Strategies for Generating Human Pluripotent Stem Cell-Derived-Organoid Culture for Disease Modeling, Drug Screening, and Regenerative Therapy
Abstract
:1. Introduction
2. The Main Features of hPSCs Derived-Organoids
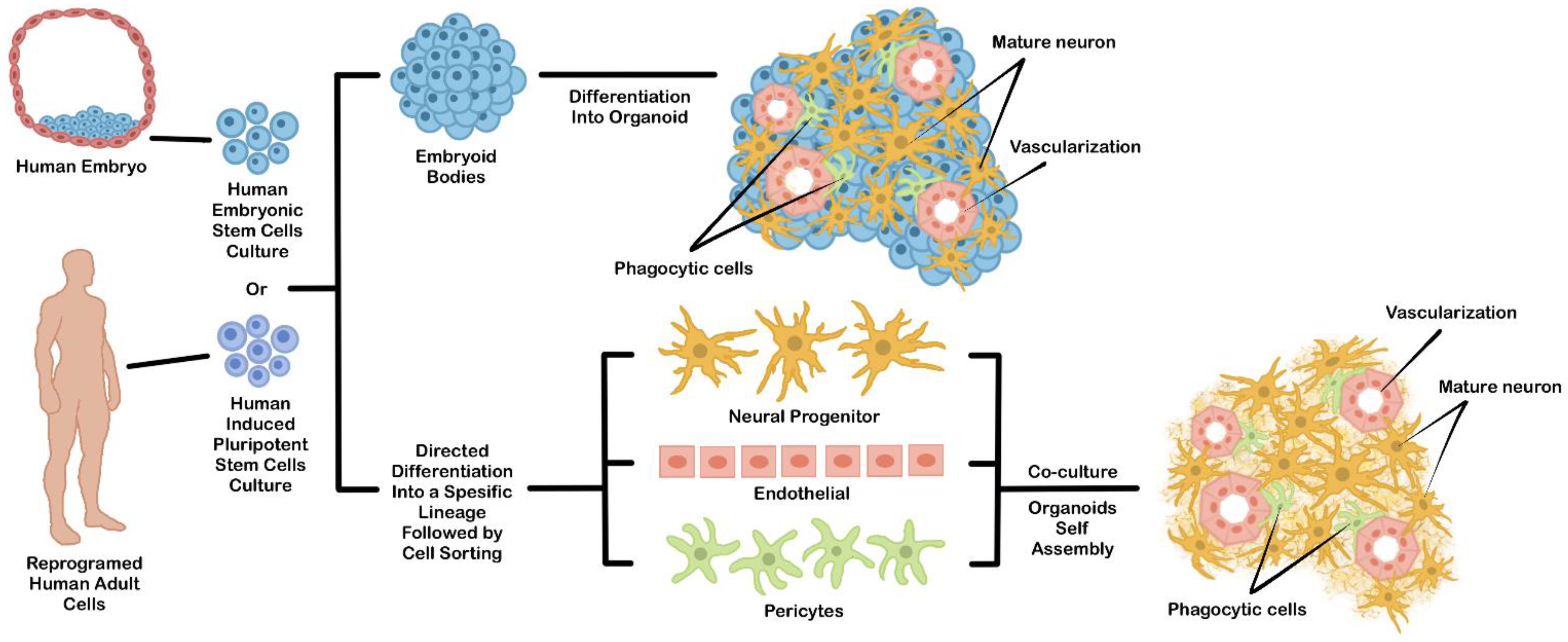
3. Culture Strategy to Generate hPSCs Derived-Organoids
3.1. Direct Organoid Differentiation from hPSCs EBs
3.2. Coculture of Multiple Differentiated Cells
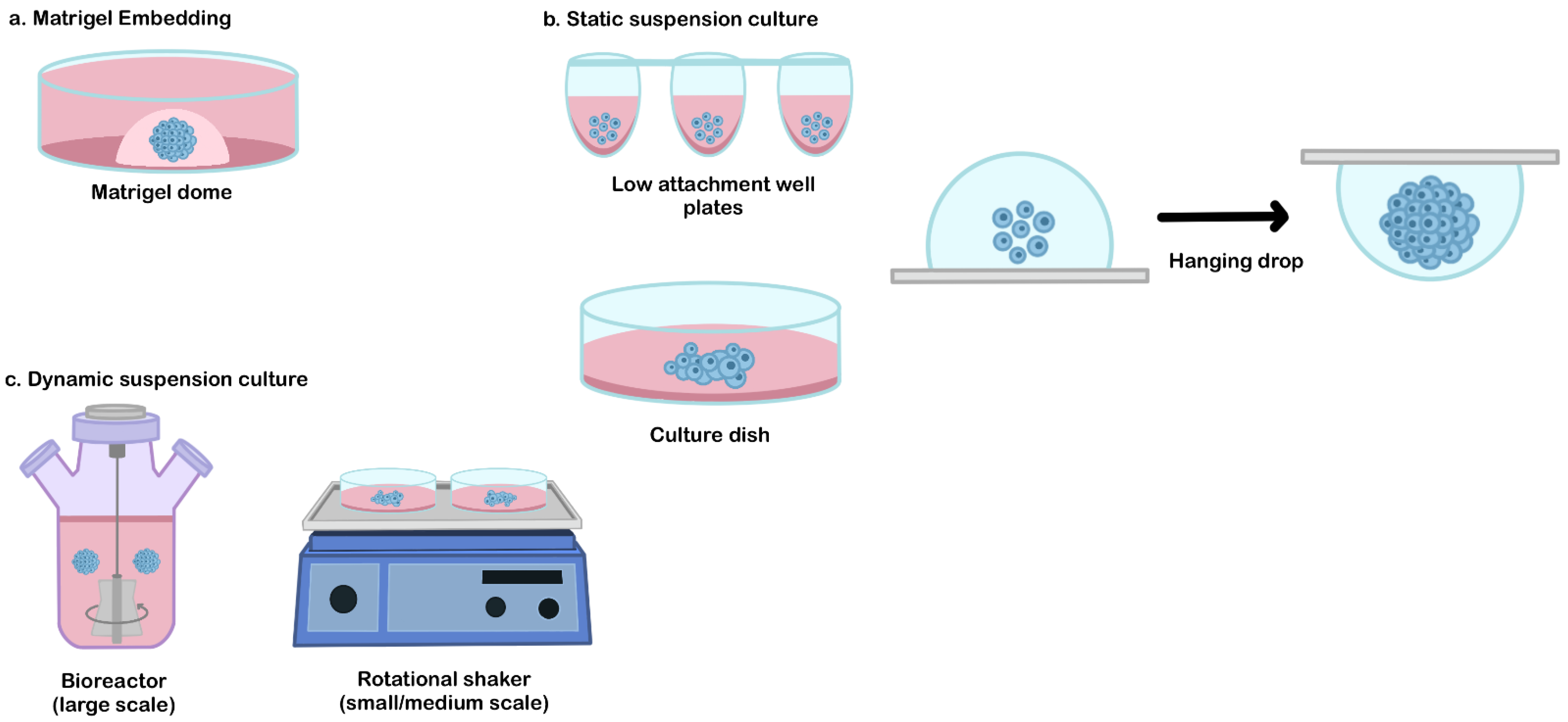
4. General Culture Platform to Generate the hPSCs Derived-Organoids
4.1. Matrigel Embedded Technique
4.2. Static Suspension Culture
4.3. Dynamic Suspension Culture
5. Other Limitations and Possible Improvements of PSCs Derived-Organoids Culture
5.1. Maturation
5.2. High-Cost Production
5.3. Tissue Complexity
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso-Moreira, M.; Sarropoulos, I.; Velten, B.; Mort, M.; Cooper, D.N.; Huber, W.; Kaessmann, H. Developmental Gene Expression Differences between Humans and Mammalian Models. Cell Rep. 2020, 33, 108308. [Google Scholar] [CrossRef]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Iefremova, V.; Manikakis, G.; Krefft, O.; Jabali, A.; Weynans, K.; Wilkens, R.; Marsoner, F.; Brändl, B.; Müller, F.J.; Koch, P.; et al. An Organoid-Based Model of Cortical Development Identifies Non-Cell-Autonomous Defects in Wnt Signaling Contributing to Miller-Dieker Syndrome. Cell Rep. 2017, 19, 50–59. [Google Scholar] [CrossRef]
- Mills, R.J.; Parker, B.L.; Quaife-Ryan, G.A.; Voges, H.K.; Needham, E.J.; Bornot, A.; Ding, M.; Andersson, H.; Polla, M.; Elliott, D.A.; et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 2019, 24, 895–907.e6. [Google Scholar] [CrossRef]
- Willenbring, H.; Soto-Gutierrez, A. Transplantable liver organoids made from only three ingredients. Cell Stem Cell 2013, 13, 139–140. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Berishvili, E.; Casiraghi, F.; Amarelli, C.; Scholz, H.; Piemonti, L.; Berney, T.; Montserrat, N. Mini-organs forum: How to advance organoid technology to organ transplant community. Transpl. Int. 2021, 34, 1588–1593. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Sasai, Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell 2013, 12, 520–530. [Google Scholar] [CrossRef]
- Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 2013, 493, 318–326. [Google Scholar] [CrossRef]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449. [Google Scholar] [CrossRef]
- Anastasaki, C.; Wilson, A.F.; Chen, A.S.; Wegscheid, M.L.; Gutmann, D.H. Generation of human induced pluripotent stem cell-derived cerebral organoids for cellular and molecular characterization. STAR Protoc. 2022, 3, 101173. [Google Scholar] [CrossRef]
- Anastasaki, C.; Wegscheid, M.L.; Hartigan, K.; Papke, J.B.; Kopp, N.D.; Chen, J.; Cobb, O.; Dougherty, J.D.; Gutmann, D.H. Human iPSC-Derived Neurons and Cerebral Organoids Establish Differential Effects of Germline NF1 Gene Mutations. Stem Cell Rep. 2020, 14, 541–550. [Google Scholar] [CrossRef]
- Wegscheid, M.L.; Anastasaki, C.; Hartigan, K.A.; Cobb, O.M.; Papke, J.B.; Traber, J.N.; Morris, S.M.; Gutmann, D.H. Patient-derived iPSC-cerebral organoid modeling of the 17q11.2 microdeletion syndrome establishes CRLF3 as a critical regulator of neurogenesis. Cell Rep. 2021, 36, 109315. [Google Scholar] [CrossRef]
- Xu, R.; Boreland, A.J.; Li, X.; Erickson, C.; Jin, M.; Atkins, C.; Pang, Z.P.; Daniels, B.P.; Jiang, P. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 2021, 16, 1923–1937. [Google Scholar] [CrossRef]
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G.L. Generation of human brain region–specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 2018, 13, 565–580. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Huang, J.Y.; Lu, A.; Ng, A.H.M.; Duenki, T.; Liu, S.; Nam, L.L.; Damaraju, S.; Church, G.M.; Lewis, J.A. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 2022, 6, 449–462. [Google Scholar] [CrossRef]
