Recent Advances in Mupirocin Delivery Strategies for the Treatment of Bacterial Skin and Soft Tissue Infection
Abstract
:1. Introduction
2. Mupirocin: A Drug of Choice for the Treatment of Skin Infection
2.1. Clinical Application and Mechanism of Action
2.2. Drug Resistance: More Than a Challenge
3. Novel Strategies to Augment Mupirocin Delivery in Bacterial Skin Infection
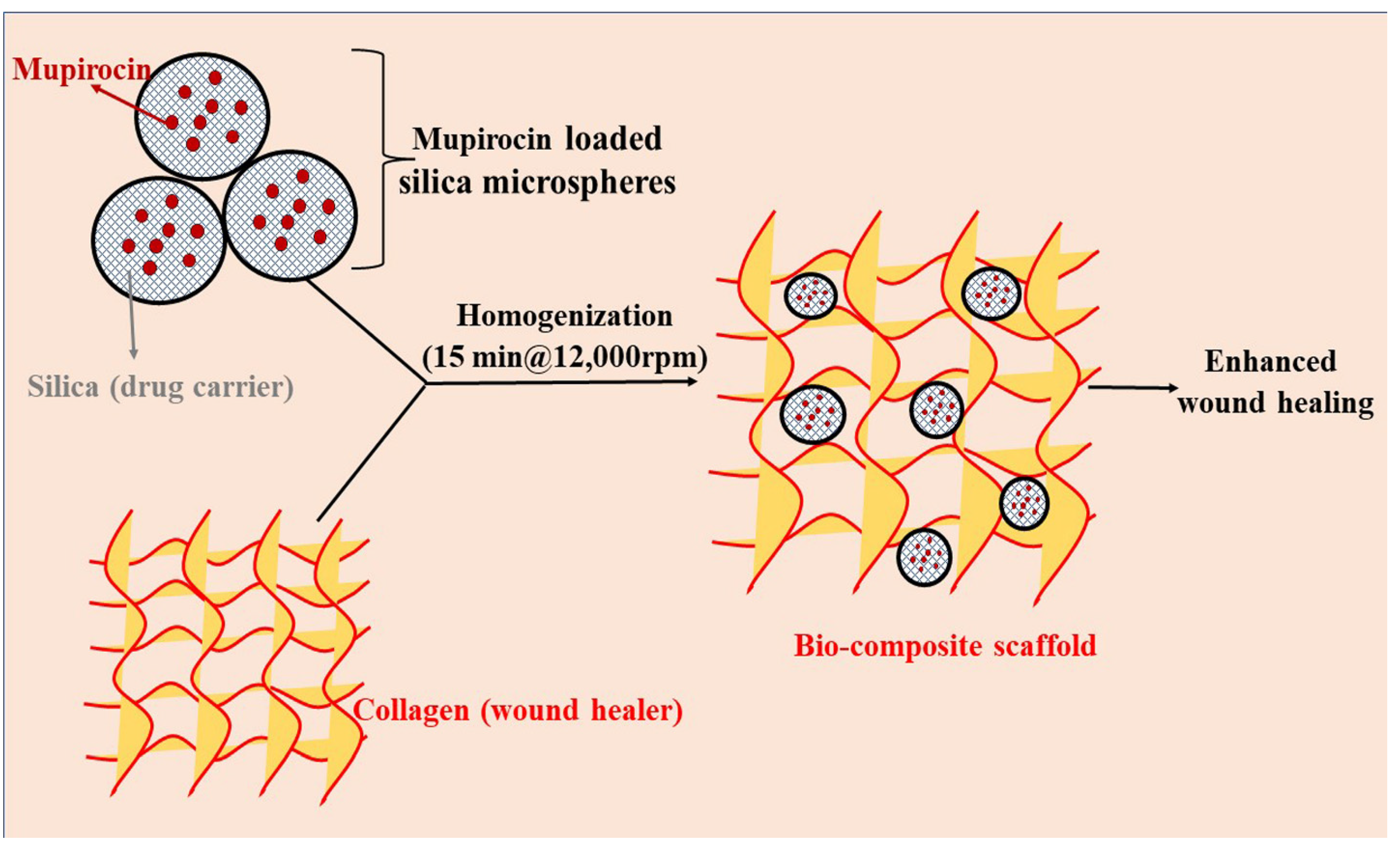
3.1. Composite Materials/Scaffolds
3.2. Hydrogel Membrane
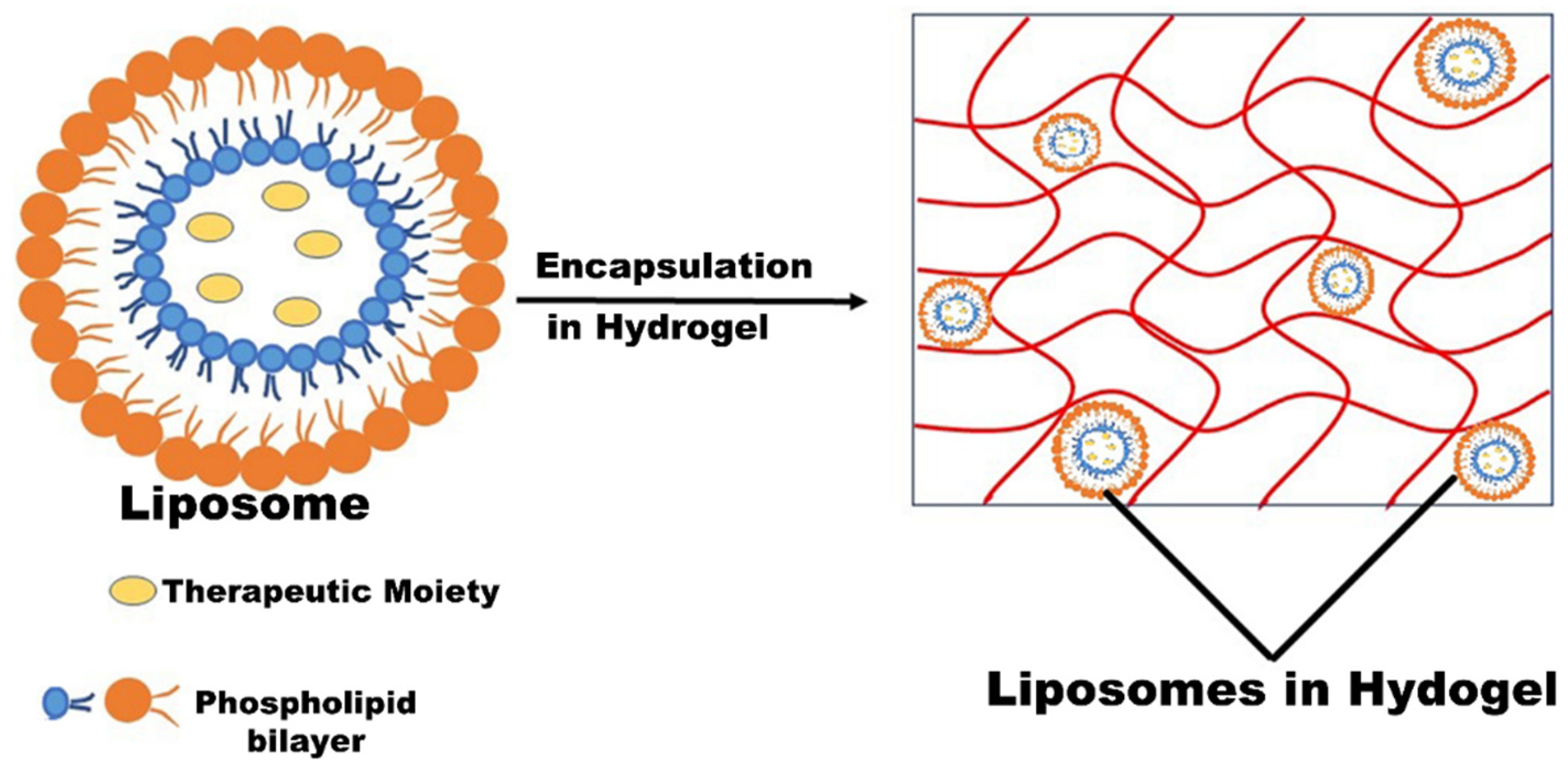
3.3. Liposomes and Liposomal Hydrogel
3.4. Microcapsule
3.5. Microsponges
3.6. Nanoparticles/Naocapsules
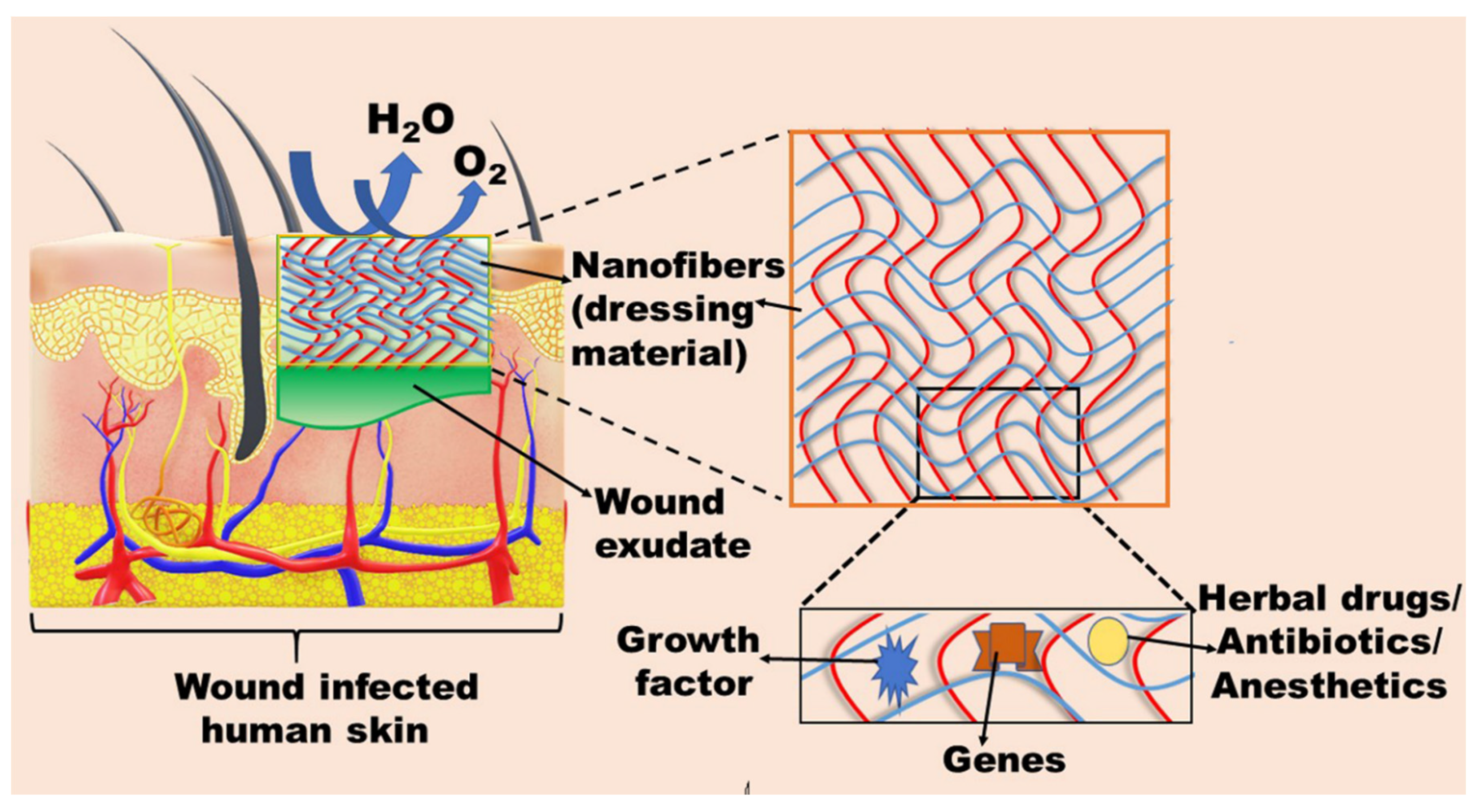
3.7. Nanofibers
3.8. Miscellaneous
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, F.; Khan, T.; Pujalte, G.G. Bacterial Skin Infections. Prim. Care 2015, 42, 485–499. [Google Scholar] [CrossRef]
- Kaye, K.S.; Petty, L.A.; Shorr, A.F.; Zilberberg, M.D. Current Epidemiology, Etiology, and Burden of Acute Skin Infections in the United States. Clin. Infect. Dis. 2019, 68, S193–S199. [Google Scholar] [CrossRef] [Green Version]
- Kollipara, R.; Downing, C.; Lee, M.; Guidry, J.; Curtis, S.; Tyring, S. Current and emerging drugs for acute bacterial skin and skin structure infections: An update. Expert Opin. Emerg. Drugs 2014, 19, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.D. Skin and Soft Tissue Infections in Ambulatory Care Settings: Setting a New Trend. J. Clin. Infect. Dis. 2019, 70, 2719–2720. [Google Scholar] [CrossRef] [PubMed]
- Edelsberg, J.; Taneja, C.; Zervos, M.; Haque, N.; Moore, C.; Reyes, K.; Spalding, J.; Jiang, J.; Oster, G. Trends in US hospital admissions for skin and soft tissue infections. Emerg. Infect. Dis. 2009, 15, 1516–1518. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Ascione, T.; Pagliano, P. Management of bacterial skin and skin structure infections with polymicrobial etiology. Expert Rev. Anti-Infect. Ther. 2019, 17, 17–25. [Google Scholar] [CrossRef]
- Sukumaran, V.; Senanayake, S. Bacterial skin and soft tissue infections. Aust. Prescr. 2016, 39, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Noviello, S.; Leone, S. Epidemiology and microbiology of skin and soft tissue infections. Curr. Opin. Infect. Dis. 2016, 29, 109–115. [Google Scholar] [CrossRef]
- Breyre, A.; Frazee, B.W. Skin and Soft Tissue Infections in the Emergency Department. Emerg. Med. Clin. N. Am. 2018, 36, 723–750. [Google Scholar] [CrossRef]
- Russo, A.; Concia, E.; Cristini, F.; De Rosa, F.G.; Esposito, S.; Menichetti, F.; Petrosillo, N.; Tumbarello, M.; Venditti, M.; Viale, P.; et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin. Microbiol. Infect. 2016, 22 (Suppl. 2), S27–S36. [Google Scholar] [CrossRef] [Green Version]
- Lipsky, B.A.; Silverman, M.H.; Joseph, W.S. A Proposed New Classification of Skin and Soft Tissue Infections Modeled on the Subset of Diabetic Foot Infection. Open Forum. Infect. Dis. 2017, 4, ofw255. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Ni Riain, U. Recommended management of common bacterial skin infections. Prescriber 2011, 22, 14–24. [Google Scholar] [CrossRef]
- Clebak, K.T.; Malone, M.A. Skin Infections. Prim. Care 2018, 45, 433–454. [Google Scholar] [CrossRef]
- Bergstein, J.M.; Baker, E.J.t.; Aprahamian, C.; Schein, M.; Wittmann, D.H. Soft tissue abscesses associated with parenteral drug abuse: Presentation, microbiology, and treatment. Am. Surg. 1995, 61, 1105–1108. [Google Scholar]
- Talan, D.A.; Summanen, P.H.; Finegold, S.M. Ampicillin/sulbactam and cefoxitin in the treatment of cutaneous and other soft-tissue abscesses in patients with or without histories of injection drug abuse. Clin. Infect. Dis. 2000, 31, 464–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. Infection in Burns. Surg. Infect. 2016, 17, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkiyaraj, D.; Sritharadol, R.; Padmavathi, A.R.; Nakpheng, T.; Srichana, T. Anti-biofilm properties of a mupirocin spray formulation against Escherichia coli wound infections. Biofouling 2017, 33, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Fung, H.B.; Chang, J.Y.; Kuczynski, S. A practical guide to the treatment of complicated skin and soft tissue infections. Drugs 2003, 63, 1459–1480. [Google Scholar] [CrossRef]
