Biochemical and Enzymatic Analyses to Understand the Accumulation of γ-Aminobutyric Acid in Wheat Grown under Flooding Stress
Abstract
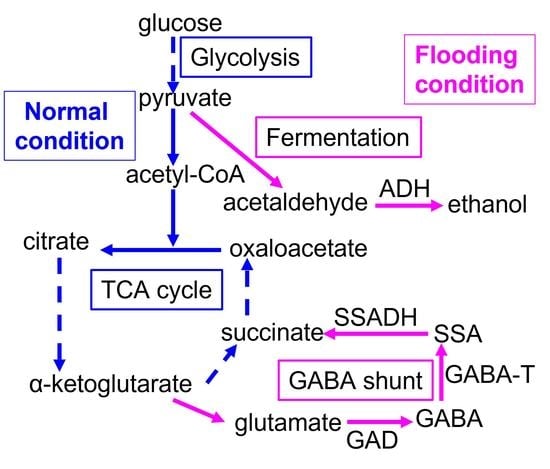
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Protein Extraction
2.3. Immunoblot Analysis
2.4. Assays of Pyruvic Acid, GABA, and Glutamic Acid
2.5. Statistical Analysis
3. Results
3.1. Pyruvic Acid Contents and ADH Accumulation in Wheat Treated with PDSS under Flooding Stress
3.2. Immunoblot Analysis of Proteins Related to GABA Synthesis and Degradation in Wheat Treated with PDSS under Flooding Stress
3.3. Assays of GABA and Glutamic Acid Contents in Wheat Treated with PDSS under Flooding Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Bouabdelli, S.; Zeroual, A.; Meddi, M.; Assani, A. Impact of temperature on agricultural drought occurrence under the effects of climate change. Theor. Appl. Climatol. 2022, 148, 191–209. [Google Scholar] [CrossRef]
- Shen, C.; Yuan, J.; Qiao, H.; Wang, Z.; Liu, Y.; Ren, X.; Wang, F.; Liu, X.; Zhang, Y.; Chen, X.; et al. Transcriptomic and anatomic profiling reveal the germination process of different wheat varieties in response to waterlogging stress. BMC Genet. 2020, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Grover, A. Molecular biology, biotechnology and genomics of flooding-associated low O2 stress response in plants. CRC Crit. Rev. Plant Sci. 2006, 25, 1–21. [Google Scholar] [CrossRef]
- Azam, A.; Shafique, M. Agriculture in pakistan and its impact on economy. A review. Int. J. Adv. Sci. Technol. 2017, 103, 47–60. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Samarah, N.H.; Tanash, A.A. Effect of drought stress on wheat (Triticum durum) growth and metabolism: Insight from GABA shunt, reactive oxygen species and dehydrin genes expression. Func. Plant. Biol. 2022. Advance online publication. [Google Scholar] [CrossRef]
- Beleggia, R.; Omranian, N.; Holtz, Y.; Gioia, T.; Fiorani, F.; Nigro, F.M.; Pecchioni, N.; De Vita, P.; Schurr, U.; David, J.L.; et al. Comparative analysis based on transcriptomics and metabolomics data reveal differences between emmer and durum wheat in response to nitrogen starvation. Int. J. Mol. Sci. 2021, 22, 4790. [Google Scholar] [CrossRef]
- Souza, S.C.; Mazzafera, P.; Sodek, L. Flooding of the root system in soybean: Biochemical and molecular aspects of N metabolism in the nodule during stress and recovery. Amino Acids 2016, 48, 1285–1295. [Google Scholar] [CrossRef]
- Komatsu, S.; Nakamura, T.; Sugimoto, Y.; Sakamoto, K. Proteomic and metabolomic analyses of soybean root tips under flooding stress. Protein. Pept. Lett. 2014, 21, 865–884. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che-Othman, M.H.; Millar, A.H.; Taylor, N.L. Connecting salt stress signalling pathways with salinity-induced changes in mitochondrial metabolic processes in C3 plants. Plant Cell Environ. 2017, 40, 2875–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Hu, Y.; Han, D.; Wei, J.; Zhang, T.; Guo, J.; Shi, L. Wild soybean salt tolerance metabolic model: Assessment of storage protein mobilization in cotyledons and C/N balance in the hypocotyl/root axis. Physiol. Plant 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Li, S.J.; Liu, S.C.; Lin, X.H.; Grierson, D.; Yin, X.R.; Chen, K.S. Citrus heat shock transcription factor CitHsfA7-mediated citric acid degradation in response to heat stress. Plant Cell Environ. 2022, 45, 95–104. [Google Scholar] [CrossRef]
- De Lange, J.H.; Boucher, C. Auto ecological studies on Audinia capitata (Bruniaceaae), plant-derived smoke as a germination cue. S. Afr. J. Bot. 1990, 56, 188–202. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.A.C. Promotion of germination of fynboss seeds by plant-derived smoke. New Phytol. 1993, 123, 575–583. [Google Scholar] [CrossRef]
- Van Staden, J.; Sparg, S.G.; Kulkarni, M.G.; Light, M.E. Post-germination effects of the smoke-derived compound 3-methyl-2H-furo[2,3-c] pyran-2-one, and its potential as a preconditioning agent. Field Crops Res. 2006, 98, 98–105. [Google Scholar] [CrossRef]
- Khatoon, A.; Rehman, S.U.; Aslam, M.M.; Jamil, M.; Komatsu, S. Plant-derived smoke affects biochemical mechanism on plant growth and seed germination. Int. J. Mol. Sci. 2020, 21, 7760. [Google Scholar] [CrossRef]
