Effect of Etanercept on Plasmodium yoelii MDR-Induced Liver Lipid Infiltration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Treatment with Etanercept
2.3. Cryosectioning of Tissue
2.4. Microtomy and Hematoxylin-Eosin (HE) Staining
2.5. Lipid Quantification
2.6. Lipid Peroxidation Assay
2.7. ELISA for Determination of Serum TNF-α
2.8. Statistical Analysis
3. Results
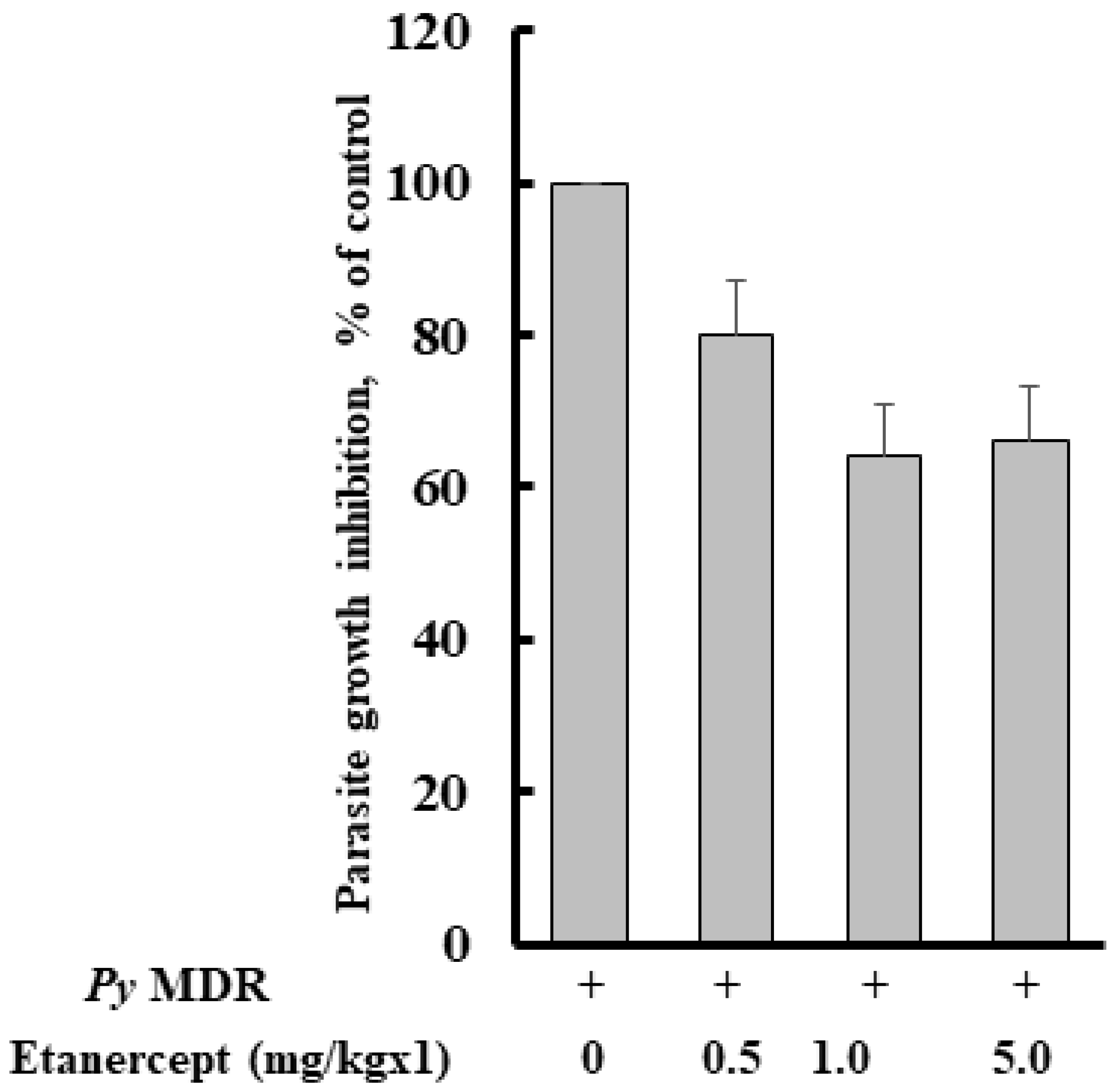
3.1. Parasitaemia Profile after Etanercept Treatment
3.2. TNF-α and Liver Triglyceride Level Increases during Py MDR Infection
3.3. Etanercept Treatment Reduces Liver Triglyceride, MDA (Malondialdehyde), and Serum TNF-α in Py MDR Infected Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monroe, A.; Williams, N.A.; Ogoma, S.; Karema, C.; Okumu, F. Reflections on the 2021 World Malaria Report and the future of malaria control. Malar. J. 2022, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Smithuis, F.M.; Woodrow, C.; Seidlein, L.V. How to Contain Artemisinin- and Multidrug-Resistant Falciparum Malaria. Trends Parasitol. 2017, 33, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Culleton, R.; Cao, J. Artemisinin-Resistant Plasmodium falciparum in Africa. N. Engl. J. Med. 2017, 377, 306. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A.; Lee, Y.S. Mosquirix RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines 2022, 10, 713. [Google Scholar] [CrossRef]
- Bajpai, R.; Dutta, G.P. Role of fatty infiltration in pathology of malaria. Life Sci. 1990, 47, 25–28. [Google Scholar] [CrossRef]
- Siddiqi, N.J.; Puri, S.K.; Dutta, G.P.; Maheshwari, R.K.; Pandey, V.C. Studies on hepatic oxidative stress and antioxidant defence system during chloroquine/poly ICLC treatment of Plasmodium yoelii nigeriensis infected mice. Mol. Cell Biochem. 1999, 194, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Vallochi, A.L.; Teixeira, L.; Oliveira, K.D.S.; Maya-Monteiro, C.M.; Bozza, P.T. Lipid Droplet, a Key Player in Host-Parasite Interactions. Front. Immunol. 2018, 9, 1022. [Google Scholar] [CrossRef]
- Asad, M.; Yamaryo-Botte, Y.; Hossain, M.E.; Thakur, V.; Jain, S.; Datta, G.; Botte, C.Y.; Mohmmed, A. An essential vesicular-trafficking phospholipase mediates neutral lipid synthesis and contributes to hemozoin formation in Plasmodium falciparum. BMC Biol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Ge, S.; Jiang, X.; Paul, D.; Song, L.; Wang, X.; Pachter, J.S. Human ES-derived MSCs correct TNF-alpha-mediated alterations in a blood-brain barrier model. Fluids Barriers CNS 2019, 16, 18. [Google Scholar] [CrossRef]
