405 | 1.89 ± 0.18 | 1.54 ± 0.30 | 2.42 ± 0.22 |
| Sudachi | 1.125 | 1223 | 2.23 ± 0.21 | 1.19 ± 0.29 | 2.30 ± 0.19 |
| Yuzu | 0.585 | 669 | 1.2 ± 0.12 | 0.42 ± 0.21 | 1.68 ± 0.18 |
| Sample | FC 1 | Ascorbic Acid 2 | CUPRAC 1 | DPPH 1 | e-CUPRAC 1 |
|---|---|---|---|---|---|
| Bergamot | 2.300 | 276 | 2.89 ± 0.19 | 2.12 ± 0.22 | 2.97 ± 0.21 |
| Faustrime lime | 1.235 | 574 | 1.52 ± 0.16 | 1.75 ± 0.26 | 1.70 ± 0.14 |
| Kumquat | 0.835 | 179 | 0.94 ± 0.15 | 1.65 ± 0.24 | 0.89 ± 0.14 |
| Lima | 1.235 | 660 | 1.69 ± 0.16 | 1.94 ± 0.28 | 2.01 ± 0.19 |
| Tahitian lime | 2.260 | 683 | 3.10 ± 0.22 | 2.61 ± 0.27 | 2.75 ± 0.18 |
| Lemon | 1.485 | 394 | 1.39 ± 0.12 | 2.59 ± 0.27 | 1.50 ± 0.20 |
| Mandarin | 1.885 | 466 | 1.87 ± 0.14 | 1.89 ± 0.23 | 1.98 ± 0.19 |
| Bitter orange | 2.065 | 617 | 2.64 ± 0.18 | 2.1 ± 0.25 | 2.73 ± 0.22 |
| Sudachi | 2.625 | 723 | 3.34 ± 0.21 | 5.18 ± 0.32 | 3.48 ± 0.20 |
| Yuzu | 1.750 | 394 | 2.63 ± 0.21 | 3.9 ± 0.29 | 2.20 ± 0.18 |
| Method | Water Extracts | Ethanolic Extracts | ||||
|---|---|---|---|---|---|---|
| Intercept | Slope | R2 | Intercept | Slope | R2 | |
| CUPRAC | 0.037 ± 0.233 | 1.751 ± 0.270 | 0.9166 | –0.198 ± 0.329 | 1.357 ± 0.178 | 0.8789 |
| DPPH | –0.289 ± 0.541 | 1.536 ± 0.621 | 0.4292 | 0.487 ± 1.044 | 1.182 ± 0.566 | 0.3524 |
| eCUPRAC | 0.414 ± 0.191 | 1.761 ± 0.221 | 0.8883 | –0.051 ± 0.297 | 1.285 ± 0.161 | 0.8886 |
| Method | Water Extracts | Ethanolic Extracts | ||||
|---|---|---|---|---|---|---|
| Intercept | Slope | R2 | Intercept | Slope | R2 | |
| CUPRAC | –0.014 ± 0.128 | 4.346 ± 0.529 | 0.8939 | –0.040± 0.313 | 1.359 ± 0.204 | 0.8480 |
| DPPH | 0.305 ± 0.526 | 0.866 ± 2.178 | 0.5182 | 0.835 ± 0.961 | 1.196 ± 0.625 | 0.3138 |
| eCUPRAC | 0.631 ± 0.228 | 3.331 ± 0.946 | 0.8984 | 0.328 ± 0.363 | 1.302 ± 0.237 | 0.8894 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, M.T.; Rodríguez Mellado, J.M. Spectrophotometric and Electrochemical Assessment of the Antioxidant Capacity of Aqueous and Ethanolic Extracts of Citrus Flavedos. Oxygen 2022, 2, 99-108. https://doi.org/10.3390/oxygen2020009
Moreno MT, Rodríguez Mellado JM. Spectrophotometric and Electrochemical Assessment of the Antioxidant Capacity of Aqueous and Ethanolic Extracts of Citrus Flavedos. Oxygen. 2022; 2(2):99-108. https://doi.org/10.3390/oxygen2020009
Chicago/Turabian StyleMoreno, María Teresa, and José Miguel Rodríguez Mellado. 2022. "Spectrophotometric and Electrochemical Assessment of the Antioxidant Capacity of Aqueous and Ethanolic Extracts of Citrus Flavedos" Oxygen 2, no. 2: 99-108. https://doi.org/10.3390/oxygen2020009






