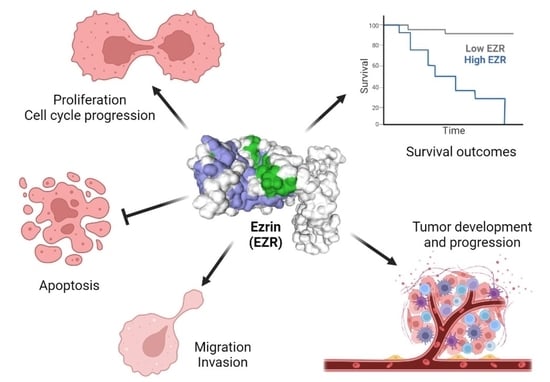
Perspectives for Targeting Ezrin in Cancer Development and Progression
Abstract
:1. Introduction
2. EZR Activation
3. Genomics of EZR
4. Expression and Functions for EZR in Human Cancers
4.1. Breast Cancer
4.2. Melanoma
4.3. Cervical Carcinoma
4.4. Colorectal Cancer
4.5. Endometrial Cancer
4.6. Gastric Cancer
4.7. Head and Neck Squamous Cell Carcinoma
4.8. Hepatocellular Carcinoma
4.9. Kidney Cancer
4.10. Leukemia
4.11. Lymphoma
4.12. Lung Cancer
4.13. Malignant Pleural Mesothelioma
4.14. Oral Squamous Cell Carcinoma
4.15. Ovarian Cancer
4.16. Pancreatic Cancer
4.17. Prostate Cancer
5. Concluding Remarks and Pharmacological Advances for EZR Inhibition
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neisch, A.L.; Fehon, R.G. Ezrin, Radixin and Moesin: Key regulators of membrane-cortex interactions and signaling. Curr. Opin. Cell Biol. 2011, 23, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, K.; Asano, S. Pathophysiological Roles of Actin-Binding Scaffold Protein, Ezrin. Int. J. Mol. Sci. 2022, 23, 3246. [Google Scholar] [CrossRef] [PubMed]
- Clucas, J.; Valderrama, F. ERM proteins in cancer progression. J. Cell Sci. 2014, 127, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, K.; Bruce, B.; Hewitt, S.; Thomas, D.; Khanna, C.; Helman, L.J. Ezrin mediates growth and survival in Ewing’s sarcoma through the AKT/mTOR, but not the MAPK, signaling pathway. Clin. Exp. Metastasis 2006, 23, 227–236. [Google Scholar] [CrossRef]
- Gautreau, A.; Poullet, P.; Louvard, D.; Arpin, M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 7300–7305. [Google Scholar] [CrossRef] [Green Version]
- Del Peso, L.; Gonzalez-Garcia, M.; Page, C.; Herrera, R.; Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278, 687–689. [Google Scholar] [CrossRef]
- Ivetic, A.; Ridley, A.J. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 2004, 112, 165–176. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho family proteins: Coordinating cell responses. Trends Cell Biol. 2001, 11, 471–477. [Google Scholar] [CrossRef]
- Li, N.; Kong, J.; Lin, Z.; Yang, Y.; Jin, T.; Xu, M.; Sun, J.; Chen, L. Ezrin promotes breast cancer progression by modulating AKT signals. Br. J. Cancer 2019, 120, 703–713. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.J.; Zhang, F.R.; Tao, L.H.; Lu, Z.; Xu, X.E.; Jian, S.; Xu, L.Y.; Li, E.M. Expression of ezrin in human embryonic, fetal, and normal adult tissues. J. Histochem. Cytochem. 2011, 59, 1001–1008. [Google Scholar] [CrossRef]
- Michie, K.A.; Bermeister, A.; Robertson, N.O.; Goodchild, S.C.; Curmi, P.M.G. Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition. Int. J. Mol. Sci. 2019, 20, 1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaseyed, T.; Viswanatha, R.; Sauvanet, C.; Filter, J.J.; Goldberg, M.L.; Bretscher, A. Ezrin activation by LOK phosphorylation involves a PIP(2)-dependent wedge mechanism. Elife 2017, 6, e22759. [Google Scholar] [CrossRef] [PubMed]