- Watanabe, M.; Buth, J.E.; Vishlaghi, N.; de la Torre-Ubieta, L.; Taxidis, J.; Khakh, B.S.; Coppola, G.; Pearson, C.A.; Yamauchi, K.; Gong, D.; et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017, 21, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Kang, Y.J.; Govindaiah, G.; Roselaar, N.; Cakir, B.; Kim, K.Y.; Lombroso, A.P.; Hwang, S.M.; et al. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 2017, 21, 383–398.e7. [Google Scholar] [CrossRef]
- Trisno, S.L.; Philo, K.E.D.; McCracken, K.W.; Catá, E.M.; Ruiz-Torres, S.; Rankin, S.A.; Han, L.; Nasr, T.; Chaturvedi, P.; Rothenberg, M.E.; et al. Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell 2018, 23, 501–515. [Google Scholar] [CrossRef]
- Koide, T.; Koyanagi-Aoi, M.; Uehara, K.; Kakeji, Y.; Aoi, T. CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells. Iscience 2022, 25, 104314. [Google Scholar] [CrossRef]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef]
- McCracken, K.W.; Aihara, E.; Martin, B.; Crawford, C.M.; Broda, T.; Treguier, J.; Zhang, X.; Shannon, J.M.; Montrose, M.H.; Wells, J.M. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature 2017, 541, 182–187. [Google Scholar] [CrossRef]
- Broda, T.R.; McCracken, K.W.; Wells, J.M. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat. Protoc. 2019, 14, 28–50. [Google Scholar] [CrossRef]
- Nadkarni, R.R.; Abed, S.; Cox, B.J.; Bhatia, S.; Lau, J.T.; Surette, M.G.; Draper, J.S. Functional Enterospheres Derived In Vitro from Human Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 897–912. [Google Scholar] [CrossRef] [Green Version]
- Mithal, A.; Capilla, A.; Heinze, D.; Berical, A.; Villacorta-Martin, C.; Vedaie, M.; Jacob, A.; Abo, K.; Szymaniak, A.; Peasley, M.; et al. Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Fonseca, B.d.F.d.; Doumont, G.; Gillotay, P.; Tourneur, A.; Eski, S.E.; Van Simaeys, G.; Chomette, L.; Lasolle, H.; Monestier, O.; et al. Transplantable human thyroid organoids generated from embryonic stem cells to rescue hypothyroidism. BioRixv. 2021. BioRxiv 2021.12.01.470729. Available online: https://www.biorxiv.org/content/10.1101/2021.12.01.470729v1 (accessed on 2 February 2022).
