The Roles of Glutathione and Oxidative Stress in Diabetes and COVID-19
Abstract
1. Introduction
The Potential Interconnections between Diabetes and COVID-19
2. The Epidemiology and Etiology of COVID-19 and Diabetes
2.1. Children and Young Adults Are as Susceptible to COVID-19 as Adults and the Elderly
2.2. The Worldwide Burden of Diabetes Is Rapidly Increasing along with the Threat of More Severe COVID-19 Outcomes
2.3. Oxidative Stress Is Increased in Both T1D and T2D
2.4. Skeletal Muscle Insulin Resistance Contributes to Postprandial Glycemia (PPG), Chronic OxS, and T2D Progression
2.5. COVID-19 Increases the Risk of Developing T2D in Patients with Prediabetes
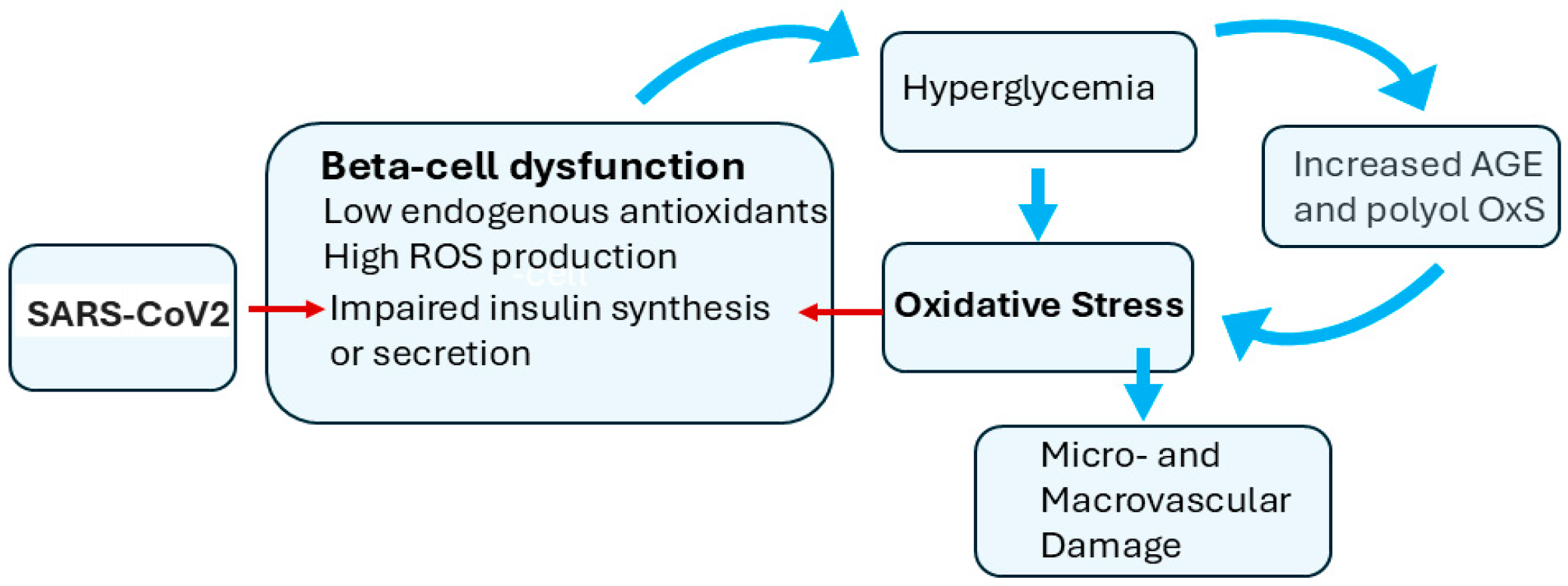
2.6. Beta-Cell Dysfunction Is a Component of T2D and COVID-19
3. COVID-19 Increases the Risk of Diabetes and Diabetes Increases COVID-19 Susceptibility
4. Glutathione Deficiency in Diabetes and COVID-19
5. Lifestyle Factors in COVID-19 and Diabetes
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stone, W.L.; Pham, T.; Mohiuddin, S.S. Biochemistry, Antioxidants; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Zhang, Z.; Huang, Q.; Zhao, D.; Lian, F.; Li, X.; Qi, W. The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications. Front. Endocrinol. 2023, 14, 1112363. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial Dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Domínguez, C.; Ruiz, E.; Gussinye, M.; Carrascosa, A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 1998, 21, 1736–1742. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef]
- Darmaun, D.; Smith, S.D.; Sweeten, S.; Hartman, B.K.; Welch, S.; Mauras, N. Poorly controlled type 1 diabetes is associated with altered glutathione homeostasis in adolescents: Apparent resistance to N-acetylcysteine supplementation. Pediatr. Diabetes 2008, 9, 577–582. [Google Scholar] [CrossRef]
- Darmaun, D.; Smith, S.D.; Sweeten, S.; Sager, B.K.; Welch, S.; Mauras, N. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes. Diabetes 2005, 54, 190–196. [Google Scholar] [CrossRef]
- Benson, M.; Hossain, J.; Darmaun, D. Improved glycemic control either alone, or combined with antioxidant supplementation, fails to restore blood glutathione or markers of oxidative stress in adolescents with poorly controlled type 1 diabetes. Nutr. Res. 2023, 117, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Osahon, O.; Vides, D.B.; Hanania, N.; Minard, C.G.; Sekhar, R.V. Severe Glutathione Deficiency, Oxidative Stress and Oxidant Damage in Adults Hospitalized with COVID-19: Implications for GlyNAC (glycine and N-acetylcysteine) supplementation. Antioxidants 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Glassman, I.; Le, N.; Mirhosseini, M.; Alcantara, C.A.; Asif, A.; Goulding, A.; Muneer, S.; Singh, M.; Robison, J.; Guilford, F.; et al. The Role of Glutathione in Prevention of COVID-19 Immunothrombosis: A Review. Front. Biosci. 2023, 28, 59. [Google Scholar] [CrossRef] [PubMed]