- Iqbal, M.; Asif, S.; Ilyas, N.; Fayyaz-Ul-Hassan; Raja, N.I.; Hussain, M.; Ejaz, M.; Saira, H. Smoke produced from plants waste material elicits growth of wheat (Triticum aestivum L.) by improving morphological, physiological and biochemical activity. Biotechnol. Rep. 2017, 17, 35–44. [Google Scholar] [CrossRef]
- Komatsu, S.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Rehman, S.U.; Ohno, T. Morphological, biochemical, and proteomic analyses to understand the promotive effects of plant-derived smoke solution on wheat growth under flooding stress. Plants 2022, 11, 1508. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nawaz, S.; Iqbal, K.; Rehman, S.; Ullah, R.; Nawaz, G.; Almeer, R.; Sayed, A.A.; Peluso, I. Plant-derived smoke solution alleviates cellular oxidative stress caused by arsenic and mercury by modulating the cellular antioxidative defense system in wheat. Plants 2022, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Kanwal, M.; Aslam, M.M.; Khan, S.S.; Malook, I.; Tu, J.; Rehman, S.U. Effect of plant-derived smoke priming on physiological and biochemical characteristics of rice under salt stress condition. Aust. J. Crop. Sci. 2014, 8, 159–170. [Google Scholar]
- Malook, J.; Shah, G.; Jan, M.; Shinwari, K.I.; Aslam, M.M.; Rehman, S.U.; Jamil, M. Smoke priming regulates growth and the expression of myeloblastosis and zinc-finger genes in rice under salt stress. Arab. J. Sci. Eng. 2017, 42, 2207–2215. [Google Scholar] [CrossRef]
- Doherty, L.C.; Cohn, M.A. Seed dormancy in rice (Oryza sativa). Commercial liquid smoke elicits germination. Seed. Sci. Res. 2000, 10, 415–421. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, S.; Malook, I.; Rehman, S.U.; Jamil, M. Pb-induced changes in roots of two cultivated rice cultivars grown in lead-contaminated soil mediated by smoke. Environ. Sci. Pollut. Res. Int. 2017, 24, 21298–21310. [Google Scholar] [CrossRef]
- Aslam, M.M.; Rehman, S.; Khatoon, A.; Jamil, M.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Li, X.; Sunohara, Y.; Matsumoto, H.; et al. Molecular responses of maize shoot to a plant derived smoke solution. Int. J. Mol. Sci. 2019, 20, 1319. [Google Scholar] [CrossRef] [Green Version]
- Çatav, Ş.S.; Elgin, E.S.; Dağ, Ç.; Stark, J.L.; Küçükakyüz, K. NMR-based metabolomics reveals that plant-derived smoke stimulates root growth via affecting carbohydrate and energy metabolism in maize. Metabolomics 2018, 14, 143. [Google Scholar] [CrossRef]
- Rehman, A.; Rehman, S.U.; Khatoon, A.; Qasim, M.; Itoh, T.; Iwasaki, Y.; Wang, X.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the promotive effect of plant-derived smoke on plant growth of chickpea. J. Proteom. 2018, 176, 56–70. [Google Scholar] [CrossRef]
- Li, X.; Rehman, S.U.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Yamaguchi, T.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the effect of plant-derived smoke on soybean during recovery from flooding stress. J. Proteom. 2018, 181, 238–248. [Google Scholar] [CrossRef]
- Zhong, Z.; Kobayashi, T.; Zhu, W.; Imai, H.; Zhao, R.; Ohno, T.; Rehman, S.U.; Uemura, M.; Tian, J.; Komatsu, S. Plant-derived smoke enhances plant growth through ornithine-synthesis pathway and ubiquitin-proteasome pathway in soybean. J. Proteom. 2020, 221, 103781. [Google Scholar] [CrossRef] [PubMed]
- Otori, M.; Murashita, Y.; Rehman, S.; Komatsu, S. Proteomic study to understand promotive effects of plant-derived smoke on soybean root growth under flooding stress. Plant Mol. Biol. Rep. 2021, 39, 24–33. [Google Scholar] [CrossRef]
- Murashita, Y.; Nishiuchi, T.; Rehman, S.U.; Komatsu, S. Subcellular proteomics to understand promotive effect of plant-derived smoke solution on soybean root. Proteomes 2021, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Komatsu, S.; Deschamps, T.; Hiraga, S.; Kato, M.; Chiba, M.; Hashiguchi, A.; Tougou, M.; Shimamura, S.; Yasue, H. Characterization of a novel flooding stress-responsive alcohol dehydrogenase expressed in soybean roots. Plant Mol. Biol. 2011, 77, 309–322. [Google Scholar] [CrossRef]
- Nishiyama, T.; Sulistyaningdyah, W.T.; Ueda, K.; Kusakabe, H. GABA enzymatic assay kit. Biosci. Biotech. Biochem. 2020, 84, 118–125. [Google Scholar] [CrossRef]
