Abstract
Lower limb muscle fatigue is the main reason for withdrawal from diving. Therefore, this study aimed to determine the local muscle oxygen saturation and hemoglobin concentration in the vastus lateralis muscle during different freediving disciplines. One freediver participated in this study, and his chronological age was 40 years, body mass 75.0 kg, body height 184.0 cm, and body fat 13.7%. The participant has been practicing freediving for 6 years. The variables in this study included anthropometric indices, heart rate, and muscle oxygen dynamics parameters (SmO2 (oxygen muscle saturation) and tHb (total hemoglobin)). The variables were measured during five diving disciplines: static apnea, bifin, dynamic no fins (DNF), monofin, and sneaking. Measurements were performed during intensive training/competition during the diving season in August 2023. The results of this study showed that when oxygen starts to decrease during the dive, the tHb increases. Furthermore, the times at which maximal tHb and minimal SmO2 were achieved are also shown. These parameters occurred at almost the same time across all disciplines: static (SmO2, 142; tHb, 150 s), bifin (SmO2, 153; tHb, 148 s), DNF (SmO2, 162; tHb, 178 s), monofin (SmO2, 96; tHb, 94 s), and sneaking (SmO2, 212; tHb, 228 s). Also, differences in tHb and SmO2 were present between diving disciplines. In particular, the highest increase in tHb was present in bifin (0.0028 AU/s), whereas monofin showed a decrease (−0.0009 AU/s). On the other hand, the highest desaturation was seen in bifin (−0.87%/s) and the lowest in sneaking (−0.29%/s) These findings emphasize the physiological characteristics of freedivers engaging in different freediving disciplines that influence muscles during the dive. Such responses could be observed through a concurrent hypoxia/hypercapnia and a transient reduction in the Fahraeus effect.
1. Introduction
Freediving is an extreme sport that places high physiological demands on athletes during their dives [1,2]. All freediving disciplines fit into the category of breath-holding or breath-hold diving (BHD). BHD is defined as resistance to a low oxygen environment, a reduction in sensitivity to hypoxia, and low mitochondrial oxygen consumption [3]. Presently, BHD is performed by many different participants (e.g., recreational divers, seafood divers, military divers, and competitive athletes) [4,5]. The periods of apnea that participants endure are why freediving is an extreme sport and why freedivers face potential danger [6,7]. In competitive BHD, the freedivers are constantly trying to break records in all disciplines, despite the possible dangers. Previously, several performance factors in freediving have been defined: the amount of oxygen that is stored in the body (e.g., lungs, blood, and tissues), tolerance of cerebral ischaemia, metabolic capacity, and the level of activation of the diving response [8].
Apart from these factors, all participants in BHD have some physiological responses, such as the diving response (DR), which comprises reduced cardiac output (CO), bradycardia, and skeletal muscle ischaemia [9,10,11,12,13]. Specifically, the DR is initiated during apnea or facial submersion in water and includes peripheral vasoconstriction, reduced CO, bradycardia, and low muscle oxygenation, and cerebral perfusion is augmented as hypercapnia develops [9,10,11,12,13,14]. Furthermore, the appropriate mechanics and technique regarding movement are also necessary. In addition, muscle ischaemia and acidosis during the dive could also be seen as important parameters of performance [13]. These parameters may lead to a loss of muscle strength, which is important for the propulsion of the fins during dynamic dives.