- Labarrere, C.A.; Kassab, G.S. Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front. Microbiol. 2022, 13, 979719. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. How to Increase Cellular Glutathione. Antioxidants 2023, 12, 1094. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Tuell, D.; Ford, G.; Los, E.; Stone, W. The Role of Glutathione and Its Precursors in Type 2 Diabetes. Antioxidants 2024, 13, 184. [Google Scholar] [CrossRef]
- Metwally, A.A.; Mehta, P.; Johnson, B.S.; Nagarjuna, A.; Snyder, M.P. COVID-19-Induced New-Onset Diabetes: Trends and Technologies. Diabetes 2021, 70, 2733–2744. [Google Scholar] [CrossRef]
- Magdy Beshbishy, A.; Oti, V.B.; Hussein, D.E.; Rehan, I.F.; Adeyemi, O.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Shah, M.A.; Abouelezz, K.; Hetta, H.F.; et al. Factors Behind the Higher COVID-19 Risk in Diabetes: A Critical Review. Front. Public. Health 2021, 9, 591982. [Google Scholar] [CrossRef]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef]
- Yan-Do, R.; MacDonald, P.E. Impaired “Glycine”-mia in Type 2 Diabetes and Potential Mechanisms Contributing to Glucose Homeostasis. Endocrinology 2017, 158, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin resistance and glycine metabolism in humans. Amino Acids 2018, 50, 11–27. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018, 18, 81–87. [Google Scholar]
- Sekhar, R.V. GlyNAC (Glycine and N-Acetylcysteine) Supplementation Improves Impaired Mitochondrial Fuel Oxidation and Lowers Insulin Resistance in Patients with Type 2 Diabetes: Results of a Pilot Study. Antioxidants 2022, 11, 154. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R.V. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J. Gerontol. Ser. A 2023, 78, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Qeadan, F.; Tingey, B.; Egbert, J.; Pezzolesi, M.G.; Burge, M.R.; Peterson, K.A.; Honda, T. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: A nationwide cohort from the US using the Cerner Real-World Data. PLoS ONE 2022, 17, e0266809. [Google Scholar] [CrossRef]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef]
- D’Souza, D.; Empringham, J.; Pechlivanoglou, P.; Uleryk, E.M.; Cohen, E.; Shulman, R. Incidence of Diabetes in Children and Adolescents During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2321281. [Google Scholar] [CrossRef]
- Ssentongo, P.; Zhang, Y.; Witmer, L.; Chinchilli, V.M.; Ba, D.M. Association of COVID-19 with diabetes: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 20191. [Google Scholar] [CrossRef]