- Macdonald, K.E.; Jordan, C.Y.; Crichton, E.; Barnes, J.E.; Harkin, G.E.; Hall, L.M.L.; Jones, J.D. A retrospective analysis of the microbiology of diabetic foot infections at a Scottish tertiary hospital. BMC Infect. Dis. 2020, 20, 218. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, E.J.; Citron, D.M.; Merriam, C.V.; Tyrrell, K.; Warren, Y. Activity of gatifloxacin compared to those of five other quinolones versus aerobic and anaerobic isolates from skin and soft tissue samples of human and animal bite wound infections. Antimicrob. Agents Chemother. 1999, 43, 1475–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, L.O. Review of quinolones in the treatment of infections of the skin and skin structure. J. Antimicrob. Chemother. 1991, 28 (Suppl. C), 97–110. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucaliuc, A.; Blaga, A.C.; Galaction, A.I.; Cascaval, D. Mupirocin: Applications and production. Biotechnol. Lett. 2019, 41, 495–502. [Google Scholar] [CrossRef]
- Rakshit, T.; Shenoy, M.S. How resistant is Staphylococcus aureus to the topical antibiotic mupirocin? J. Glob. Antimicrob. Resist. 2017, 8, 102–103. [Google Scholar] [CrossRef]
- Ha, K.R.; Psaltis, A.J.; Butcher, A.R.; Wormald, P.-J.; Tan, L.W. In Vitro Activity of Mupirocin on Clinical Isolates of Staphylococcus aureus and its Potential Implications in Chronic Rhinosinusitis. Laryngoscope 2008, 118, 535–540. [Google Scholar] [CrossRef]
- Cern, A.; Connolly, K.L.; Jerse, A.E.; Barenholz, Y. In Vitro Susceptibility of Neisseria gonorrhoeae Strains to Mupirocin, an Antibiotic Reformulated for Parenteral Administration in Nanoliposomes. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, J.A.; Gelone, S.P.; Safdar, A. 37-Topical Antibacterials. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 452–462.e452. [Google Scholar]
- Breneman, D.L. Use of mupirocin ointment in the treatment of secondarily infected dermatoses. J. Am. Acad. Dermatol. 1990, 22, 886–892. [Google Scholar] [CrossRef]
- David, S.R.; Malek, N.; Mahadi, A.H.; Chakravarthi, S.; Rajabalaya, R. Development of controlled release silicone adhesive-based mupirocin patch demonstrates antibacterial activity on live rat skin against Staphylococcus aureus. Drug. Des. Devel. Ther. 2018, 12, 481–494. [Google Scholar] [CrossRef] [Green Version]
- Tacconelli, E.; Foschi, F.; Forstner, C.; Finch, R.G.; Pletz, M.W. 136-Principles of Anti-infective Therapy and Surgical Prophylaxis. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1145–1161.e1142. [Google Scholar]
- Khoshnood, S.; Heidary, M.; Asadi, A.; Soleimani, S.; Motahar, M.; Savari, M.; Saki, M.; Abdi, M. A review on mechanism of action, resistance, synergism, and clinical implications of mupirocin against Staphylococcus aureus. Biomed. Pharmacother. 2019, 109, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Gurney, R.; Thomas, C.M. Mupirocin: Biosynthesis, special features and applications of an antibiotic from a gram-negative bacterium. Appl. Microbiol. Biotechnol. 2011, 90, 11–21. [Google Scholar] [CrossRef]
- Greywal, T.; Cohen, P.R. Erythrasma: A report of nine men successfully managed with mupirocin 2% ointment monotherapy. Dermatol. Online J. 2017, 23. [Google Scholar] [CrossRef]
- Bari, O.; Cohen, P.R. Successful Management of Zoon’s Balanitis with Topical Mupirocin Ointment: A Case Report and Literature Review of Mupirocin-Responsive Balanitis Circumscripta Plasmacelluaris. Dermatol. Ther. (Heidelb.) 2017, 7, 203–210. [Google Scholar] [CrossRef] [Green Version]