Near-infrared spectroscopy (NIRS) is a non-invasive assessment tool for viewing changes in oxygenation and hemodynamics in skeletal muscle tissue [15,16]. NIRS measures blood volume, muscle oxygenation, and muscle oxygen saturation (SmO2), which represents oxygen delivery to the precise muscle area beneath the sensor during exercise [17]. Moreover, continuous-wave NIRS does not provide absolute measurements; therefore, only relative NIRS blood volume measures were reported. SmO2, which has a value range of 1% to 100%, precisely represents the dynamic equilibrium between the supply and consumption of oxygen in muscle tissue and is unaffected by the path of the near-infrared photons (the algorithm was developed to solve the problem of the unknown differential path length factor) [18]. Apart from SmO2, NIRS could determine the fluctuation of hemoglobin in the measured region by measuring total hemoglobin (tHb). tHb is a parameter estimated from hemoglobin concentration in the blood and myoglobin in the muscle, and it indirectly shows hemodynamics or changes in local muscle blood volume [19]. Previously, NIRS variables were measured in the right vastus lateralis muscle of the freedivers, which is the most common site for the examination of local muscle oxygenation dynamics [20]. So far, there is sparse evidence of NIRS usage in freediving, especially in specific conditions (i.e., diving disciplines). Prior research on several bodily sites (particularly cerebral) defined NIRS measures in freediving [21]. The primary goals of these investigations were to ascertain and comprehend human physiology in low-oxygen settings [11,22]. Also, some NIRS studies were performed using laboratory tests that included non-specific procedures [20,23]. Following that, there is a need for an examination of NIRS parameters during specific freediving disciplines. This is mainly because of fatigue in the lower limb muscles after prolonged dives. Therefore, this study aimed to determine the local muscle oxygen saturation and hemoglobin concentration in the right vastus lateralis muscle of freedivers during different freediving disciplines. Also, the secondary aim was to observe SmO2 and tHb parameters in relation to heart rate.
2. Materials and Methods
2.1. Participant
One freediver participated in this study, and his chronological age was 40 years, body mass 75.0 kg, body height 184.0 cm, and body fat 13.7%. The participant has been practicing freediving for 6 years, and he could be considered as a moderate athlete. Measurements were performed during intensive training/competition during the diving season in August 2023. He was informed about the procedures and purpose of the study and provided informed consent before the investigation began. Before the beginning of the procedure, the participant was informed about all the risks and possible dangers of the procedure and provided signed written consent. All experimental procedures were completed according to the Declaration of Helsinki, and they were approved by the corresponding author’s institutional research ethics board (ethics board approval no. 2181-205-02-05-22-035).
2.2. Variables
The variables in this study included anthropometric indices, heart rate, blood lactate levels, and muscle oxygen dynamics parameters (SmO2 and tHb).
Anthropometric indices included body height, body mass, and body fat percentage. Body mass and body fat percentage were assessed with the bioimpedance scale (Tanita BC 418 scale; Tokyo, Japan). Body height was determined with a Tanita HR-001 anthropometer (Tanita; Tokyo, Japan). Heart rate was assessed with a Garmin Descent Mk1 watch-sized dive computer (Garmin Ltd., Olathe, KS, USA). Lactate levels were analyzed after every discipline of freediving. The analysis was performed by professional personnel using the lactate Accutrend analyzer (Roche, Boehringer Mannheim, Indianapolis, IN, USA).
Muscle oxygenation and blood volume changes in the lower limb muscles were measured by the commercially available continuous wave NIRS sensor (Moxy Monitor; Fortiori Design LLC, Hutchinson, MN, USA). The functioning, usage, validity, and reliability of the NIRS sensor have been previously described [18,24]. The main parameter generated by the NIRS sensor is SmO2, which is calculated as a ratio of relative oxyhemoglobin to oxymyoglobin and relative deoxyhemoglobin to deoxymyoglobin [18]. Apart from SmO2, tHb (total blood hemoglobin) is also a parameter derived from the NIRS sensor readings and indicates changes in blood volume in a particular muscle under the sensor [15]. Specifically, tHb is a parameter estimated from hemoglobin and myoglobin concentration in the blood, and it indirectly shows hemodynamics or changes in local muscle blood volume [19].
Additionally, the following muscle oxygen parameters were calculated: baseline SmO2 (average muscle oxygen saturation before the test), initial desaturation SmO2 rate (desat slope) (inclination of the slope at the beginning of the test to first breakpoint in the curve), minimal SmO2 (minimal oxygen saturation during the dive), and maximal SmO2 (maximal oxygen saturation during recovery). tHb measurements included baseline tHb (average muscle oxygen saturation before the test), maximal tHb (maximal hemoglobin during the test), minimal tHb (minimal hemoglobin before the test), and tHb slope (inclination of the slope at the beginning of the test to first breakpoint in the curve). Furthermore, breakpoints were determined via least squares piecewise regression analysis at the minimally attainable SmO2 [24] (see Figure 1).