- Montel-Hagen, A.; Seet, C.S.; Li, S.; Chick, B.; Zhu, Y.; Chang, P.; Tsai, S.; Sun, V.; Lopez, S.; Chen, H.C.; et al. Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells. Cell Stem Cell 2019, 24, 376–389. [Google Scholar] [CrossRef]
- Drakhlis, L.; Biswanath, S.; Farr, C.M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Drakhlis, L.; Devadas, S.B.; Zweigerdt, R. Generation of heart-forming organoids from human pluripotent stem cells. Nat. Protoc. 2021, 16, 5652–5672. [Google Scholar] [CrossRef]
- Hoang, P.; Kowalczewski, A.; Sun, S.; Winston, T.S.; Archilla, A.M.; Lemus, S.M.; Ercan-Sencicek, A.G.; Gupta, A.R.; Liu, W.; Kontaridis, M.I.; et al. Engineering spatial-organized cardiac organoids for developmental toxicity testing. Stem Cell Rep. 2021, 16, 1228–1244. [Google Scholar] [CrossRef] [PubMed]
- Voges, H.K.; Mills, R.J.; Elliott, D.A.; Parton, R.G.; Porrello, E.R.; Hudson, J.E. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Miller, A.J.; Hill, D.R.; Nagy, M.S.; Aoki, Y.; Dye, B.R.; Chin, A.M.; Huang, S.; Zhu, F.; White, E.S.; Lama, V.; et al. In Vitro Induction and In Vivo Engraftment of Lung Bud Tip Progenitor Cells Derived from Human Pluripotent Stem Cells. Stem Cell Reports 2018, 10, 101–119. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, D.; Garfin, P.M.; Ehmer, U.; Hurwitz, M.; Enns, G.; Michie, S.; Wu, M.; Zheng, M.; Nishimura, T.; et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2017, 2, e94954. [Google Scholar] [CrossRef]
- Mun, S.J.; Ryu, J.S.; Lee, M.O.; Son, Y.S.; Oh, S.J.; Cho, H.S.; Son, M.Y.; Kim, D.S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef]
- Ramli, M.N.B.; Lim, Y.S.; Lim, C.T.; Demircioglu, D.; Tng, W.; Gonzales, K.A.U.; Tan, C.P.; Szczerbinska, I.; Liang, H.; Soe, E.L.; et al. Human Pluripotent Stem Cell-Derived Organoids as Models of Liver Disease. Gastroenterology 2020, 159, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Torizal, F.G.; Utami, T.; You, L.Q.; Inamura, K.; Nishikawa, M.; Sakai, Y. Generation of high-density human induced pluripotent stem cell derived-liver organoid by enabling growth factors accumulation in a simple dialysis medium refinement culture platform. Preprint 2022, 1–10. [Google Scholar] [CrossRef]
- Wiedenmann, S.; Breunig, M.; Merkle, J.; von Toerne, C.; Georgiev, T.; Moussus, M.; Schulte, L.; Seufferlein, T.; Sterr, M.; Lickert, H.; et al. Single-cell-resolved differentiation of human induced pluripotent stem cells into pancreatic duct-like organoids on a microwell chip. Nat. Biomed. Eng. 2021, 5, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Przepiorski, A.; Sander, V.; Tran, T.; Hollywood, J.A.; Sorrenson, B.; Shih, J.H.; Wolvetang, E.J.; McMahon, A.P.; Holm, T.M.; Davidson, A.J. A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 470–484. [Google Scholar] [CrossRef]
- Sander, V.; Przepiorski, A.; Crunk, A.E.; Hukriede, N.A.; Holm, T.M.; Davidson, A.J. Protocol for Large-Scale Production of Kidney Organoids from Human Pluripotent Stem Cells. STAR Protoc. 2020, 1, 100150. [Google Scholar] [CrossRef]
- Zhang, R.R.; Koido, M.; Tadokoro, T.; Ouchi, R.; Matsuno, T.; Ueno, Y.; Sekine, K.; Takebe, T.; Taniguchi, H. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Rep. 2018, 10, 780–793. [Google Scholar] [CrossRef]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Kimura, M.; Kodaka, A.; Nishii, S.; Thompson, W.L.; Takebe, T. Engineering human hepato-biliary-pancreatic organoids from pluripotent stem cells. Nat. Protoc. 2021, 16, 919–936. [Google Scholar] [CrossRef]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Giesbrecht, K.; Saiki, N.; Ferguson, A.; Kimura, M.; Thompson, W.L.; Wells, J.M.; et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut–midgut boundary. Nature 2019, 574, 112–116. [Google Scholar] [CrossRef]
- Takebe, T.; Wells, J.M. Organoids by design. Science 2019, 364, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Li, G.; Gokulnath, P.; Xiao, J. Generating 3D human cardiac constructs from pluripotent stem cells. eBioMedicine 2022, 76, 103813. [Google Scholar] [CrossRef] [PubMed]
- Salaris, F.; Rosa, A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: Challenges and opportunities. Brain Res. 2019, 1723, 146393. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Nelson, C.M. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis 2012, 8, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Garreta, E.; Kamm, R.D.; Chuva de Sousa Lopes, S.M.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat. Mater. 2021, 20, 145–155. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Recapitulating early cardiogenesis in vitro. Nat. Methods 2021, 18, 331. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Torizal, F.G.; Horiguchi, I.; Sakai, Y. Physiological Microenvironmental Conditions in Different Scalable Culture Systems for Pluripotent Stem Cell Expansion and Differentiation. Open Biomed. Eng. J. 2017, 13, 41–54. [Google Scholar] [CrossRef]