- Shabardina, V.; Kramer, C.; Gerdes, B.; Braunger, J.; Cordes, A.; Schafer, J.; Mey, I.; Grill, D.; Gerke, V.; Steinem, C. Mode of Ezrin-Membrane Interaction as a Function of PIP2 Binding and Pseudophosphorylation. Biophys. J. 2016, 110, 2710–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrig, A.; Janke, M.; Austermann, J.; Gerke, V.; Janshoff, A.; Steinem, C. Cooperative adsorption of ezrin on PIP2-containing membranes. Biochemistry 2006, 45, 13025–13034. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A.; Reczek, D.; Berryman, M. Ezrin: A protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 1997, 110 Pt 24, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, R.; Ohouo, P.Y.; Smolka, M.B.; Bretscher, A. Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 2012, 199, 969–984. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, S.; Xing, R.; Zhang, Q. High expression of EZR (ezrin) gene is correlated with the poor overall survival of breast cancer patients. Thorac. Cancer 2019, 10, 1953–1961. [Google Scholar] [CrossRef] [Green Version]
- Elliott, B.E.; Meens, J.A.; SenGupta, S.K.; Louvard, D.; Arpin, M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast. Cancer Res. 2005, 7, R365. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wu, M.; Wang, H.; Xu, G.; Zhu, T.; Zhang, Y.; Liu, P.; Song, A.; Gang, C.; Han, Z.; et al. Ezrin silencing by small hairpin RNA reverses metastatic behaviors of human breast cancer cells. Cancer Lett. 2008, 261, 55–63. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, T. Clinical implications of Ezrin and CD44 coexpression in breast cancer. Oncol. Rep. 2013, 30, 1899–1905. [Google Scholar] [CrossRef]
- Ilmonen, S.; Vaheri, A.; Asko-Seljavaara, S.; Carpen, O. Ezrin in primary cutaneous melanoma. Mod. Pathol. 2005, 18, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Federici, C.; Brambilla, D.; Lozupone, F.; Matarrese, P.; de Milito, A.; Lugini, L.; Iessi, E.; Cecchetti, S.; Marino, M.; Perdicchio, M.; et al. Pleiotropic function of ezrin in human metastatic melanomas. Int. J. Cancer 2009, 124, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Lozupone, F.; Matarrese, P.; Funaro, C.; Luciani, F.; Malorni, W.; Rivoltini, L.; Castelli, C.; Tinari, A.; Piris, A.; et al. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: A key role of ezrin. Lab. Invest. 2003, 83, 1555–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, G. MicroRNA-183 inhibits A375 human melanoma cell migration and invasion by targeting Ezrin and MMP-9. Oncol. Lett. 2019, 17, 548–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.; Di, C.; Piao, J.; Sun, J.; Han, L.; Chen, L.; Yan, G.; Lin, Z. Ezrin contributes to cervical cancer progression through induction of epithelial-mesenchymal transition. Oncotarget 2016, 7, 19631–19642. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Li, Y.; Liu, S.; Jin, H.; Shang, Y.; Quan, C.; Lin, Z. High expression of ezrin predicts poor prognosis in uterine cervical cancer. BMC Cancer 2013, 13, 520. [Google Scholar] [CrossRef] [Green Version]
- Auvinen, E.; Carpen, O.; Korpela, T.; Ronty, M.; Vaheri, A.; Tarkkanen, J. Altered expression of ezrin, E-Cadherin and beta-Catenin in cervical neoplasia. Neoplasma 2013, 60, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Greco, D.; Kivi, N.; Qian, K.; Leivonen, S.K.; Auvinen, P.; Auvinen, E. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS ONE 2011, 6, e21646. [Google Scholar] [CrossRef] [Green Version]