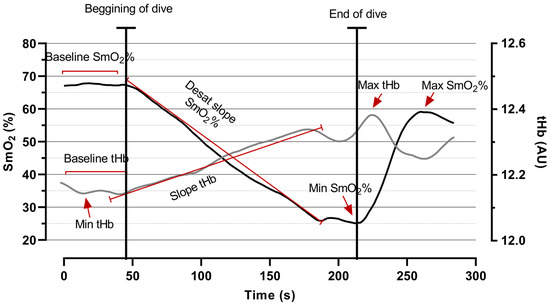
Figure 1.
A graphical example of the time course of SmO2 (black line) and tHb (grey line), with variables (baseline, slope, minimal, and maximal) gathered from the right vastus lateralis muscle.
2.3. Procedure
A NIRS sensor was placed over the right vastus lateralis muscle, consistent with previous studies [25,26]. This muscle was used mainly because it is at the highest contraction during the tests. The oxygenation of the muscle was measured, and the appropriate light shield was placed over the NIRS sensor to prevent ambient light intrusion and interference with the sensor reading. Furthermore, the Moxy probe is waterproof and does not need any additional protection. The sensor was fixed in place with 3M medical tape. The device uses four wavelengths (680, 720, 760, and 800 nm) to assess the ratio of oxyhemoglobin to oxymyoglobin and deoxyhemoglobin to deoxymyoglobin via a modified Beer–Lambert equation, resulting in a percent concentration of SmO2. The device detectors were spaced 12.5 mm and 25 mm from the emitter. The sampling rate was set using the default mode, which samples the four wavelengths over 80 cycles to generate an average output every two seconds (0.5 Hz). The data were gathered using the Moxy Portal app, which provides a data export feature in xlsx format. The participant’s body fat percentage was low (13.7%), and therefore, adipose tissue thickness was considered negligible for the NIRS results.
The procedure was conducted in a swimming pool during the participant’s anaerobic apnea training period. He engaged in 5 disciplines of freediving training (static apnea, bifin, dynamic no fins (DNF), monofin, and sneaking), one after another. Static apnea was performed on dry land while the athlete was holding his breath in a supine position. The bifin and monofin disciplines were performed in the swimming pool with the use of two fins and one fin. DNF and sneaking were performed without additional diving equipment. Furthermore, during sneaking, the athlete slowly crawled on the floor of the swimming pool. All disciplines were submaximal for the participant.
During the intense diving training/competition season in August 2023, local muscle oxygenation and hemodynamics were measured together with heart rate. After each discipline, the diver rested for at least 10 min to fully recover so that the measured parameters could return to their baseline values. The NIRS data acquisition was performed for 2 min before the start of the dive and 2 min after the dive was performed, and heart rate was measured in the same manner (see Figure 2).
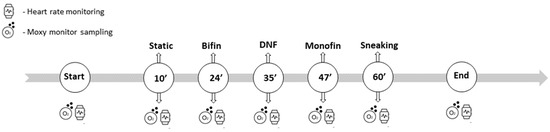
Figure 2.
Data collection timeline with the order of the tested disciplines.
2.4. Statistical Analysis
Descriptive statistics were measured to assess the arithmetic means and standard deviations of all measured variables. The K–S (Kolmogorov–Smirnov) test for normality was used to determine the normal distribution of the data. The data in the figures are presented as normalized values with a range from 0% to 100%. The normalization was performed according to the formula below:
Statistica version 13.0 (Dell Inc., Austin, TX, USA) was used for the analyses, and a level of 95% (p < 0.05) was applied.