- van Bambeke, F.; Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. 137-Mechanisms of Action. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1162–1180.e1161. [Google Scholar]
- Thomas, C.M.; Hothersall, J.; Willis, C.L.; Simpson, T.J. Resistance to and synthesis of the antibiotic mupirocin. Nat. Rev. Microbiol. 2010, 8, 281–289. [Google Scholar] [CrossRef]
- Poovelikunnel, T.; Gethin, G.; Humphreys, H. Mupirocin resistance: Clinical implications and potential alternatives for the eradication of MRSA. J. Antimicrob. Chemother. 2015, 70, 2681–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadass, S.K.; Perumal, S.; Gopinath, A.; Nisal, A.; Subramanian, S.; Madhan, B. Sol-gel assisted fabrication of collagen hydrolysate composite scaffold: A novel therapeutic alternative to the traditional collagen scaffold. ACS Appl. Mater. Interfaces 2014, 6, 15015–15025. [Google Scholar] [CrossRef]
- Perumal, S.; Ramadass, S.; Madhan, B. Sol-gel processed mupirocin silica microspheres loaded collagen scaffold: A synergistic bio-composite for wound healing. Eur. J. Pharm. Sci. 2014, 52, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ Okur, N.; Hökenek, N.; Okur, M.E.; Ayla, Ş.; Yoltaş, A.; Siafaka, P.I.; Cevher, E. An alternative approach to wound healing field; new composite films from natural polymers for mupirocin dermal delivery. Saudi Pharm. J. 2019, 27, 738–752. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Abdullah, O.; Badshah, S.F. Preparation and Evaluation of Skin Wound Healing Chitosan-Based Hydrogel Membranes. AAPS PharmSciTech 2018, 19, 3199–3209. [Google Scholar] [CrossRef]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Khalid, Q.; Abdullah, O. Synthesis and evaluation of topical hydrogel membranes; a novel approach to treat skin disorders. J. Mater. Sci. Mater. Med. 2018, 29, 191. [Google Scholar] [CrossRef]
- Ahmad, S.; Usman Minhas, M.; Ahmad, M.; Sohail, M.; Abdullah, O.; Khan, K.U. Topical hydrogel patches of vinyl monomers containing mupirocin for skin injuries: Synthesis and evaluation. Adv. Polym. Technol. 2018, 37, 3401–3411. [Google Scholar] [CrossRef]
- Cern, A.; Nativ-Roth, E.; Goldblum, A.; Barenholz, Y. Effect of solubilizing agents on mupirocin loading into and release from PEGylated nanoliposomes. J. Pharm. Sci. 2014, 103, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Cern, A.; Michael-Gayego, A.; Bavli, Y.; Koren, E.; Goldblum, A.; Moses, A.E.; Xiong, Y.Q.; Barenholz, Y. Nano-mupirocin: Enabling the parenteral activity of mupirocin. J. Eur. J. Nanomed. 2016, 8, 139. [Google Scholar] [CrossRef]
- Goldmann, O.; Cern, A.; Müsken, M.; Rohde, M.; Weiss, W.; Barenholz, Y.; Medina, E. Liposomal mupirocin holds promise for systemic treatment of invasive Staphylococcus aureus infections. J. Control. Release 2019, 316, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Berg, O.A.; Skar, M.; Conradi, A.H.; Johnsen, P.J.; Skalko-Basnet, N. Improved burns therapy: Liposomes-in-hydrogel delivery system for mupirocin. J. Pharm. Sci. 2012, 101, 3906–3915. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Sørensen, K.K.; Fallarero, A.; Vuorela, P.; Škalko-Basnet, N. Liposomes-in-Hydrogel Delivery System with Mupirocin:In Vitro Antibiofilm Studies and In Vivo Evaluation in Mice Burn Model. BioMed. Res. Int. 2013, 2013, 498485. [Google Scholar] [CrossRef] [Green Version]
- Dürrigl, M.; Kwokal, A.; Hafner, A.; Segvić Klarić, M.; Dumičić, A.; Cetina-Čižmek, B.; Filipović-Grčić, J. Spray dried microparticles for controlled delivery of mupirocin calcium: Process-tailored modulation of drug release. J. Microencapsul. 2011, 28, 108–121. [Google Scholar] [CrossRef]