- Heo, J.H.; Kang, D.; Seo, S.J.; Jin, Y. Engineering the Extracellular Matrix for Organoid Culture. Int. J. Stem Cells 2022, 15, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Poudel, H.; Sanford, K.; Szwedo, P.K.; Pathak, R.; Ghosh, A. Synthetic Matrices for Intestinal Organoid Culture: Implications for Better Performance. ACS Omega 2022, 7, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, K.; Spee, B.; Costa, P.; Sachs, N.; Clevers, H.; Malda, J. Converging biofabrication and organoid technologies: The next frontier in hepatic and intestinal tissue engineering? Biofabrication 2017, 9, 013001. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Ramanathan, R.; Fisher, R.A.; Mangino, M.J. Scalable Differentiation of Human iPSCs in a Multicellular Spheroid- based 3D Culture into Hepatocyte- like Cells through Direct Wnt/β—Catenin Pathway Inhibition. Sci. Rep. 2016, 6, 32888. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Lehoux, S.; Ramanathan, R.; Salem, M.M.; He, L.X.; Muse, O.; Flaumenhaft, R.; Thompson, M.T.; Rouse, E.A.; Cummings, R.D.; et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019, 9, 8920. [Google Scholar] [CrossRef]
- Otsuji, T.G.; Bin, J.; Yoshimura, A.; Tomura, M.; Tateyama, D.; Minami, I.; Yoshikawa, Y.; Aiba, K.; Heuser, J.E.; Nishino, T.; et al. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014, 2, 734–745. [Google Scholar] [CrossRef]
- Vosough, M.; Omidinia, E.; Kadivar, M.; Shokrgozar, M.-A.; Pournasr, B.; Aghdami, N.; Baharvand, H. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cells Dev. 2013, 22, 2693–2705. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar] [CrossRef]
- Torizal, F.G.; Kimura, K.; Horiguchi, I.; Sakai, Y. Size-dependent hepatic differentiation of human induced pluripotent stem cells spheroid in suspension culture. Regen. Ther. 2019, 16, 66–73. [Google Scholar] [CrossRef]
- Torizal, F.G.; Kim, S.M.; Horiguchi, I.; Inamura, K.; Suzuki, I.; Morimura, T.; Nishikawa, M.; Sakai, Y. Production of homogenous size-controlled human induced pluripotent stem cell aggregates using ring-shaped culture vessel. J. Tissue Eng. Regen. Med. 2022, 16, 254–266. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.P.; Leleux, J.; Nerem, R.M.; Ahsan, T. Effects of shear stress on germ lineage specification of embryonic stem cells. Integr. Biol. 2012, 4, 1263–1273. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.A.; Kim, S.J.; Lee, H.A. Improving Generation of Cardiac Organoids from Human Pluripotent Stem Cells Using the Aurora Kinase Inhibitor ZM447439. Biomedicines 2021, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.H.; Ko, U.H.; Oh, Y.; Lim, A.; Sohn, J.W.; Shin, J.H.; Kim, H.H.; Han, Y.M. Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci. Rep. 2016, 6, 35145. [Google Scholar] [CrossRef]
- Nam, S.A.; Seo, E.; Kim, J.W.; Kim, H.W.; Kim, H.L.; Kim, K.; Kim, T.M.; Ju, J.H.; Gomez, I.G.; Uchimura, K.; et al. Graft immaturity and safety concerns in transplanted human kidney organoids. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vreeker, A.; Van Stuijvenberg, L.; Hund, T.J.; Mohler, P.J.; Nikkels, P.G.J.; Van Veen, T.A.B. Assembly of the cardiac intercalated disk during preand postnatal development of the human heart. PLoS ONE 2014, 9, e94722. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef]
- Ergir, E.; Oliver-De La Cruz, J.; Fernandes, S.; Cassani, M.; Niro, F.; Sousa, D.; Vrbský, J.; Vinarský, V.; Perestrelo, A.R.; Debellis, D.; et al. Generation and Maturation of Human iPSC-derived Cardiac Organoids in Long Term Culture. BioRxiv 2022. BioRxiv 2022.03.07.483273. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mondragon-Gonzalez, R.; Xu, B.; Magli, A.; Kim, H.; Lainé, J.; Kiley, J.; McKee, H.; Rinaldi, F.; Aho, J.; et al. Screening identifies small molecules that enhance the maturation of human pluripotent stem cell-derived myotubes. Elife 2019, 8, e47970. [Google Scholar] [CrossRef] [PubMed]
- Hergenreder, E.; Zorina, Y.; Zhao, Z.; Munguba, H.; Calder, L.; Baggiolini, A.; Minotti, A.P.; Walsh, R.M.; Levitz, J.; Garippa, R.; et al. Combined small molecule treatment accelerates timing of maturation in human pluripotent stem cell-derived neurons. BioRxiv 2022. BioRxiv 2022.06.02.494616. [Google Scholar] [CrossRef]
- Huang, C.Y.; Peres Moreno Maia-Joca, R.; Ong, C.S.; Wilson, I.; DiSilvestre, D.; Tomaselli, G.F.; Reich, D.H. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J. Mol. Cell. Cardiol. 2020, 138, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel-Rad, N.; Zahmatkesh, E.; Moeinvaziri, F.; Haghparast, N.; Baharvand, H.; Aghdami, N.; Moghadasali, R. Promoting Maturation of Human Pluripotent Stem Cell-Derived Renal Microtissue by Incorporation of Endothelial and Mesenchymal Cells. Stem Cells Dev. 2021, 30, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nuebel, E.; Daley, G.Q.; Koehler, C.M.; Teitell, M.A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 2012, 11, 589–595. [Google Scholar] [CrossRef]
- Wu, J.; Ocampo, A.; Belmonte, J.C.I. Cellular Metabolism and Induced Pluripotency. Cell 2016, 166, 1371–1385. [Google Scholar] [CrossRef]
- Mills, R.J.; Titmarsh, D.M.; Koenig, X.; Parker, B.L.; Ryall, J.G.; Quaife-Ryan, G.A.; Voges, H.K.; Hodson, M.P.; Ferguson, C.; Drowley, L.; et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. USA 2017, 114, E8372–E8381. [Google Scholar] [CrossRef]