3. Results
Table 1, Table 2 and Table 3 show results of the measured variables (duration, heart rate, tHb, and SmO2) in the five freediving disciplines. The static and sneaking disciplines were the longest in duration at 218.0 s and 165.0 s, respectively. Furthermore, the participant experienced a decrease in heart rate during all disciplines. On the other hand, the highest heart rate decrease was observed in the static (−0.33 bpm/s) and DNF (−0.31 bpm/s) disciplines (see Table 1).

Table 1.
Results of apnea duration and heart rate parameters in all measured disciplines.

Table 2.
Results of total hemoglobin parameters (tHb) in all measured disciplines.

Table 3.
Results of oxygen saturation parameters (SmO2) in all measured disciplines.
The tHb results showed an increased trend in all disciplines, except monofin (−0.0009 AU/s). The largest increase was seen in bifin (0.0028 AU/s) (see Table 2). Table 3 shows the SmO2 variables (baseline SmO2 %, desaturation slope, minimal SmO2 %, and maximal SmO2 %). The results showed that the hemoglobin in the vastus lateralis muscle desaturated the fastest in bifin (−0.87%/s) and the slowest in sneaking (−0.29%/s).
Figure 3 presents tHb and SmO2 trends in all diving disciplines. The analysis showed that when oxygen started to decrease during the dive, the tHb increased. By contrast, after the dive, the SmO2 % increased and the tHb decreased. Furthermore, the times at which the maximal tHb and the minimal SmO2 were reached are also shown. These parameters occurred at almost the same time across all disciplines: static (SmO2, 142 s; tHb, 150 s), bifin (SmO2, 153 s; tHb, 148 s), DNF (SmO2, 162 s; tHb, 178 s), monofin (SmO2, 96 s; tHb, 94 s), and sneaking (SmO2, 212 s; tHb, 228 s).
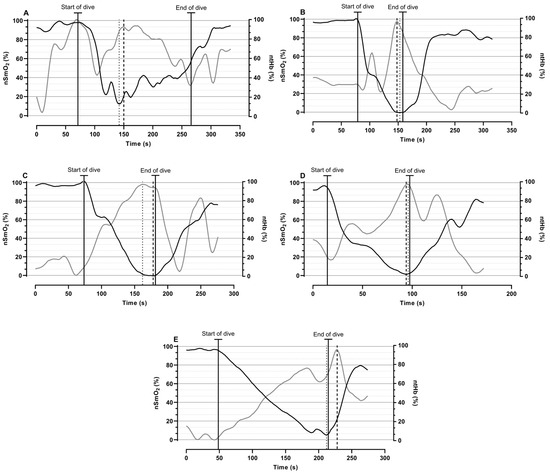
Figure 3.
A time course of normalized SmO2 (black line) and tHb (grey line) variables gathered from the right vastus lateralis muscle during rest, test, and rest in (A) static, (B) bifin, (C) DNF, (D) monofin, and (E) sneaking disciplines; times of minimal SmO2 (dotted line) and maximal tHb (dashed line) are shown.
After the diver performed the dive, his lactate levels ranged from 1.6 to 7.7 mmol/L (static, 1.6 mmol/L; bifin, 7.6 mmol/L; DNF, 7.7 mmol/L; monofin, 6.4 mmol/L; and sneaking, 6.7 mmol/L).
4. Discussion
Different freediving disciplines place different demands on athletes. The fatigue and acidosis of lower limb muscles are the number one reason for stopping the apnea during dives. Such extreme demands placed on athletes require an examination of local muscle hemodynamics during diving to enhance athletes’ performance. Therefore, this study aimed to examine both tHb and SmO2 fluctuations and relations during freediving in the right vastus lateralis muscle during the intense training/competition season in August 2023. The main findings of this study are that SmO2 and tHb have opposing trends during different diving disciplines.