- Dürrigl, M.; Kregar, M.L.; Hafner, A.; Klarić, M.; Filipović-Grčić, J. Mupirocin calcium microencapsulation via spray drying: Feed solvent influence on microparticle properties, stability and antimicrobial activity. Drug Dev. Ind. Pharm. 2011, 37, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Amrutiya, N.; Bajaj, A.; Madan, M. Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech 2009, 10, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Singaravelu, S.; Ramanathan, G.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Kaveri, K.; Sivagnanam, U.T. Biomimetic interconnected porous keratin-fibrin-gelatin 3D sponge for tissue engineering application. Int. J. Biol. Macromol. 2016, 86, 810–819. [Google Scholar] [CrossRef]
- Rubenick, J.B.; Rubim, A.M.; Bellé, F.; Nogueira-Librelotto, D.R.; Rolim, C.M.B. Preparation of mupirocin-loaded polymeric nanocapsules using essential oil of rosemary. J Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef] [Green Version]
- Golmohammadi, R.; Najar-Peerayeh, S.; Tohidi Moghadam, T.; Hosseini, S.M.J. Synergistic Antibacterial Activity and Wound Healing Properties of Selenium-Chitosan-Mupirocin Nanohybrid System: An in Vivo Study on Rat Diabetic Staphylococcus aureus Wound Infection Model. Sci. Rep. 2020, 10, 2854. [Google Scholar] [CrossRef] [Green Version]
- Kamlungmak, S.; Rugmai, S.; Tinpun, K.; Nakpheng, T.; Srichana, T. Phase behavior, in vitro drug release, and antibacterial activity of thermoresponsive poloxamer–polyvinyl alcohol hydrogel-loaded mupirocin nanoparticles. Appl. Polym. 2020, 137, 49325. [Google Scholar] [CrossRef]
- Kamlungmak, S.; Nakpheng, T.; Kaewpaiboon, S.; Mudhar Bintang, M.A.K.; Prom-in, S.; Chunhachaichana, C.; Suwandecha, T.; Srichana, T. Safety and Biocompatibility of Mupirocin Nanoparticle-Loaded Hydrogel on Burn Wound in Rat Model. Biol. Pharm. Bull. 2021, 44, 1707–1716. [Google Scholar] [CrossRef]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, R.; Wang, X.; Li, X.; Peng, F.; Jin, Z.; Gao, X.; Yu, J.; Wang, C. Electrospun mupirocin loaded polyurethane fiber mats for anti-infection burn wound dressing application. J. Biomater. Sci. Polym. Ed. 2017, 28, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Yang, S.; Liu, P.; Zhang, B. Electrospun PCL/mupirocin and chitosan/lidocaine hydrochloride multifunctional double layer nanofibrous scaffolds for wound dressing applications. Int. J. Nanomed. 2018, 13, 5287–5299. [Google Scholar] [CrossRef] [Green Version]
- Amajuoyi, J.N.; Ilomuanya, M.O.; Asantewaa-Osei, Y.; Azubuike, C.P.; Adeosun, S.O.; Igwilo, C.I. Development of electrospun keratin/coenzyme Q10/poly vinyl alcohol nanofibrous scaffold containing mupirocin as potential dressing for infected wounds. Future J. Pharm. Sci. 2020, 6, 25. [Google Scholar] [CrossRef]
- Sritharadol, R.; Nakpheng, T.; Wan Sia Heng, P.; Srichana, T. Development of a topical mupirocin spray for antibacterial and wound-healing applications. Drug Dev. Ind. Pharm. 2017, 43, 1715–1728. [Google Scholar] [CrossRef]
- Alcantara, K.P.; Zulfakar, M.H.; Castillo, A.L. Development, characterization and pharmacokinetics of mupirocin-loaded nanostructured lipid carriers (NLCs) for intravascular administration. Int. J. Pharm. 2019, 571, 118705. [Google Scholar] [CrossRef] [PubMed]
- Salernitano, E.; Migliaresi, C. Composite Materials for Biomedical Applications: A Review. J. Appl. Biomater. Funct. Mater. 2003, 1, 3–18. [Google Scholar] [CrossRef]
- Serpooshan, V.; Zhao, M.; Metzler, S.A.; Wei, K.; Shah, P.B.; Wang, A.; Mahmoudi, M.; Malkovskiy, A.V.; Rajadas, J.; Butte, M.J.; et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 2013, 34, 9048–9055. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Li, C.; Zhao, Y.; Hu, J.; Zhang, L.-M. Co-electrospun Nanofibrous Membranes of Collagen and Zein for Wound Healing. ACS Appl. Mater. Interfaces 2012, 4, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Ficai, A.; Albu, M.G.; Birsan, M.; Sonmez, M.; Ficai, D.; Trandafir, V.; Andronescu, E. Collagen hydrolysate based collagen/hydroxyapatite composite materials. J. Mol. Struct. 2013, 1037, 154–159. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114, 111023. [Google Scholar] [CrossRef]
- Mohamad, N.; Mohd Amin, M.C.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef]