- Kwan, A.C.; Ebinger, J.E.; Botting, P.; Navarrette, J.; Claggett, B.; Cheng, S. Association of COVID-19 Vaccination With Risk for Incident Diabetes After COVID-19 Infection. JAMA Netw. Open 2023, 6, e2255965. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Lam, E.; Bramante, C.T.; Johnson, S.G.; Reusch, J.; Wilkins, K.J.; Yeh, H.C. Does COVID-19 Infection Increase the Risk of Diabetes? Current Evidence. Curr. Diab Rep. 2023, 23, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sharbatdar, Y.; Mousavian, R.; Noorbakhsh Varnosfaderani, S.M.; Aziziyan, F.; Liaghat, M.; Baziyar, P.; Yousefi Rad, A.; Tavakol, C.; Moeini, A.M.; Nabi-Afjadi, M.; et al. Diabetes as one of the long-term COVID-19 complications: From the potential reason of more diabetic patients’ susceptibility to COVID-19 to the possible caution of future global diabetes tsunami. Inflammopharmacology 2023, 31, 1029–1052. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Shao, M.; Guo, Q.; Shi, J.; Zhao, Y.; Xiaokereti, J.; Tang, B. Diabetes Mellitus is Associated with Severe Infection and Mortality in Patients with COVID-19: A Systematic Review and Meta-analysis. Arch. Med. Res. 2020, 51, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Gęca, T.; Wojtowicz, K.; Guzik, P.; Góra, T. Increased Risk of COVID-19 in Patients with Diabetes Mellitus-Current Challenges in Pathophysiology, Treatment and Prevention. Int. J. Environ. Res. Public. Health 2022, 19, 6555. [Google Scholar] [CrossRef]
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, e3000003. [Google Scholar] [CrossRef]
- Mahilkar, S.; Agrawal, S.; Chaudhary, S.; Parikh, S.; Sonkar, S.C.; Verma, D.K.; Chitalia, V.; Mehta, D.; Koner, B.C.; Vijay, N.; et al. SARS-CoV-2 variants: Impact on biological and clinical outcome. Front. Med. 2022, 9, 995960. [Google Scholar] [CrossRef]
- Fotea, S.; Ghiciuc, C.M.; Stefanescu, G.; Cianga, A.L.; Mihai, C.M.; Lupu, A.; Butnariu, L.I.; Starcea, I.M.; Salaru, D.L.; Mocanu, A.; et al. Pediatric COVID-19 and Diabetes: An Investigation into the Intersection of Two Pandemics. Diagnostics 2023, 13, 2436. [Google Scholar] [CrossRef]
- Singer, M.E.; Dorrance, K.A.; Oxenreiter, M.M.; Yan, K.R.; Close, K.L. The type 2 diabetes ‘modern preventable pandemic’ and replicable lessons from the COVID-19 crisis. Prev. Med. Rep. 2022, 25, 101636. [Google Scholar] [CrossRef]
- Chandrashekhar Joshi, S.; Pozzilli, P. COVID-19 induced Diabetes: A novel presentation. Diabetes Res. Clin. Pract. 2022, 191, 110034. [Google Scholar] [CrossRef]
- Paul, A.K.; Hossain, M.K.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; de Lourdes Pereira, M.; et al. Does Oxidative Stress Management Help Alleviation of COVID-19 Symptoms in Patients Experiencing Diabetes? Nutrients 2022, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- StatPearls. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551501/ (accessed on 14 July 2024).
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Ashmore, P.; Sherwood, E. An overview of COVID-19 global epidemiology and discussion of potential drivers of variable global pandemic impacts. J. Antimicrob. Chemother. 2023, 78, ii2–ii11. [Google Scholar] [CrossRef] [PubMed]
- Statista. Coronavirus (COVID-19) in the U.S.—Statistics & Facts. Available online: https://www.statista.com/topics/6084/coronavirus-covid-19-in-the-us/#topicOverview (accessed on 28 February 2023).