As expected, the results showed that tHb and SmO2 demonstrated opposing trends during all disciplines since extreme skeletal muscle ischemia occurs during a dive in which divers swim after taking a single breath; at the same time, muscle hyperemia (increased muscle blood flow) compensates for the local lack of oxygen during dynamic exercise. In particular, the decrease in SmO2 corresponded with an increase in tHb. Additionally, tHb is derived as the sum of oxygenated (O2Hb) and deoxygenated hemoglobin (HHb) in the measured region (tHb = O2Hb + HHb), and it indicates a change in the blood flow to the muscle [27]. On the other hand, SmO2 is the fraction of O2Hb compared to tHb (SmO2 = O2Hb/tHb) and is defined as the percentage of oxygenated hemoglobin and myoglobin relative to the total quantity of chromophores present in the muscle tissue at a certain moment [18]. However, current technology does not allow us to determine if there are increases in O2Hb or HHb (deoxyHb). Therefore, these muscle oxygenation trends should be explained with a comprehensive physiological evaluation.
The trend observed regarding the increase in blood flow could be seen as hyperemic reactivity of a muscle due to extreme local hypoxia/hypercapnia. According to Alvares, et al. [28], tHb has a strong correlation with Doppler ultrasound. Additionally, the authors demonstrated that there was an increase in tHb and blood flow post exercise due to hyperemia of the muscle tissue. These findings were explained by (i) increased cardiac output (CO) (with concomitant redirection of blood flow to the working muscle) and vasodilation of downstream resistance arterioles brought about by concurrent hypoxia/hypercapnia, which typically occurs during muscle contraction and (ii) a transient reduction in the Fahraeus effect (i.e., a decrease in the hematocrit level as blood flows from larger to smaller vessels) [29]. It was previously established that during freediving, divers experience involuntary breathing movements (IBM) that increase SV to maintain CO and increase blood flow [11]. Therefore, the increase in tHb in our study could be connected with the renormalization of CO and the need for blood flow maintenance of working muscles.
However, the increase also occured during static apnea (SA). Therefore, other factors may have influenced the opposing trend of SmO2 and tHb. During diving, oxygen delivery is limited to one breath that is taken before the dive. The oxygen consumption during this single breath limits the quantity of O2 in the tissue, causing extreme local hypoxia and accumulation of CO2. Molchanova, et al. [30] reported that the partial pressure of CO2 increases during prolonged dives. As a result, the increase in blood flow during both static and dynamic disciplines may be connected to the need for the removal of accumulated CO2.
Further data analysis showed that minimal SmO2 and maximal tHb occurred at almost the same time, with differences ranging from 2 s to 16 s. Previously, it was demonstrated that tHb usually drops with SmO2, after which it increases rapidly [28]. However, our study showed the opposite results, with minimal and maximal values occurring at similar times. Furthermore, this correspondence of SmO2 and tHb values happened during different periods of the dives (i.e., beginning, middle, or end). Such results most likely occurred because of the demands that freediving places on athletes. Also, the variability in the timing most likely happened because of differences in the execution of the different dives. Specifically, different diving disciplines require different techniques concerning the use of lower limb muscles. Furthermore, differences between active and passive dives play a key role in the explanation of the deoxygenation trend. It should be noted that stronger deoxygenation trends and lower SmO2 values were observed during passive dives (i.e., sneaking and static apnea).
There are a limited number of studies that assess the cardiovascular responses to SA and DA in pools by measuring underwater hemodynamic physiological parameters such as ECG (electrocardiogram), arterial oxygen saturation (SaO2), SV, HR, and CO [9,31,32]. Kiviniemi, Breskovic, Uglesic, Kuch, Maslov, Sieber, Seppänen, Tulppo and Dujic [31] investigated heart rate variability changes in the settings of SA and DA. They concluded that apnea blunts the effects of exercise on cardiac vagal activity at the end of DA; however, a higher HR during DA compared with SA indicates higher cardiac sympathetic activity during DA. Breskovic, Uglesic, Zubin, Kuch, Kraljevic, Zanchi, Ljubkovic, Sieber and Dujic [9] found moderate increases in blood pressure (BP) during SA and DA as well as a faster desaturation during DA vs. SA. These cardiovascular changes during immersed SA and DA are in agreement with those reported for dry SA and DA. Tocco, Marongiu, Pinna, Roberto, Pusceddu, Angius, Migliaccio, Milia, Concu and Crisafulli [32] also found a decrement in SV (−8%) and CO (−25%), but mean blood pressure (MBP) was maintained because of an increase in stroke volume rate (SVR) in SA. Sympathetic activation was induced by exercise during DA, which partially obscured the effects of the diving response.