- Turner, G.D.; Morrison, H.; Jones, M.; Davis, T.M.; Looareesuwan, S.; Buley, I.D.; Gatter, K.C.; Newbold, C.I.; Pukritayakamee, S.; Nagachinta, B.; et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 1994, 145, 1057–1069. [Google Scholar]
- Pappa, S.; Hatzistilianou, M.; Kouvatsi, A.; Pantzartzi, C.; Sakellaropoulou, A.; Pavlou, E.; Mavromichales, I.; Athanassiadou, F. Tumour necrosis factor gene polymorphisms and migraine in Greek children. Arch. Med. Sci. 2010, 6, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cao, Y.; Bell, B.; Chen, X.; Weiss, R.M.; Felder, R.B.; Wei, S.G. Brain TACE (Tumor Necrosis Factor-alpha-Converting Enzyme) Contributes to Sympathetic Excitation in Heart Failure Rats. Hypertension 2019, 74, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Buks, J.; Wilczak, M.; Rzymski, P.; Opala, T. Do soluble p55 and p75 TNF-alpha receptor concentrations play a role in women with primary sterility? Arch. Med. Sci. 2010, 6, 264–269. [Google Scholar] [CrossRef]
- Marotte, H.; Cimaz, R. Etanercept—TNF receptor and IgG1 Fc fusion protein: Is it different from other TNF blockers? Expert Opin. Biol. Ther. 2014, 14, 569–572. [Google Scholar] [CrossRef]
- Zhao, S.; Mysler, E.; Moots, R.J. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy 2018, 10, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Von Brand, T.; Mercado, T.I. Quantitative and histochemical studies on liver lipids of rats infected with Plasmodium berghei. Am. J. Hyg. 1958, 67, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Utley, H.G.; Bernheim, F.; Hochstein, P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967, 18, 4. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Grau, G.E.; Taylor, T.E.; Molyneux, M.E.; Wirima, J.J.; Vassalli, P.; Hommel, M.; Lambert, P.H. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 1989, 320, 1586–1591. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N. Anti-TNF alpha therapy of rheumatoid arthritis: What have we learned? Annu. Rev. Immunol. 2001, 19, 163–196. [Google Scholar] [CrossRef] [PubMed]
- Victor, F.C.; Gottlieb, A.B.; Menter, A. Changing paradigms in dermatology: Tumor necrosis factor alpha (TNF-alpha) blockade in psoriasis and psoriatic arthritis. Clin. Derm. 2003, 21, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Kremer, J.M.; Bankhurst, A.D.; Bulpitt, K.J.; Fleischmann, R.M.; Fox, R.I.; Jackson, C.G.; Lange, M.; Burge, D.J. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 1999, 340, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, E.M.; Ristow, B.; Gordon, S.M.; Aronowitz, P. Overwhelming parasitemia with Plasmodium falciparum infection in a patient receiving infliximab therapy for rheumatoid arthritis. Clin. Infect. Dis. 2007, 44, e82–e84. [Google Scholar] [CrossRef]