- Ma, Q.; Liu, J.; Liu, Q.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections Among the Tested Population and Individuals With Confirmed COVID-19 Diagnosis: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Ricks, K.M.; Kuehnert, P.A.; Eick-Cost, A.A.; Scheckelhoff, M.R.; Wiesen, A.R.; Clements, T.L.; Hu, Z.; Zak, S.E.; Olschner, S.P.; et al. Seroprevalence as an Indicator of Undercounting of COVID-19 Cases in a Large Well-Described Cohort. AJPM Focus. 2023, 2, 100141. [Google Scholar] [CrossRef]
- Justman, J.; Skalland, T.; Moore, A.; Amos, C.I.; Marzinke, M.A.; Zangeneh, S.Z.; Kelley, C.F.; Singer, R.; Mayer, S.; Hirsch-Moverman, Y.; et al. Prevalence of SARS-CoV-2 Infection among Children and Adults in 15 US Communities, 2021. Emerg. Infect. Dis. 2024, 30, 245–254. [Google Scholar] [CrossRef]
- Wimmers, F.; Burrell, A.R.; Feng, Y.; Zheng, H.; Arunachalam, P.S.; Hu, M.; Spranger, S.; Nyhoff, L.E.; Joshi, D.; Trisal, M.; et al. Multi-omics analysis of mucosal and systemic immunity to SARS-CoV-2 after birth. Cell 2023, 186, 4632–4651.e23. [Google Scholar] [CrossRef]
- Flaxman, S.; Whittaker, C.; Semenova, E.; Rashid, T.; Parks, R.M.; Blenkinsop, A.; Unwin, H.J.T.; Mishra, S.; Bhatt, S.; Gurdasani, D.; et al. Assessment of COVID-19 as the Underlying Cause of Death Among Children and Young People Aged 0 to 19 Years in the US. JAMA Netw. Open 2023, 6, e2253590. [Google Scholar] [CrossRef]
- Harris, E. Millions of US Children Experience Range of Long COVID Effects. JAMA 2024, 331, 726. [Google Scholar] [CrossRef]
- NIH. Available online: https://www.niddk.nih.gov/health-information/professionals/diabetes-discoveries-practice/research-on-covid-and-diabetes#:~:text=A%20study%20of%20over%20181%2C000,with%20those%20who%20didn’t (accessed on 1 April 2024).
- Li, R.; Shen, M.; Yang, Q.; Fairley, C.K.; Chai, Z.; McIntyre, R.; Ong, J.J.; Liu, H.; Lu, P.; Hu, W.; et al. Global Diabetes Prevalence in COVID-19 Patients and Contribution to COVID-19- Related Severity and Mortality: A Systematic Review and Meta-analysis. Diabetes Care 2023, 46, 890–897. [Google Scholar] [CrossRef]
- Atwah, B.; Iqbal, M.S.; Kabrah, S.; Kabrah, A.; Alghamdi, S.; Tabassum, A.; Baghdadi, M.A.; Alzahrani, H. Susceptibility of Diabetic Patients to COVID-19 Infections: Clinico-Hematological and Complications Analysis. Vaccines 2023, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- IDF. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 14 February 2024).
- An, Y.; Xu, B.T.; Wan, S.R.; Ma, X.M.; Long, Y.; Xu, Y.; Jiang, Z.Z. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef]
- Brooks-Worrell, B.; Palmer, J.P. Is diabetes mellitus a continuous spectrum? Clin. Chem. 2011, 57, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Jones, A.G. The challenges of identifying and studying type 1 diabetes in adults. Diabetologia 2023, 66, 2200–2212. [Google Scholar] [CrossRef]
- Perng, W.; Conway, R.; Mayer-Davis, E.; Dabelea, D. Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care 2023, 46, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Toren, E.; Burnette, K.S.; Banerjee, R.R.; Hunter, C.S.; Tse, H.M. Partners in Crime: Beta-Cells and Autoimmune Responses Complicit in Type 1 Diabetes Pathogenesis. Front. Immunol. 2021, 12, 756548. [Google Scholar] [CrossRef] [PubMed]
- Alu, S.N.; Los, E.A.; Ford, G.A.; Stone, W.L. Oxidative Stress in Type 2 Diabetes: The Case for Future Pediatric Redoxomics Studies. Antioxidants 2022, 11, 1336. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Jubran, A.S.; Almulla, A.F.; Moustafa, S.R.; Maes, M. Increased insulin resistance due to Long COVID is associated with depressive symptoms and partly predicted by the inflammatory response during acute infection. Braz. J. Psychiatry 2023, 45, 205–215. [Google Scholar] [CrossRef]