Additionally, different disciplines place different demands on freedivers, which is evident from observations of SmO2 and tHb values. Based on these results, there is an implication that bifin is the most oxygen demanding since desaturation occurs more rapidly during this discipline compared to the other disciplines. Also, the rate of tHb increase was highest during bifin, indicating a large blood flow increase in the measured muscle. Such a conclusion is logical since divers use both legs simultaneously. Furthermore, there was a similar yet smaller increasing/decreasing trend during the static disciplines (static and sneaking). The slower desaturation rate during sneaking is mostly due to the diving response that occurs in water, whereas dry-land apnea results in faster desaturation. Moreover, it should be noted that both static and dynamic apneas influence the SmO2 decrease differently. Such an indication was seen with the relation between apnea duration and minimal SmO2. The results indicate that more prolonged breath holds will induce lower values of SmO2. Such trends and differences between disciplines could potentially implicate diving limiting factors. Specifically, more static disciplines may have less of an impact on skeletal muscle deoxygenation, and other factors may influence the duration of the dive. On the other hand, active dives lead to a higher work rate of skeletal muscle, and skeletal muscle deoxygenation may influence the cessation of the dive. Hence, different disciplines place different demands on athletes in terms of the usage of skeletal muscles, which are important to the duration of dives. Also, these deoxygenation trends and apnea breakpoints may be detrimental to performance in dynamic diving disciplines.
Limitations and Strengths
The main limitation of this study is the sample of participants, which is one athlete. Also, the intensity of the study procedure could influence the observed results. In particular, performing multiple disciplines one after the other could be seen as intensive and could have longer-lasting residual effects on the subsequent disciplines. Furthermore, the inclusion of measurements of other physiological parameters could more precisely explain SmO2 and tHb trends in future studies. On the other hand, this study is the first one that explains these results in specific freediving disciplines. Also, the usage of NIRS underwater is lacking in the literature, apart from studies including cerebral measurements [21]. Therefore, these are the main strengths of the study.
5. Conclusions
Conclusively, this study aimed to determine the local muscle oxygen saturation and hemoglobin concentration during freediving disciplines. According to the aims, the results of this study demonstrated opposing SmO2 and tHb trends. Furthermore, the minimal SmO2 and maximal tHb occurred almost simultaneously during the dive. Additionally, the trends in the rates of increase/decrease in SmO2 and tHb were different during the different disciplines.
These findings outline the physiological characteristics of freediving that influence muscles during the dive. Such responses may have been observed because of a concurrent extreme local hypoxia/hypercapnia and a transient reduction in the Fahraeus effect. Furthermore, there were different trends in the timing of low oxygen and high blood flow during the different disciplines. These results could be used in freediving training to monitor the safety of the divers and to influence the resistance to hypoxia/hypercapnia, resulting in an increase in the duration of dives.