- Yasasvini, S.; Anusa, R.S.; VedhaHari, B.N.; Prabhu, P.C.; RamyaDevi, D. Topical hydrogel matrix loaded with Simvastatin microparticles for enhanced wound healing activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Dorrani, M.; Kaul, M.; Parhi, A.; LaVoie, E.J.; Pilch, D.S.; Michniak-Kohn, B. TXA497 as a topical antibacterial agent: Comparative antistaphylococcal, skin deposition, and skin permeation studies with mupirocin. Int. J. Pharm. 2014, 476, 199–204. [Google Scholar] [CrossRef]
- Kennedy, P.; Brammah, S.; Wills, E. Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010, 36, 49–56. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Wang, D.Y.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-Based Antimicrobial Delivery-Systems for the Treatment of Bacterial Infections. Front. Chem. 2019, 7, 872. [Google Scholar] [CrossRef]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef] [Green Version]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Singhvi, G.; Manchanda, P.; Hans, N.; Dubey, S.K.; Gupta, G. Microsponge: An emerging drug delivery strategy. Drug Dev. Res. 2019, 80, 200–208. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, P.; Ludeña, D.; Fernández, A.; Aranda, J.L.; Varela, G.; Iglesias, J. Autologous fibrin scaffolds cultured dermal fibroblasts and enriched with encapsulated bFGF for tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99A, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Burman, U.; Kaul, R.K. Chapter 19 - Ecological Risks of Nanoparticles: Effect on Soil Microorganisms. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 429–452. [Google Scholar]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electrospun scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Chinsriwongkul, A.; Chareanputtakhun, P.; Ngawhirunpat, T.; Rojanarata, T.; Sila-on, W.; Ruktanonchai, U.; Opanasopit, P. Nanostructured Lipid Carriers (NLC) for Parenteral Delivery of an Anticancer Drug. AAPS PharmSciTech 2012, 13, 150–158. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sen, S.; Hazra, A.; Das, A.K. Randomized controlled trial of topical mupirocin versus mupirocin with sucralfate combination in chronic skin ulcers. Indian J. Pharmacol. 2019, 51, 316–322. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Klarić, M. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Jagdale, S.C.; Kothekar, P.V. Development of Emulgel Delivery of Mupirocin for Treatment of Skin Infection. Recent Pat. Antiinfect. Drug Discov. 2020, 15, 137–156. [Google Scholar] [CrossRef]



| SSTIs | Infection | Pathogen | Description | Ref. |
|---|---|---|---|---|
| Non-purulent SSTIs | Impetigo | Staphylococcus aureus, Streptococcus pyogenes | Superficial infection developed via direct or indirect invasion of bacteria. It is the most common infection in children and presents in two forms, i.e., bullous and non-bullous impetigo. | [7,9,13] |
| Cellulitis | Staphylococcus aureus, beta-hemolytic streptococci (groups A, B, C, or G) | Subcutaneous infections are accompanied by lymphadenopathy and lymphangitis. It is characterized by redness, edema, or induration and usually affects lower limbs. | [7] | |
| Erysipelas | Staphylococcus aureus, Streptococcus pyogenes | Superficial lymphatics and upper dermis infection, usually affects the face and sometimes lower limbs. It possesses well-defined sharp raised borders in contrast to non-infected areas. | [7,13] | |
| Folliculitis | Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes | It is a superficial inflammation of hair follicles, mainly affecting moist skin with hair. | [7,9,14] | |
| Purulent SSTIs | Furuncle | Staphylococcus aureus | Furuncle or boil is a deep inflammatory infection developed from folliculitis. Initially, it is a firm, tender, erythematous nodule that becomes fluctuant and painful. It usually infects the face, buttocks, and axillae. | [9,13] |