- Štáfková, J.; Mach, J.; Biran, M.; Verner, Z.; Bringaud, F.; Tachezy, J. Mitochondrial pyruvate carrier in Trypanosoma brucei. Mol. Microbiol. 2016, 100, 442–456. [Google Scholar] [CrossRef] [Green Version]
- Parveen, M.; Miyagi, A.; Kawai-Yamada, M.; Rashid, M.H.; Asaeda, T. Metabolic and biochemical responses of Potamogeton anguillanus Koidz. (Potamogetonaceae) to low oxygen conditions. J. Plant Physiol. 2019, 232, 171–179. [Google Scholar] [CrossRef]
- Ismond, K.P.; Dolferus, R.; de Pauw, M.; Dennis, E.S.; Good, A.G. Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol. 2003, 132, 1292–1302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wei, X.; Liu, Z.; Wu, X.; Bao, C.; Sun, Y.; Su, N.; Cui, J. Transcriptome analysis reveals the molecular mechanism of GABA accumulation during quinoa (Chenopodium quinoa Willd.) germination. J. Agric. Food Chem. 2021, 69, 12171–12186. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Sokolov, S.S.; Balakireva, A.V.; Markova, O.V.; Severin, F.F. Negative feedback of glycolysis and oxidative phosphorylation: Mechanisms of and reasons for it. Biochemistry 2015, 80, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shen, L.; Zhang, D.; Wang, X.; Wang, Q.; Qin, W.; Gao, Y.; Li, X. Ammonia-induced oxidative stress triggered proinflammatory response and apoptosis in pig lungs. J. Environ. Sci. 2023, 126, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Roudier, E.; Perrin, A. Considering the role of pyruvate in tumor cells during hypoxia. Biochim. Biophys. Acta 2009, 1796, 55–62. [Google Scholar]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Wang, L.; Tan, F.; Lu, S.; Lv, X.; Zaynab, M.; Cheng, C.L.; Abubakar, Y.S.; Chen, S.; Chen, W. Phosphoproteomics unveils stable energy supply as key to flooding tolerance in Kandelia candel. J. Proteom. 2018, 176, 1–12. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, C.; Wang, P.; Gu, Z.; Yang, R. GABA regulates phenolics accumulation in soybean sprouts under nacl stress. Antioxidants 2021, 10, 990. [Google Scholar] [CrossRef]
- Hossain, M.A.; Uddin, S.N. Mechanisms of waterlogging tolerance in wheat: Morphological and metabolic adaptations under hypoxia or anoxia. Aust. J. Crop. Sci. 2011, 5, 1094–1101. [Google Scholar]
- Sun, L.; Li, X.; Wang, X.; Xiang, L.; Yang, J.; Min, Q.; Chen, G.; Chen, F.; Huang, C.; Wang, G. Growth and respiratory metabolic adaptation strategies of riparian plant Distylium chinense to submergence by the field study and controlled experiments. Plant Physiol. Biochem. 2020, 157, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R. Identification of psychoactive metabolites from Cannabis sativa, its smoke, and other phytocannabinoids using machine learning and multivariate methods. ACS Omega 2020, 5, 281–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelp, B.J.; Bown, A.W.; Zarei, A. 4-Aminobutyrate (GABA): A metabolite and signal with practical significance. Botany 2017, 95, 1015–1032. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The Versatile GABA in Plants. Plant Signal Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef] [PubMed]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Valderrama, R.; Corpas, F.J.; Carreras, A. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ. 2006, 29, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Fromm, H.; Walter, D. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, S.; Nishiyama, N.; Diniyah, A. Biochemical and Enzymatic Analyses to Understand the Accumulation of γ-Aminobutyric Acid in Wheat Grown under Flooding Stress. Oxygen 2023, 3, 120-132. https://doi.org/10.3390/oxygen3010009
Komatsu S, Nishiyama N, Diniyah A. Biochemical and Enzymatic Analyses to Understand the Accumulation of γ-Aminobutyric Acid in Wheat Grown under Flooding Stress. Oxygen. 2023; 3(1):120-132. https://doi.org/10.3390/oxygen3010009
Chicago/Turabian StyleKomatsu, Setsuko, Natsuru Nishiyama, and Azzahrah Diniyah. 2023. "Biochemical and Enzymatic Analyses to Understand the Accumulation of γ-Aminobutyric Acid in Wheat Grown under Flooding Stress" Oxygen 3, no. 1: 120-132. https://doi.org/10.3390/oxygen3010009
APA StyleKomatsu, S., Nishiyama, N., & Diniyah, A. (2023). Biochemical and Enzymatic Analyses to Understand the Accumulation of γ-Aminobutyric Acid in Wheat Grown under Flooding Stress. Oxygen, 3(1), 120-132. https://doi.org/10.3390/oxygen3010009