- Tuell, D.S.; Los, E.A.; Ford, G.A.; Stone, W.L. The Role of Natural Antioxidant Products That Optimize Redox Status in the Prevention and Management of Type 2 Diabetes. Antioxidants 2023, 12, 1139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Dubsky, M.; Veleba, J.; Sojakova, D.; Marhefkova, N.; Fejfarova, V.; Jude, E.B. Endothelial Dysfunction in Diabetes Mellitus: New Insights. Int. J. Mol. Sci. 2023, 24, 10705. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.Y.; Wang, S.H.; Duong, T.Q. Patients with prediabetes are at greater risk of developing diabetes 5 months postacute SARS-CoV-2 infection: A retrospective cohort study. BMJ Open Diabetes Res. Care 2023, 11, e003257. [Google Scholar] [CrossRef]
- Ng, H.Y.; Chan, L.T.W. Prediabetes in children and adolescents: An updated review. World J. Clin. Pediatr. 2023, 12, 263–272. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Saisho, Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J. Diabetes 2015, 6, 109–124. [Google Scholar] [CrossRef]
- White, M.G.; Shaw, J.A.; Taylor, R. Type 2 Diabetes: The Pathologic Basis of Reversible β-Cell Dysfunction. Diabetes Care 2016, 39, 2080–2088. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E.J. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for β Cell Recovery. Cell Metab. 2018, 28, 667. [Google Scholar] [CrossRef]
- Khin, P.P.; Lee, J.H.; Jun, H.-S. Pancreatic Beta-cell Dysfunction in Type 2 Diabetes. Eur. J. Inflamm. 2023, 21, 1721727X231154152. [Google Scholar] [CrossRef]
- Leenders, F.; Groen, N.; de Graaf, N.; Engelse, M.A.; Rabelink, T.J.; de Koning, E.J.P.; Carlotti, F. Oxidative Stress Leads to β-Cell Dysfunction Through Loss of β-Cell Identity. Front. Immunol. 2021, 12, 690379. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010, 460, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef]
- Gojda, J.; Koudelková, K.; Ouřadová, A.; Lang, A.; Krbcová, M.; Gvozdeva, A.; Šebo, V.; Slagmolen, L.; Potočková, J.; Tůma, P.; et al. Severe COVID-19 associated hyperglycemia is caused by beta cell dysfunction: A prospective cohort study. Nutr. Diabetes 2023, 13, 11. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, Q.; Zhang, Z.; Walline, J.H.; Liu, Y.; Zhu, H.; Zhang, S. Risk for newly diagnosed diabetes after COVID-19: A systematic review and meta-analysis. BMC Med. 2022, 20, 444. [Google Scholar] [CrossRef]
- Lugar, M.; Eugster, A.; Achenbach, P.; von dem Berge, T.; Berner, R.; Besser, R.E.J.; Casteels, K.; Elding Larsson, H.; Gemulla, G.; Kordonouri, O.; et al. SARS-CoV-2 Infection and Development of Islet Autoimmunity in Early Childhood. JAMA 2023, 330, 1151–1160. [Google Scholar] [CrossRef]
- DeForest, N.; Majithia, A.R. Genetics of Type 2 Diabetes: Implications from Large-Scale Studies. Curr. Diab. Rep. 2022, 22, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Minniakhmetov, I.; Yalaev, B.; Khusainova, R.; Bondarenko, E.; Melnichenko, G.; Dedov, I.; Mokrysheva, N. Genetic and Epigenetic Aspects of Type 1 Diabetes Mellitus: Modern View on the Problem. Biomedicines 2024, 12, 399. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Wang, G.; Zhai, J.; Du, B. COVID-19 as a Trigger for Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R.; Kalita, P.; Pathak, M.P. Diabetes mellitus and COVID-19: Understanding the association in light of current evidence. World J. Clin. Cases 2021, 9, 8327–8339. [Google Scholar] [CrossRef] [PubMed]
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef]