| Carbuncle | Staphylococcus aureus, Streptococcus pyogenes | It is an aggregation of multiple furuncles, involves infection of the hair follicle, and is further extended to subcutaneous tissues. The infection is painful and tender but the patient is well. It is usually observed at the neck, back, and thighs. | [7,9,13] | |
| Abscess | Staphylococcus aureus, Streptococcus pyogenes, Streptococcus milleri, viridans, streptococci, coagulase–staphylococci | Focal collection of pus in dermis and hypodermis, characterized by tender, red nodules surrounded by erythematous swelling. | [7,9,15,16] | |
| Complex SSTIs | Burn wound | Anaerobes | Burn wound infection possesses a high bacteria concentration (>105 colonies forming unit). It arises immediately after the injury due to the damage of the cutaneous barrier and adaptive immunity. The surrounding tissues of the burn wound exhibit warmth, tenderness, induration, and erythema. | [6,17] |
| Surgical site infection | Escherichia coli | It usually arises 4 days after surgery and is categorized into superficial incisional, deep incisional, and organ or space infection. It is diagnosed by incisional discharge, swelling, tenderness, and erythema. | [18] | |
| Diabetic foot infection | Staphylococcus aureus, Enterococci, Pseudomonas aeruginosa, Enterobacteriaceae, Acinetobacter spp., Bacteroides spp. | This infection is most common in diabetic patients and possesses high mortality. This infection encompasses a range from nails to necrotizing limbs. Nails serve as an entry portal for bacterial infection due to poor hygiene. | [6,19,20] | |
| Necrotizing SSTIs | Monomicrobial, Polymicrobial | Staphylococcus aureus, Streptococcus pyogenes Gram-negatives, Clostridium species, Anaerobic bacteria | Necrosis of soft tissues or muscles is initially characterized by erythema and induration with pain followed by skin color change to blue/purple. The patient suffers from systemic toxicity, multi-organ failure, and hemodynamic instability. | [6,7,9] |
| Bite wounds | Human and animal bite | Eikenella corrodens, Pasteurella multocida, Pasteurella canis, Capnocytophaga canimorsus, Staphylococcus aureus | It usually arises after biting. | [7,21,22] |
| Drug Delivery System | Infection | Pathogen | Biomaterial | Outcome | Ref. |
|---|---|---|---|---|---|
| Composite biomaterials/scaffold | Wound healing | Staphylococcus aureus, Bacillus subtilis, Escherichia coli | Collagen, Silica | The collagen scaffolds exhibited more therapeutic potential for the treatment of wound infection and displayed a promising carrier approach for tissue engineering. | [39] |
| The developed bio-composite exhibited enhanced water uptake, sustained release, and antimicrobial activity. In vivo results stipulated that the biomaterial showed enhanced adhesion and wound contraction rate, supported by histopathological analysis. | [40] | ||||
| Hydrogel dressings | Wound healing | Escherichia coli (ATCC 8739), Enterococcus hirae (ATCC 10541), S. aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27853), Bacillus cereus (ATCC 7064), Klebsiella pneumonia | Chitosan, sodium alginate, carbopol | The developed composite film accelerated the regeneration of the epidermal layer in contrast to the marketed commercial formulation. | [41] |
| Diabetic wound | Polyvinyl alcohol | The developed gel was effective for the treatment of diabetic wound and accelerated the wound closure. | [42] | ||
| Primary and secondary | Gram-positive and Gram-negative bacteria | Chitosan | The prepared polymeric membrane was spherical, stable, and elastic, along with having the controlled release property. Furthermore, the membrane exhibited magnified retention of the drug in the skin without any irritation. | [43] | |
| Surgical wound | Staphylococcus aureus | Chitosan | The formulated spherical membrane exhibited superior adhesion and elasticity along with progressive drug release. The Draize patch test revealed that the developed membrane was non-irritant to the skin, along with having magnified antimicrobial efficiency and enhanced retention to the skin. | [44] | |