- Patara, M.; Santos, E.M.; Coudry Rde, A.; Soares, F.A.; Ferreira, F.O.; Rossi, B.M. Ezrin expression as a prognostic marker in colorectal adenocarcinoma. Pathol. Oncol. Res. 2011, 17, 827–833. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Chen, B.; Chen, S.; Jiang, Z.; Zhou, T.; Hou, Z.; Wang, Y. Ezrin/NF-kB activation regulates epithelial- mesenchymal transition induced by EGF and promotes metastasis of colorectal cancer. Biomed. Pharmacother. 2017, 92, 140–148. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhu, J.S.; Zhang, Q.; Sun, Q.; Guo, H. High level of ezrin expression in colorectal cancer tissues is closely related to tumor malignancy. World J. Gastroenterol. 2009, 15, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Ben-Shmuel, A.; Lemmon, V.; Brabletz, T.; Ben-Ze’ev, A. Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J. Cell Sci. 2010, 123, 2135–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiphrakpam, P.D.; Rajput, A.; Mathiesen, M.; Agarwal, E.; Lazenby, A.J.; Are, C.; Brattain, M.G.; Chowdhury, S. Ezrin expression and cell survival regulation in colorectal cancer. Cell Signal 2014, 26, 868–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtani, K.; Sakamoto, H.; Rutherford, T.; Chen, Z.; Satoh, K.; Naftolin, F. Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 1999, 147, 31–38. [Google Scholar] [CrossRef]
- Kobel, M.; Langhammer, T.; Huttelmaier, S.; Schmitt, W.D.; Kriese, K.; Dittmer, J.; Strauss, H.G.; Thomssen, C.; Hauptmann, S. Ezrin expression is related to poor prognosis in FIGO stage I endometrioid carcinomas. Mod. Pathol. 2006, 19, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Bal, N.; Yildirim, S.; Nursal, T.Z.; Bolat, F.; Kayaselcuk, F. Association of ezrin expression in intestinal and diffuse gastric carcinoma with clinicopathological parameters and tumor type. World J. Gastroenterol. 2007, 13, 3726–3729. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.Y.; Zhao, Z.S.; Ma, J. Ezrin is associated with gastric cancer progression and prognosis. Pathol. Oncol. Res. 2011, 17, 909–915. [Google Scholar] [CrossRef]
- Jin, J.; Jin, T.; Quan, M.; Piao, Y.; Lin, Z. Ezrin overexpression predicts the poor prognosis of gastric adenocarcinoma. Diagn. Pathol. 2012, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Madan, R.; Brandwein-Gensler, M.; Schlecht, N.F.; Elias, K.; Gorbovitsky, E.; Belbin, T.J.; Mahmood, R.; Breining, D.; Qian, H.; Childs, G.; et al. Differential tissue and subcellular expression of ERM proteins in normal and malignant tissues: Cytoplasmic ezrin expression has prognostic signficance for head and neck squamous cell carcinoma. Head Neck 2006, 28, 1018–1027. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Brandwein-Gensler, M.; Smith, R.V.; Kawachi, N.; Broughel, D.; Lin, J.; Keller, C.E.; Reynolds, P.A.; Gunn-Moore, F.J.; Harris, T.; et al. Cytoplasmic ezrin and moesin correlate with poor survival in head and neck squamous cell carcinoma. Head Neck Pathol. 2012, 6, 232–243. [Google Scholar] [CrossRef]
- Bakheet, A.M.H.; Mahmoud, S.A.; Huang, Y.; Zhang, J.; Wang, J.; Wei, Y.; Gamallat, Y.; Awadasseid, A.; Owusu, L.; Khidir, Y.; et al. Ezrin as a possible diagnostic and/or prognostic biomarker in mice lymphatic metastatic hepatocellular carcinoma in vivo. Biofactors 2017, 43, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, S.; Ye, H.; Xu, S.; Ye, G. Ezrin expression in the primary hepatocellular carcinoma patients and associated with clinical, pathological characteristics. J. Cancer Res. Ther. 2016, 12, 291–294. [Google Scholar]