- Tan, K.S.; Lee, K.O.; Low, K.C.; Gamage, A.M.; Liu, Y.; Tan, G.Y.; Koh, H.Q.; Alonso, S.; Gan, Y.H. Glutathione deficiency in type 2 diabetes impairs cytokine responses and control of intracellular bacteria. J. Clin. Investig. 2012, 122, 2289–2300. [Google Scholar] [CrossRef]
- Kalamkar, S.; Acharya, J.; Kolappurath Madathil, A.; Gajjar, V.; Divate, U.; Karandikar-Iyer, S.; Goel, P.; Ghaskadbi, S. Randomized Clinical Trial of How Long-Term Glutathione Supplementation Offers Protection from Oxidative Damage and Improves HbA1c in Elderly Type 2 Diabetic Patients. Antioxidants 2022, 11, 1026. [Google Scholar] [CrossRef]
- Neves, F.F.; Pott-Junior, H.; Yamashita, K.M.C.; de Sousa Santos, S.; Cominetti, M.R.; de Melo Freire, C.C.; Cunha, A.F.D.; Jordão Júnior, A.A. Do the oxidative stress biomarkers predict COVID-19 outcome? An in-hospital cohort study. Free Radic. Biol. Med. 2023, 207, 194–199. [Google Scholar] [CrossRef]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005, 94, 29–86. [Google Scholar] [CrossRef]
- Gamarra-Morales, Y.; Herrera-Quintana, L.; Molina-López, J.; Vázquez-Lorente, H.; Machado-Casas, J.F.; Castaño-Pérez, J.; Pérez-Villares, J.M.; Planells, E. Response to Intravenous N-Acetylcysteine Supplementation in Critically Ill Patients with COVID-19. Nutrients 2023, 15, 2235. [Google Scholar] [CrossRef]
- Lange, K.W. Food science and COVID-19. Food Sci. Hum. Wellness 2021, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Nakamura, Y. Lifestyle factors in the prevention of COVID-19. Glob. Health J. 2020, 4, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, W.; Tsegaye, M.; Gizaw, A.; Adera, T. Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: Systematic review and meta-analysis. BMJ Nutr. Prev. Health 2022, 5, 10–18. [Google Scholar] [CrossRef]
- Aminian, A.; Bena, J.; Pantalone, K.M.; Burguera, B. Association of obesity with postacute sequelae of COVID-19. Diabetes Obes. Metab. 2021, 23, 2183–2188. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=Facts%20about%20overweight%20and%20obesity&text=About%2016%25%20of%20adults%20aged,of%205%20years%20were%20overweight (accessed on 13 March 2024).
- Zhao, L.; Freedman, D.S.; Blanck, H.M.; Park, S. Trends in Severe Obesity Among Children Aged 2 to 4 Years in WIC: 2010 to 2020. Pediatrics 2024, 153, e2023062461. [Google Scholar] [CrossRef]
- Fauci, G.; Montalti, M.; Di Valerio, Z.; Gori, D.; Salomoni, M.G.; Salussolia, A.; Soldà, G.; Guaraldi, F. Obesity and COVID-19 in Children and Adolescents: Reciprocal Detrimental Influence-Systematic Literature Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7603. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Landolfi, F.; Ciani, F.; Iorio, E.L. Redoxomics and Oxidative Stress: From the Basic Research to the Clinical Practice. In Free Radicals and Diseases; Ahmad, R., Ed.; Intech: London, UK, 2016. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Los, E.; Ford, G.; Tuell, D.; Macariola, D., Jr.; Stone, W. The Roles of Glutathione and Oxidative Stress in Diabetes and COVID-19. Oxygen 2024, 4, 351-362. https://doi.org/10.3390/oxygen4030021
Los E, Ford G, Tuell D, Macariola D Jr., Stone W. The Roles of Glutathione and Oxidative Stress in Diabetes and COVID-19. Oxygen. 2024; 4(3):351-362. https://doi.org/10.3390/oxygen4030021
Chicago/Turabian StyleLos, Evan, George Ford, Dawn Tuell, Demetrio Macariola, Jr., and William Stone. 2024. "The Roles of Glutathione and Oxidative Stress in Diabetes and COVID-19" Oxygen 4, no. 3: 351-362. https://doi.org/10.3390/oxygen4030021
APA StyleLos, E., Ford, G., Tuell, D., Macariola, D., Jr., & Stone, W. (2024). The Roles of Glutathione and Oxidative Stress in Diabetes and COVID-19. Oxygen, 4(3), 351-362. https://doi.org/10.3390/oxygen4030021