| Skin injuries | Acrylic acid | The developed patches exhibited good elasticity and tensile strength, along with enhanced permeation and retention into the skin. The patches were non-irritant to the skin, evidenced by the Draize patch test. | [45] | ||
| Liposomes | Methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus aureus | Hydrogenated soy phosphatidylcholine, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [methoxy (PEG)-2000], cholesterol | Mupirocin was administered intravenously the first time with a distinctive mechanism of action that resulted in a better approach for the treatment of resistant bacterial infection. Further, the results stipulated that nano-mupirocin extended the topical application of mupirocin to the systemic application for the treatment of MRSA infections by changing the pharmacodynamics of mupirocin. | [46,47,48] | |
| Liposomal hydrogel | Burn therapy | Staphylococcus aureus and Bacillus subtilis | Chitosan | Mupirocin-loaded liposomal hydrogel system exhibited prolonged release and superior bio-adhesiveness in contrast to the marketed formulation of mupirocin. In vitro and in vivo studies stipulated that the developed system was significantly safe, more therapeutically active along with shorter healing time, and exhibited antibiofilm activity against the bacterial pathogen. | [49,50] |
| Microparticles/Microspheres | Wound healing | Staphylococcus aureus | Eudragit | The developed formulation exhibited the sustained release of mupirocin along with magnified storage. The morphology, drug release, and antimicrobial activity of the developed formulation were dependent on the drug loading and the solvent. Time-kill assay results revealed that there was no loss of the antimicrobial activity of mupirocin during the encapsulation. | [51,52] |
| Microsponges | Surgical wound | Staphylococcus aureus | Ethylcellulose | Mupirocin microsponge exhibited a diffusion-controlled release profile along with ~5 times magnified retention on rat skin in contrast to the marketed formulation. The formulation was found stable and non-irritant, evidenced by the Draize patch test. | [53] |
| Wound healing | Staphylococcus aureus, Escherichia coli | Keratin, fibrin, and gelatin | The developed formulation exhibited a prolonged release pattern along with enhanced biocompatibility and cell adhesion properties. The antimicrobial activity results demonstrated that the mupirocin-loaded sponge was a promising medicated dressing material for the treatment of wound infection. | [54] | |
| Nanocapsule/nanoparticles | Wound healing | Poly(ε-caprolactone) | The developed nanocapsules showed excellent stability at 40 °C and room temperature. | [55] | |
| Methicillin-resistant Staphylococcus aureus (MRSA) | Chitosan, selenium | The tailored formulation showed remarkable therapeutic potential in terms of diabetic wound healing and wound contraction compared to the native mupirocin. | [56] | ||
| Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Escherichia coli | Poly(ethylene oxide)–poly (propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) | The tailored formulation exhibited reduced minimum inhibitory concentrations and minimum bactericidal concentrations against S. aureus, S. epidermidis, Pseudomonas aeruginosa, and E. coli compared to the mupirocin ointment. Further, the developed formulation was safe, effective, and biocompatible for the treatment of wound infection. | [57,58] | ||
| Nanofibers | Wound healing | Staphylococcus aureus | Poly-l-lactic acid | The tailored scaffold exhibited a different release profile for both drugs, suggesting that the release kinetics of one drug was altered by keeping the two different drugs in the same polymer matrix. The dual drug scaffold released a significantly higher drug and even compensated the inactive monic acid to act on the applied area, resulting in the maintainence of a sufficient concentration of mupirocin in the infected wound for more than a 72 h period, resulting in profound wound healing. | [59] |