- Kang, Y.K.; Hong, S.W.; Lee, H.; Kim, W.H. Prognostic implications of ezrin expression in human hepatocellular carcinoma. Mol. Carcinog. 2010, 49, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, N.; Han, A.; Wang, Y.; Lin, Z.; Yang, Y. Ezrin promotes hepatocellular carcinoma progression by modulating glycolytic reprogramming. Cancer Sci. 2020, 111, 4061–4074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.Y.; Wu, W.Z.; Wang, Z.J.; Zhou, K.; Zha, X.L.; Liu, K.D. The membrane-cytoskeleton organizer ezrin is necessary for hepatocellular carcinoma cell growth and invasiveness. J. Cancer Res. Clin. Oncol. 2006, 132, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Song, X.; Li, Y.; Cao, Y.; Chu, F.; Durojaye, O.A.; Su, Z.; Shi, X.; Wang, J.; Cheng, J.; et al. Celastrol inhibits ezrin-mediated migration of hepatocellular carcinoma cells. Sci. Rep. 2020, 10, 11273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Guo, Z.; Zhao, J.; Wu, B.; Deng, H.; Zhou, T.; Xiang, H.; Gao, F.; Yu, X.; et al. Rho kinase phosphorylation promotes ezrin-mediated metastasis in hepatocellular carcinoma. Cancer Res. 2011, 71, 1721–1729. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Yang, C.; Du, P.; Zhang, J.L.; Zhang, J.C. Effects of arsenic trioxide on the expression of ezrin in hepatocellular carcinoma. Medicine 2017, 96, e7602. [Google Scholar] [CrossRef]
- Cetin, B.; Gonul, I.I.; Gumusay, O.; Afsar, B.; Bilgetekin, I.; Ozet, A.; Uner, A. Ezrin is a prognostic biomarker in patients with clear cell metastatic renal cell carcinoma receiving sunitinib. J. Cancer Res. Ther. 2021, 17, 408–413. [Google Scholar] [CrossRef]
- Ferrari, M.V.O.; da Costa, W.H.; Matushita, M.A.M.; Meduna, R.R.; Brazao, E.S., Jr.; Bezerra, S.M.; da Cunha, I.W.; Zequi, S.C. Immunohistochemical negative expression of ezrin predicts poor prognosis in clear cell renal cell carcinoma. Urol. Oncol. 2020, 38, 75.e1–75.e7. [Google Scholar] [CrossRef]
- Altaf, E.; Huang, X.; Xiong, J.; Yang, X.; Deng, X.; Xiong, M.; Zhou, L.; Pan, S.; Yuan, W.; Li, X.; et al. NHE1 has a notable role in metastasis and drug resistance of T-cell acute lymphoblastic leukemia. Oncol. Lett. 2017, 14, 4256–4262. [Google Scholar] [CrossRef] [PubMed]
- Habif, G.; Grasset, M.F.; Kieffer-Jaquinod, S.; Kuhn, L.; Mouchiroud, G.; Gobert-Gosse, S. Phosphoproteome analyses reveal specific implications of Hcls1, p21-activated kinase 1 and Ezrin in proliferation of a myeloid progenitor cell line downstream of wild-type and ITD mutant Fms-like tyrosine kinase 3 receptors. J. Proteomics 2013, 78, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Cotter, T.G. FLT3-driven redox-modulation of Ezrin regulates leukaemic cell migration. Free Radic Res. 2013, 47, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Monni, R.; Haddaoui, L.; Naba, A.; Gallais, I.; Arpin, M.; Mayeux, P.; Moreau-Gachelin, F. Ezrin is a target for oncogenic Kit mutants in murine erythroleukemia. Blood 2008, 111, 3163–3172. [Google Scholar] [CrossRef] [Green Version]
- Lipreri da Silva, J.C.; Coelho-Silva, J.L.; Lima, K.; Vicari, H.P.; Lazarini, M.; Costa-Lotufo, L.V.; Traina, F.; Machado-Neto, J.A. Comprehensive analysis of cytoskeleton regulatory genes identifies ezrin as a prognostic marker and molecular target in acute myeloid leukemia. Cell Oncol. 2021, 44, 1105–1117. [Google Scholar] [CrossRef]