- Dye, B.R.; Dedhia, P.H.; Miller, A.J.; Nagy, M.S.; White, E.S.; Shea, L.D.; Spence, J.R. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife 2016, 5, e19732. [Google Scholar] [CrossRef]
- Kempf, H.; Andree, B.; Zweigerdt, R. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv. Drug Deliv. Rev. 2016, 96, 18–30. [Google Scholar] [CrossRef]
- Zweigerdt, R. Large Scale Production of Stem Cells and Their Derivatives. Adv. Biochem. Eng. Biotechnol. 2009, 123, 127–141. [Google Scholar] [CrossRef]
- Iansante, V.; Chandrashekran, A.; Dhawan, A. Cell-based liver therapies: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170229. [Google Scholar] [CrossRef]
- Siller, R.; Greenhough, S.; Naumovska, E.; Sullivan, G.J. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Rep. 2015, 4, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Torizal, F.G.; Lau, Q.Y.; Ibuki, M.; Kawai, Y.; Horikawa, M.; Minami, M.; Michiue, T.; Horiguchi, I.; Nishikawa, M.; Sakai, Y. A miniature dialysis-culture device allows high-density human-induced pluripotent stem cells expansion from growth factor accumulation. Commun. Biol. 2021, 4, 1316. [Google Scholar] [CrossRef] [PubMed]
- Torizal, F.G.; Lau, Q.Y.; Ibuki, M.; Kawai, Y.; Horikawa, M.; Minami, M.; Horiguchi, I.; Nishikawa, M.; Sakai, Y. High-density hiPSCs expansion supported by growth factors accumulation in a simple dialysis- culture platform. Preprint 2020, 1–17. [Google Scholar] [CrossRef]
- Choi, H.; Torizal, F.G.; Shinohara, M.; Sakai, Y. Differentiation of Human Induced Pluripotent Stem Cells into Definitive Endoderm Using Simple Dialysis Culture Device. Methods Mol. Biol. 2021, 2158, 141–153. [Google Scholar] [CrossRef]
- Miki, T.; Ring, A.; Gerlach, J.; Ph, D.; Ring, A.; Ph, D.; Gerlach, J. Hepatic differentiation of human embryonic stem cells is promoted by three-dimensional dynamic perfusion culture conditions. Tissue Eng. Part C. Methods 2011, 17, 557–568. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef]
- Daviaud, N.; Friedel, R.H.; Zou, H. Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. Eneuro 2018, 5, 0219-18. [Google Scholar] [CrossRef]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Takebe, T.; Zhang, R.-R.; Koike, H.; Kimura, M.; Yoshizawa, E.; Enomura, M.; Koike, N.; Sekine, K.; Taniguchi, H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 2014, 9, 396–409. [Google Scholar] [CrossRef]
- Wörsdörfer, P.; Dalda, N.; Kern, A.; Krüger, S.; Wagner, N.; Kwok, C.K.; Henke, E.; Ergün, S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019, 9, 15663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, L.; Yi, Q. Engineering the vasculature of stem-cell-derived liver organoids. Biomolecules 2021, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Grebenyuk, S.; Ranga, A. Engineering organoid vascularization. Front. Bioeng. Biotechnol. 2019, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fujishima, K.; Kengaku, M. Modeling intestinal stem cell function with organoids. Int. J. Mol. Sci. 2021, 22, 10912. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef]
- Jung, K.B.; Lee, H.; Son, Y.S.; Lee, M.O.; Kim, Y.D.; Oh, S.J.; Kwon, O.; Cho, S.; Cho, H.S.; Kim, D.S.; et al. Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat. Commun. 2018, 9, 3039. [Google Scholar] [CrossRef]
- Stanley, A.C.; Lacy, P. Pathways for cytokine secretion. Physiology 2010, 25, 218–229. [Google Scholar] [CrossRef]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. Biomed Res. Int. 2017, 2017, 9042851. [Google Scholar] [CrossRef]
- Fligor, C.M.; Lavekar, S.S.; Harkin, J.; Shields, P.K.; VanderWall, K.B.; Huang, K.C.; Gomes, C.; Meyer, J.S. Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Rep. 2021, 16, 2228–2241. [Google Scholar] [CrossRef]
| Organoid Type | Culture Method | Targeted Application | Results | Ref |
|---|---|---|---|---|
| Brain | Static 96-well V-bottom plates | Disease modeling | The cerebral organoid was successfully delineated defects caused by deletions of the distal tip of chromosome | [13] |
| Static ultra-low attachment 96 well plate and dynamic 24 well plates | Disease modeling | Human cerebral organoids established differential effects in Neurofibromatosis type 1 (NF1) developmental disorder | [14,15,16] | |
| Static culture low-adhesion 6-cm plates followed by Matrigel embedded | Disease modeling | A patient-specific forebrain organoid was utilized to investigate the pathological changes associated with Miller-Dieker syndrome (MDS), caused by chromosomal deletion | [5] | |
| Ultra-low-attachment 96-well plates | Disease modeling | The introduction of hPSCs derived-microglia into hPSCs derived-cerebral organoids reflected an innate immune response system in the central nervous system which showed a phagocytic activity during Zika virus infection. | [17] | |
| Miniaturized spinning bioreactor | Disease modeling and developmental study | Resulted brain organoids were successfully recapitulating key dynamic features of the developing human brain at the molecular, cellular, and structural levels | [18] | |