Author Contributions
Conceptualization, D.V. and N.F.; methodology, N.F.; software, D.V.; validation, N.F. and Ž.D.; formal analysis, D.V.; investigation, D.V. and N.F.; resources, Ž.D.; data curation, Ž.D.; writing—original draft preparation, D.V.; writing—review and editing, Ž.D.; visualization, D.V.; supervision, Ž.D.; project administration, N.F.; funding acquisition, N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional research ethics board of the Faculty of Kinesiology, University of Split (ethics board approval no. 2181-205-02-05-22-035; approval date: 20 September 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are presented in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schagatay, E. Predicting performance in competitive apnea diving, part II: Dynamic apnoea. Diving Hyperb. Med. 2010, 40, 11–22. [Google Scholar] [PubMed]
- Bain, A.R.; Drvis, I.; Dujic, Z.; MacLeod, D.B.; Ainslie, P.N. Physiology of static breath holding in elite apneists. Exp. Physiol. 2018, 103, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Kjeld, T.; Stride, N.; Gudiksen, A.; Hansen, E.G.; Arendrup, H.C.; Horstmann, P.F.; Zerahn, B.; Jensen, L.T.; Nordsborg, N.; Bejder, J.; et al. Oxygen conserving mitochondrial adaptations in the skeletal muscles of breath hold divers. PLoS ONE 2018, 13, e0201401. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Clarke, J.R. Breath-hold diving. Compr. Physiol. 2018, 8, 585–630. [Google Scholar] [PubMed]
- Schagatay, E. Human breath-hold diving ability and the underlying physiology. Hum. Evol. 2014, 29, 125–140. [Google Scholar]
- Giunta, A.A.; Liberati, L.; Pellegrino, C.; Ricci, G.; Rizzo, S. Eustachian tube balloon dilation in treatment of equalization problems of freediving spearfishermen. Diving Hyperb. Med. 2019, 49, 9. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.; Lundgren, C. Alveolar gas composition before and after maximal breath-holds in competitive divers. Undersea Hyperb. Med. 2006, 33, 463. [Google Scholar] [PubMed]
- Schagatay, E.; Andersson, J.P.; Nielsen, B. Hematological response and diving response during apnea and apnea with face immersion. Eur. J. Appl. Physiol. 2007, 101, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Breskovic, T.; Uglesic, L.; Zubin, P.; Kuch, B.; Kraljevic, J.; Zanchi, J.; Ljubkovic, M.; Sieber, A.; Dujic, Z. Cardiovascular changes during underwater static and dynamic breath-hold dives in trained divers. J. Appl. Physiol. 2011, 111, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Dujic, Z.; Ivancev, V.; Heusser, K.; Dzamonja, G.; Palada, I.; Valic, Z.; Tank, J.; Obad, A.; Bakovic, D.; Diedrich, A.; et al. Central chemoreflex sensitivity and sympathetic neural outflow in elite breath-hold divers. J. Appl. Physiol. 2008, 104, 205–211. [Google Scholar] [CrossRef]
- Dujic, Z.; Uglesic, L.; Breskovic, T.; Valic, Z.; Heusser, K.; Marinovic, J.; Ljubkovic, M.; Palada, I. Involuntary breathing movements improve cerebral oxygenation during apnea struggle phase in elite divers. J. Appl. Physiol. 2009, 107, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Steinback, C.D.; Salmanpour, A.; Breskovic, T.; Dujic, Z.; Shoemaker, J.K. Sympathetic neural activation: An ordered affair. J. Physiol. 2010, 588, 4825–4836. [Google Scholar] [CrossRef] [PubMed]
- Hoiland, R.L.; Bain, A.R.; Rieger, M.G.; Bailey, D.M.; Ainslie, P.N. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 310, R398–R413. [Google Scholar] [CrossRef] [PubMed]
- Willie, C.K.; Ainslie, P.N.; Drvis, I.; MacLeod, D.B.; Bain, A.R.; Madden, D.; Maslov, P.Z.; Dujic, Z. Regulation of brain blood flow and oxygen delivery in elite breath-hold divers. J. Cereb. Blood Flow. Metab. 2015, 35, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Hamaoka, T. Near-infrared spectroscopy: What can it tell us about oxygen saturation in skeletal muscle? Exerc. Sport Sci. Rev. 2000, 28, 123–127. [Google Scholar]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Schmitz, R.; Erlacher, D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: Reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 115001. [Google Scholar] [CrossRef] [PubMed]