- Lipreri da Silva, J.C.; Saldanha-Araujo, F.; de Melo, R.C.B.; Vicari, H.P.; Silva-Carvalho, A.E.; Rego, E.M.; Buccheri, V.; Machado-Neto, J.A. Ezrin is highly expressed and a druggable target in chronic lymphocytic leukemia. Life Sci. 2022, 311, 121146. [Google Scholar] [CrossRef]
- Pore, D.; Bodo, J.; Danda, A.; Yan, D.; Phillips, J.G.; Lindner, D.; Hill, B.T.; Smith, M.R.; Hsi, E.D.; Gupta, N. Identification of Ezrin-Radixin-Moesin proteins as novel regulators of pathogenic B-cell receptor signaling and tumor growth in diffuse large B-cell lymphoma. Leukemia 2015, 29, 1857–1867. [Google Scholar] [CrossRef] [Green Version]
- Pore, D.; Gupta, N. The ezrin-radixin-moesin family of proteins in the regulation of B-cell immune response. Crit. Rev. Immunol. 2015, 35, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Kim, E.H.; Oh, M.H. Clinicopathologic implication of ezrin expression in non-small cell lung cancer. Korean J. Pathol. 2012, 46, 470–477. [Google Scholar] [CrossRef]
- Jin, T.; Jin, J.; Li, X.; Zhang, S.; Choi, Y.H.; Piao, Y.; Shen, X.; Lin, Z. Prognostic implications of ezrin and phosphorylated ezrin expression in non-small cell lung cancer. BMC Cancer 2014, 14, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolegova, E.S.; Kakurina, G.V.; Kostromitskiy, D.N.; Dobrodeev, A.Y.; Kondakova, I.V. Increases in mRNA and Protein Levels of the Genes for the Actin-Binding Proteins Profilin, Fascin, and Ezrin Promote Metastasis in Non-Small Cell Lung Cancer. Mol. Biol. 2020, 54, 285–292. [Google Scholar] [CrossRef]
- Pignochino, Y.; Dell’Aglio, C.; Inghilleri, S.; Zorzetto, M.; Basirico, M.; Capozzi, F.; Canta, M.; Piloni, D.; Cemmi, F.; Sangiolo, D.; et al. The combination of sorafenib and everolimus shows antitumor activity in preclinical models of malignant pleural mesothelioma. BMC Cancer 2015, 15, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safi, A.F.; Nickenig, H.J.; Rothamel, D.; Zirk, M.; Thiele, O.; Grandoch, A.; Scheer, M.; Zinser, M.; Zoller, J.; Drebber, U.; et al. Expression of ezrin in oral squamous cell carcinoma: Prognostic impact and clinicopathological correlations. J. Craniomaxillofac. Surg. 2015, 43, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamamoto, H.; Mukaisho, K.; Sato, S.; Higo, T.; Hattori, T.; Yamamoto, G.; Sugihara, H. Mechanisms underlying cancer progression caused by ezrin overexpression in tongue squamous cell carcinoma. PLoS ONE 2013, 8, e54881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noi, M.; Mukaisho, K.I.; Yoshida, S.; Murakami, S.; Koshinuma, S.; Adachi, T.; Machida, Y.; Yamori, M.; Nakayama, T.; Yamamoto, G.; et al. ERK phosphorylation functions in invadopodia formation in tongue cancer cells in a novel silicate fibre-based 3D cell culture system. Int. J. Oral. Sci. 2018, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Noi, M.; Mukaisho, K.I.; Murakami, S.; Koshinuma, S.; Machida, Y.; Yamori, M.; Nakayama, T.; Ogawa, T.; Nakata, Y.; Shimizu, T.; et al. Expressions of ezrin, ERK, STAT3, and AKT in tongue cancer and association with tumor characteristics and patient survival. Clin. Exp. Dent. Res. 2020, 6, 420–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assao, A.; Nonogaki, S.; Lauris, J.R.P.; Carvalho, A.L.; Pinto, C.A.L.; Soares, F.A.; Kowalski, L.P.; Oliveira, D.T. Podoplanin, ezrin, and Rho-A proteins may have joint participation in tumor invasion of lip cancer. Clin. Oral. Investig. 2017, 21, 1647–1657. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Sun, L.; Fan, S.; Huang, Z.; Zhang, D.; Yang, Z.; Li, J.; Chen, W. Akt/Ezrin Tyr353/NF-kappaB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br. J. Cancer 2014, 110, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Fadiel, A.; Edusa, V.; Chen, Z.; So, J.; Sakamoto, H.; Fishman, D.A.; Naftolin, F. Estradiol-induced ezrin overexpression in ovarian cancer: A new signaling domain for estrogen. Cancer Lett. 2005, 220, 57–65. [Google Scholar] [CrossRef]
- Kobel, M.; Gradhand, E.; Zeng, K.; Schmitt, W.D.; Kriese, K.; Lantzsch, T.; Wolters, M.; Dittmer, J.; Strauss, H.G.; Thomssen, C.; et al. Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int. J. Gynecol. Pathol. 2006, 25, 121–130. [Google Scholar] [CrossRef]
- Li, M.J.; Xiong, D.; Huang, H.; Wen, Z.Y. Ezrin Promotes the Proliferation, Migration, and Invasion of Ovarian Cancer Cells. Biomed. Environ. Sci. 2021, 34, 139–151. [Google Scholar] [PubMed]
- Horwitz, V.; Davidson, B.; Stern, D.; Trope, C.G.; Tavor Re’em, T.; Reich, R. Ezrin Is Associated with Disease Progression in Ovarian Carcinoma. PLoS ONE 2016, 11, e0162502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Feng, Y.; Tao, K.; Su, Z.; Yu, X.; Zheng, J.; Zhang, L.; Yang, D. The expression and phosphorylation of ezrin and merlin in human pancreatic cancer. Int. J. Oncol. 2014, 44, 2059–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, C.; Sun, J.; Lin, Z.; Jin, T.; Dong, B.; Meng, Z.; Piao, J. Ezrin promotes pancreatic cancer cell proliferation and invasion through activating the Akt/mTOR pathway and inducing YAP translocation. Cancer Manag. Res. 2019, 11, 6553–6566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Li, T.; Zhang, D.; Han, J. Expression of Ezrin and phosphorylated Ezrin (pEzrin) in pancreatic ductal adenocarcinoma. Cancer Invest. 2010, 28, 242–247. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, Z.; Yu, S.; Zhang, Q.; Ma, Y.; Chen, J. Ezrin promotes invasion and metastasis of pancreatic cancer cells. J. Transl. Med. 2010, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Lipreri da Silva, J.C.; Carvalho, M.F.L.; de Miranda, L.B.L.; de Almeida, B.O.; Lima, K.; Machado-Neto, J.A. NSC305787, a pharmacological ezrin inhibitor, exhibits antineoplastic activity in pancreatic cancer cells. Invest. New Drugs 2022, 40, 728–737. [Google Scholar] [CrossRef]
- Valdman, A.; Fang, X.; Pang, S.T.; Nilsson, B.; Ekman, P.; Egevad, L. Ezrin expression in prostate cancer and benign prostatic tissue. Eur. Urol. 2005, 48, 852–857. [Google Scholar] [CrossRef]
- Pang, S.T.; Fang, X.; Valdman, A.; Norstedt, G.; Pousette, A.; Egevad, L.; Ekman, P. Expression of ezrin in prostatic intraepithelial neoplasia. Urology 2004, 63, 609–612. [Google Scholar] [CrossRef]
- Chuan, Y.C.; Pang, S.T.; Cedazo-Minguez, A.; Norstedt, G.; Pousette, A.; Flores-Morales, A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J. Biol. Chem. 2006, 281, 29938–29948. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wang, J.; Lu, Y.; Lai, C.; Qu, L.; Zhuo, Y. Ezrin expression in circulating tumor cells is a predictor of prostate cancer metastasis. Bioengineered 2022, 13, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Chen, R.; Yang, T.N.; Zhang, F.; Zhao, D. Baicalein inhibits the proliferative activity of human prostate cancer cell line PC3 by downregulating Ezrin. J. Biol. Regul. Homeost. Agents 2020, 34, 885–892. [Google Scholar] [PubMed]