- De Sanctis, S.; Marcovecchio, M.L.; Gaspari, S.; Del Torto, M.; Mohn, A.; Chiarelli, F.; Breda, L. Etanercept improves lipid profile and oxidative stress measures in patients with juvenile idiopathic arthritis. J. Rheumatol. 2013, 40, 943–948. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Gawel, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57, 779S–785S; discussion 785S–786S. [Google Scholar] [CrossRef]
- Narsaria, N.; Mohanty, C.; Das, B.K.; Mishra, S.P.; Prasad, R. Oxidative stress in children with severe malaria. J. Trop. Pediatr. 2012, 58, 147–150. [Google Scholar] [CrossRef]
- Pabon, A.; Carmona, J.; Burgos, L.C.; Blair, S. Oxidative stress in patients with non-complicated malaria. Clin. Biochem. 2003, 36, 71–78. [Google Scholar] [CrossRef]
- Kageyama, Y.; Takahashi, M.; Nagafusa, T.; Torikai, E.; Nagano, A. Etanercept reduces the oxidative stress marker levels in patients with rheumatoid arthritis. Rheumatol. Int. 2008, 28, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Viriyavejakul, P.; Khachonsaksumet, V.; Punsawad, C. Liver changes in severe Plasmodium falciparum malaria: Histopathology, apoptosis and nuclear factor kappa B expression. Malar. J. 2014, 13, 106. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, B.S.; Gunjan, S.; Singh, S.K.; Pandey, S.K.; Tripathi, R. Effect of Etanercept on Plasmodium yoelii MDR-Induced Liver Lipid Infiltration. Future Pharmacol. 2022, 2, 499-510. https://doi.org/10.3390/futurepharmacol2040031
Chauhan BS, Gunjan S, Singh SK, Pandey SK, Tripathi R. Effect of Etanercept on Plasmodium yoelii MDR-Induced Liver Lipid Infiltration. Future Pharmacology. 2022; 2(4):499-510. https://doi.org/10.3390/futurepharmacol2040031
Chicago/Turabian StyleChauhan, Bhavana Singh, Sarika Gunjan, Sunil Kumar Singh, Swaroop Kumar Pandey, and Renu Tripathi. 2022. "Effect of Etanercept on Plasmodium yoelii MDR-Induced Liver Lipid Infiltration" Future Pharmacology 2, no. 4: 499-510. https://doi.org/10.3390/futurepharmacol2040031
APA StyleChauhan, B. S., Gunjan, S., Singh, S. K., Pandey, S. K., & Tripathi, R. (2022). Effect of Etanercept on Plasmodium yoelii MDR-Induced Liver Lipid Infiltration. Future Pharmacology, 2(4), 499-510. https://doi.org/10.3390/futurepharmacol2040031