| Combined biopolymer scaffold and Matrigel embedded | Developmental study and disease model | The cerebral organoid derived by guided cortical plate formation using a biopolymer scaffold showed a distinctive radial organization of the cerebral cortex and allowed the study of neuronal migration. | [19] | |
| Matrigel embedded | Transplantation model | Organoid grafts showed a progressive neural maturation and regeneration, followed by neovascularization after they were transplanted into mouse | [20] | |
| Orbital suspension culture and guided bioprinting | Drug screening and regenerative therapy | Patterned vascularized cortical organoid consisted of neural stem cells, endothelial, and neurons that mimic the vascularized brain showed its potential in drug testing and regeneration model. | [21] | |
| V-bottomed 96-well plate | Drug Screening and disease modeling | Cerebral organoid demonstrated its capability for modeling the teratogenic effects of Zika virus infection as well as testing the therapeutic compounds | [22] | |
| U-bottom ultra-low-attachment 96-well plate | Disease modeling | The presence of vasculature-like structures resulted in an enhanced functional cortical organoids maturation for disease modeling applications | [23] | |
| Ultra-low-attachment 96-well plate | Disease modeling | The 3D system created a reciprocal interaction between the thalamus and cortex by fusing the two distinct region-specific organoids that represent the developing thalamus or cortex. | [24] | |
| Esophagus | Matrigel embedded | Disease modeling | Human esophageal organoids were utilized for modeling the human esophageal birth defects, with enhanced survival. | [25] |
| Gastrointestinal | Matrigel embedded | Disease modeling | The gastric organoid was successfully modeled intestinal metaplasia during Doxorubicin induction | [26] |
| Static suspension in 24 well plates | Disease modeling | A robust in vitro system was developed to elucidate the mechanisms underlying human stomach development and disease including peptic ulcer disease and gastric cancer | [27] | |
| Matrigel embedded | Drug Screening and disease modeling | The human fundic-type gastric organoids (hFGOs) platform was reported to be a potential tractable human model system in terms identify and study signaling mechanisms such as normal cellular homeostasis in the fundus and antrum. | [28] | |
| Matrigel embedded | Disease modeling | Human antral and fundic gastric organoids (hGOs) have been effectively used to study stem cells, recovering gastric cell function and showing a cellular response to injury during H. pylori infection in a patient-specific manner | [29] | |
| Static suspension in a culture insert | Disease modeling | Human intestinal organoids displayed an innate immune response when treated by enteric pathogens | [30] | |
| Matrigel embedded | Disease modeling | Human intestinal organoids (hIOs) were successfully modeled the cystic fibrosis by CFTR alteration as well as the functional recovery after the correction of its mutation | [31] | |
| Thyroid | Hanging drop | Transplantation model | hESC-derived thyroid follicles have successfully produced thyroid hormone in vitro and in vivo after transplantation into thyroid gland ablated mice. | [32] |
| Thymus | Static transwell-6 well plates | Transplantation model | The thymic organoids were validated for in vitro generation of antigen-specific T cells | [33] |
| Heart | Micropatterned | Drug screening | The cardiac organoid successfully represented a decent in vitro pro regenerative drug development and potential reduction of their adverse effects | [6] |
| U shape 96 well and Matrigel-embedded | Gene targeting and Drug testing. | Heart-forming organoids were providing simultaneous monitoring of genetic mutation defects, as well as drug screening of teratogenic substances | [34,35] | |
| 2D micropatterned | Developmental study and drug-induced cardiac developmental toxicity. | The development of cardiac organoids presented a platform for comprehensive risk assessment in cardiac developmental toxicity assay, which better predicts thalidomide toxicity in the fetal heart model. | [36] | |
| 3D micropatterning using PDMS molds and poles | Disease modeling and regeneration | Human cardiac organoids were proposed as the physiologically relevant model of the human heart and completely recover the cardiac function after cryoinjury | [37] | |
| 3D micropattern by agarose hydrogel molds | Drug screening | The cardiac organoids recapitulated 3D tissue-level responses including drug-induced/exacerbated cardiotoxicity and fibrotic effects. This organoid can be applied for studying doxorubicin induced-myocardial infarction | [38] | |
| Lung | Matrigel embedded | Transplantation model | Human PSCs-derived lung bud tip organoid was able to be fully engrafted into the airways of the mouse model | [39] |
| Liver | Suspension culture and Matrigel embedded | Disease modeling | Hepatic organoid consisting of hepatocytes and cholangiocytes was successfully utilized for the disease modeling of liver genetic disease | [40] |