- Muthalib, M.; Lee, H.; Millet, G.Y.; Ferrari, M.; Nosaka, K. Comparison between maximal lengthening and shortening contractions for biceps brachii muscle oxygenation and hemodynamics. J. Appl. Physiol. 2010, 109, 710–720. [Google Scholar] [CrossRef]
- Uljević, O.; Vrdoljak, D.; Drviš, I.; Foretić, N.; Dujić, Ž. Local Muscle Oxygenation Differences between Lower Limbs according to Muscle Mass in Breath-Hold Divers. Symmetry 2024, 16, 377. [Google Scholar] [CrossRef]
- McKnight, J.C.; Mulder, E.; Ruesch, A.; Kainerstorfer, J.M.; Wu, J.; Hakimi, N.; Balfour, S.; Bronkhorst, M.; Horschig, J.M.; Pernett, F.; et al. When the human brain goes diving: Using near-infrared spectroscopy to measure cerebral and systemic cardiovascular responses to deep, breath-hold diving in elite freedivers. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200349. [Google Scholar] [CrossRef] [PubMed]
- Palada, I.; Obad, A.; Bakovic, D.; Valic, Z.; Ivancev, V.; Dujic, Z. Cerebral and peripheral hemodynamics and oxygenation during maximal dry breath-holds. Respir. Physiol. Neurobiol. 2007, 157, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, D.; Foretić, N.; Drviš, I.; Ivančev, V.; Perić, M.; Dujić, Ž. Do freedivers and spearfishermen differ in local muscle oxygen saturation and anaerobic power? J. Sports Med. Phys. Fit. 2024, 64, 21–29. [Google Scholar] [CrossRef]
- Feldmann, A.M.; Erlacher, D.; Pfister, S.; Lehmann, R. Muscle oxygen dynamics in elite climbers during finger-hang tests at varying intensities. Sci. Rep. 2020, 10, 3040. [Google Scholar] [CrossRef] [PubMed]
- Luck, J.C.; Miller, A.J.; Aziz, F.; Radtka, J.F., 3rd; Proctor, D.N.; Leuenberger, U.A.; Sinoway, L.I.; Muller, M.D. Blood pressure and calf muscle oxygen extraction during plantar flexion exercise in peripheral artery disease. J. Appl. Physiol. 2017, 123, 2–10. [Google Scholar] [CrossRef]
- Rębiś, K.; Sadowska, D.; Starczewski, M.; Klusiewicz, A. Usefulness of Portable Device to Establish Differences in Muscle Oxygenation Between the Wingate Test and Graded Exercise Test: Effect of Gender on Anaerobic and Aerobic Capacity in Speed Skaters. Front. Physiol. 2022, 13, 809864. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur. J. Sport. Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef]
- Alvares, T.S.; Oliveira, G.V.d.; Soares, R.; Murias, J.M. Near-infrared spectroscopy-derived total haemoglobin as an indicator of changes in muscle blood flow during exercise-induced hyperaemia. J. Sports Sci. 2020, 38, 751–758. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Davis, M.J.; Secher, N.H.; van Lieshout, J.J.; Arce-Esquivel, A.A.; Simmons, G.H.; Bender, S.B.; Padilla, J.; Bache, R.J.; Merkus, D.; et al. Peripheral circulation. Compr. Physiol. 2012, 2, 321–447. [Google Scholar] [CrossRef]
- Molchanova, N.; Rybakov, V.; Kalinin, E.; Kamenshchikova, A. Features of gas exchange in advanced level freediver during monofin practice in pool. Theory Pract. Extrem. Sports (Aka Extrem. Hum. Act. GTSOLIFK) 2013. printed. [Google Scholar] [CrossRef]
- Kiviniemi, A.M.; Breskovic, T.; Uglesic, L.; Kuch, B.; Maslov, P.Z.; Sieber, A.; Seppänen, T.; Tulppo, M.P.; Dujic, Z. Heart rate variability during static and dynamic breath-hold dives in elite divers. Auton. Neurosci. 2012, 169, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Tocco, F.; Marongiu, E.; Pinna, M.; Roberto, S.; Pusceddu, M.; Angius, L.; Migliaccio, G.; Milia, R.; Concu, A.; Crisafulli, A. Assessment of circulatory adjustments during underwater apnoea in elite divers by means of a portable device. Acta Physiol. 2013, 207, 290–298. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).