| Burn wound | Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli | Polyurethane | The developed fiber mat was enough for wound hydration via providing adequate environmental humidity. Moreover, the tailored nanofiber exhibited sufficient cell spreading and attachment. The cytotoxicity results revealed that the antibacterial activity of the scaffold was increased proportionally with the increase in mupirocin concentration (2–5%). Further, the histopathological study revealed that the nanofibrous mat was enough for burn wound healing due to negligible inflammation. | [60] | |
| Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli | Polycaprolactone | The tailored multifunctional double-layer nanofibrous scaffold (MDLS) was effective for the management of wound infection, along with superior tensile strength with enhanced contact angle and swelling ratio. Furthermore, cytotoxicity results revealed that the MDLS was more biocompatible due to the addition of chitosan in contrast to polycaprolactone nanofibers. | [61] | ||
| Staphylococcus aureus, and Escherichia coli | Keratin, and coenzyme Q10, and polyvinyl alcohol | The tailored formulations were biocompatible, evidenced by the skin irritancy test. Further, the therapeutic efficacy of the tailored formulation was assessed by antimicrobial activity against various strains of S. aureus (2583, 2586, 2587, 2590), MRSA 2555, and E. coli 1808. Moreover, cell proliferation results evidenced the ability of nanofibers to support the keratinocytes’ growth due to the presence of coenzyme Q10. | [62] | ||
| Topical spray | Burn wound | Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli, and Streptococcus suis (S. suis) | Eudragit E100 | The developed spray exhibited magnified antimicrobial activity (18-fold) against S. suis, in contrast to the marketed formulation due to close contact between spray and skin, leading to the formation of a thin film on the infected surface. Moreover, the topical formulation was found non-irritant to the human skin without any toxicity to the monocytes, keratinocytes, and fibroblasts cells. Additionally, the safety profile of the formulation was also confirmed by zero production of nitric oxide and inflammatory cytokines (IL-1b and TNF-a) due to its antiendotoxin effect. | [18,63] |
| Nanostructured lipid carrier | Cetyl palmitate, caprylic acid | Nanostructured lipid carrier (NLC) reduced the metabolic degradation of MUP via the protective lipid layer of NLC which resulted in a 40-fold and 55-fold area under the curve and half-life, respectively, in contrast to native MUP. | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangwar, A.; Kumar, P.; Singh, R.; Kush, P. Recent Advances in Mupirocin Delivery Strategies for the Treatment of Bacterial Skin and Soft Tissue Infection. Future Pharmacol. 2021, 1, 80-103. https://doi.org/10.3390/futurepharmacol1010007
Gangwar A, Kumar P, Singh R, Kush P. Recent Advances in Mupirocin Delivery Strategies for the Treatment of Bacterial Skin and Soft Tissue Infection. Future Pharmacology. 2021; 1(1):80-103. https://doi.org/10.3390/futurepharmacol1010007
Chicago/Turabian StyleGangwar, Aishwarya, Parveen Kumar, Ranjit Singh, and Preeti Kush. 2021. "Recent Advances in Mupirocin Delivery Strategies for the Treatment of Bacterial Skin and Soft Tissue Infection" Future Pharmacology 1, no. 1: 80-103. https://doi.org/10.3390/futurepharmacol1010007
APA StyleGangwar, A., Kumar, P., Singh, R., & Kush, P. (2021). Recent Advances in Mupirocin Delivery Strategies for the Treatment of Bacterial Skin and Soft Tissue Infection. Future Pharmacology, 1(1), 80-103. https://doi.org/10.3390/futurepharmacol1010007