- Bulut, G.; Hong, S.H.; Chen, K.; Beauchamp, E.M.; Rahim, S.; Kosturko, G.W.; Glasgow, E.; Dakshanamurthy, S.; Lee, H.S.; Daar, I.; et al. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene 2012, 31, 269–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celik, H.; Bulut, G.; Han, J.; Graham, G.T.; Minas, T.Z.; Conn, E.J.; Hong, S.H.; Pauly, G.T.; Hayran, M.; Li, X.; et al. Ezrin Inhibition Up-regulates Stress Response Gene Expression. J. Biol. Chem. 2016, 291, 13257–13270. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, K.; Sakamoto, H.; Rutherford, T.; Chen, Z.; Kikuchi, A.; Yamamoto, T.; Satoh, K.; Naftolin, F. Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 2002, 179, 79–86. [Google Scholar] [CrossRef]




| Publications | Cancer Type | Cell Lines | Primary Tissues | Techniques | Notes |
|---|---|---|---|---|---|
| Zhang et al. [17] | Breast cancer | No | Yes, 120 samples from breast cancer patients. | IHC | EZR was upregulated in breast cancer and may be used as a potential biomarker for overall survival. |
| Li et al. [19] | Breast cancer | Yes, MCF-7, MDA-MB-453, MDA-MB-435s, and MDA-MB-231 | Yes, tumor samples from 23 patients with invasive human ductal breast cancer. | RT-PCR, IHC | EZR protein expression was significantly higher in primary cancer tissues than in lymph node metastases. |
| Ilmonen et al. [21] | Melanoma | No | Yes, 95 samples from patients with primary melanoma of the skin. | IHC | EZR expression correlated with tumor thickness and level of invasion. |
| Kong et al. [26] | Cervical cancer | No | Yes, 586 samples were collected from routinely processed and diagnosed uterine cervical lesion tissues from pretreatment surgical resections. | qRT-PCR, IHC | EZR was a potentially effective predictor of the poor prognosis of cervical cancer patients, especially those with early-stage disease. The determination of EZR expression levels may help to identify high-risk cervical cancer. |
| Patara et al. [29] | Colorectal cancer | No | Yes, samples from tumors and construction of a TMA. | TMA, IHC | Increased cytoplasmatic EZR expression correlated with higher tumor aggressiveness and a worse prognosis in colorectal cancer patients. |
| Gavert et al. [32] | Colorectal cancer | Yes, Ls174T and SW620 | Yes, samples from tumor and normal colon tissues. | Invading tumor cells expressed high EZR levels. | |
| Leiphrakpam et al. [33]. | Colorectal cancer | Yes, GEO, GEORI, CBS, HCT166, and HCT166b | Yes, samples of tumors and construction of a TMA. | TMA, IHC | Increased expression of p-EZRT567 was found in liver metastasis of orthotopic implantation studies in vivo and IHC studies of human CRC patient specimens. |
| Ohtani et al. [85] | Endometrial cancer | No | Yes, 20 samples from uterine endometrioid adenocarcinoma, 7 samples from simple endometrial hyperplasias, 7 samples from complex endometrial hyperplasias, 7 samples from atypical endometrial hyperplasias, and 12 samples of normal endometrium. | IHC | EZR protein was explicitly expressed in uterine endometrioid adenocarcinoma and its precursor lesions. EZR protein expression in metastatic lesions increased and shifted from the cytosol to the membrane. |