| Embedding inside the Matrigel dome | Drug screening and disease modeling | Screening of the drugs that was previously retracted from the market, such as troglitazone, trovafloxacin, and levofloxacin, was successfully employed using liver organoids. Additionally, this organoid was also utilized for modeling hepatic steatosis. | [41] | |
| Dynamic suspension culture in Erlenmeyer flask using an orbital shaker | Transplantation, drug screening, and disease modeling | The functional vascularized liver organoid showed liver-like functional features, such as the production of serum proteins and the coagulation factors, as well as supported ureagenesis and bilirubin uptake. | [7] | |
| Induced pluripotent stem cells | Disease modeling and drug screening | The hepatobiliary organoid was organized as a functional bile canaliculi system, which was disrupted by cholestasis-inducing drugs. | [42] | |
| Combined static microwell culture, 24-well, 6-well, and 1-well plate. | Transplantation | Vascularized and functional liver organoids generated entirely from iPSCs significantly improved hepatic functionalization and represent functional rescue against acute liver failure via transplantation. | [43] | |
| Suspension culture in the miniature dialysis device | Drug testing and transplantation | The scalable human liver organoid (hLOs) consisted of functional hepatocytes and cholangiocytes showed a response to drug exposure and showed a bile canaliculi activity | [44] | |
| Pancreas | Microwell chip | Disease modeling | Pancreatic duct-like organoids showed mucins regulations and cystic fibrosis transmembrane conductance. | [45] |
| Blood vessel | Coculture techniques in static ultra-low attachment six-well plate | Transplantation model, Modelling diabetic vasculopathy, Drug screening | Transplanted blood vessel organoids which were grown in streptozotocin-treated mice successfully represented a model of diabetic vasculopathy and its drug treatment. | [46] |
| Kidney organoids | Ultra-low-attachment 6-well plates followed by spinner flask bioreactor | Disease modeling and drug screening | The organoid successfully modeled the congenital kidney defect that interferes with tubulogenesis and potential drug treatment. | [47,48] |
| Assembloid (multiple organoid fusion) | Matrigel embedded | Transplantation model | Transplantation of gut and liver organoids showed an effective therapeutic potential against fulminant liver failure | [49] |
| 96-well round bottom low attachment plates | Disease modeling, drug development, and therapeutic transplantation. | Human hepato-biliary-pancreatic organoids represented the therapeutic responses to new potential treatments, modeling biliary atresia and promoting pancreatic fate commitment in Hes1-deficient mice. | [50,51] |
| Consideration | Animal Model | hPSCs Differentiation or Coculture in Monolayer | hPSCs Derived-Organoid | ||
|---|---|---|---|---|---|
| Matrigel Embedded /Dome | Static Suspension Culture | Dynamic Suspension Culture | |||
| Culture maintenance | Not required, but difficult animal handling | Easy | Relatively easy | Relatively Easy | Relatively easy |
| Represent actual human organ’s physiology | Partial, limited by interspecies variability | Poor | good | Good | Good |
| Tissue complexity | Very good | Poor | good | Good | Good |
| ECM and cell-cell interaction | Very good, native tissue condition | Poor | good | Good | Good |
| Ability to represent organogenesis/developmental biology | No | No | Yes | Yes | Yes |
| Represent actual human organs physiology | Partial, limited by interspecies variability | Poor | good | Good | Good |
| Scalability | Not available | Low | Low | High | High |
| Relative cost production | high | Relatively high | Relatively low | Low | Low |
| Application for personalized medicine and non-xenogeneic transplantation | Not possible | possible | Not possible | Possible | Possible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gania, Z.; Noorintan, S.T.; Septiari, N.P.D.P.; Fitriany, D.S.; Torizal, F.G. Strategies for Generating Human Pluripotent Stem Cell-Derived-Organoid Culture for Disease Modeling, Drug Screening, and Regenerative Therapy. Future Pharmacol. 2022, 2, 360-376. https://doi.org/10.3390/futurepharmacol2030025
Gania Z, Noorintan ST, Septiari NPDP, Fitriany DS, Torizal FG. Strategies for Generating Human Pluripotent Stem Cell-Derived-Organoid Culture for Disease Modeling, Drug Screening, and Regenerative Therapy. Future Pharmacology. 2022; 2(3):360-376. https://doi.org/10.3390/futurepharmacol2030025
Chicago/Turabian StyleGania, Zakiya, Syarifah Tiara Noorintan, Ni Putu Diah Pradnya Septiari, Dhea Sandra Fitriany, and Fuad Gandhi Torizal. 2022. "Strategies for Generating Human Pluripotent Stem Cell-Derived-Organoid Culture for Disease Modeling, Drug Screening, and Regenerative Therapy" Future Pharmacology 2, no. 3: 360-376. https://doi.org/10.3390/futurepharmacol2030025
APA StyleGania, Z., Noorintan, S. T., Septiari, N. P. D. P., Fitriany, D. S., & Torizal, F. G. (2022). Strategies for Generating Human Pluripotent Stem Cell-Derived-Organoid Culture for Disease Modeling, Drug Screening, and Regenerative Therapy. Future Pharmacology, 2(3), 360-376. https://doi.org/10.3390/futurepharmacol2030025