| Bal et al. [36]. | Gastric cancer | No | Yes, samples from 53 intestinal-type adenocarcinoma and 22 diffuse-type carcinoma patients. | IHC | H. pylori-infected gastric carcinomas presented increased EZR expression. |
| Li et al. [37] | Gastric cancer | No | Yes, samples from 436 gastric cancer patients. | TMA, IHC | EZR was upregulated in gastric cancer tissues compared with normal gastric tissues and correlated significantly with prognosis. In addition, high levels of EZR expression were associated with age, tumor size, location, depth of invasion, vessel invasion, lymph node and distant metastasis, and TNM stage. |
| Schlecht et al. [40] | Head and neck squamous cell carcinoma | No | Yes, samples from 128 primary HNSCC were collected at initial diagnosis and treatment. | TMA, global RNA expression | No association with prognosis for HNSCC was found due to the inherent heterogeneity in disease management. |
| Lee et al. [59] | Lung cancer | No | Yes, 112 NSCLC specimens. | TMA, IHC | EZR was overexpressed in tumor tissues. EZR expression correlated with pleural invasion and pathological stage. The negative group for EZR expression presented more prolonged survival. |
| Jin et al. [60] | Lung cancer | No | Yes, samples from 150 NSCLC tumors and 14 normal lungs. | IHC, qRT-PCR | EZR was upregulated in NSCLC. Increased EZR levels correlated with NSCLC late stage and poor differentiation. p-EZRT567 correlated with the presence of lymph node metastases. EZR was associated with reduced survival time for patients with early-stage NSCLC. |
| Kolegova et al. [61] | Lung cancer | No | Yes, samples from 46 NSCLC patients | qRT-PCR, Western blotting | Increased EZR gene and protein expression was found in patients with distant metastasis. |
| Lipreri da Silva et al. [55] | Acute myeloid leukemia | Yes, Kasumi-1 and MOLM-13 | Yes, the cohort deposited in TCGA. | Bioinformatics | EZR expression was a prognostic marker in AML. In intermediate-risk AML patients, high EZR expression distinguished a group with a poor prognosis. |
| Lipreri da Silva et al. [56] | Chronic lymphocytic leukemia | Yes, MEC-1 | Yes, samples from 56 CLL patients and ten age-matched healthy donors. | Bioinformatics, cDNA microarray, qPCR | EZR was highly expressed and positively associated with relevant signaling pathways related to CLL development and progression, including TP53, PI3K/AKT/mTOR, NFκB, and MAPK. |
| Safi et al. [63] | Oral squamous cell carcinoma | No | Yes, resection specimens from 80 treatment-naive OSCC patients. | IHC | EZR expression had a significant impact on overall survival. Increased EZR expression was associated with malignant progression, leading to a higher risk of metastases. |
| Saito et al. [64] | Oral squamous cell carcinoma | Yes, HSC-3 | Yes, 79 samples (10 normal tongue tissues and 69 tongue SCC tissues) | IHC, microarray | EZR was overexpressed in 46.4% of the tumors examined. EZR expression was correlated with proliferative activity. |
| Noi et al. [65] | Oral squamous cell carcinoma | Yes, HSC-3 and HSC-4 (3D culture) | Yes, human tongue cancer tissue (CIS (12 cases) and invasive SCC of the tongue (T1: ten cases, T2: three cases)). | IHC | Higher expression of EZR was found in invasive squamous cell carcinoma than in carcinoma in situ. |
| Noi et al. [66] | Oral squamous cell carcinoma | No | Yes, in situ tongue carcinoma patients (CIS, n = 17) and tongue squamous cell carcinoma patients (SCC, n = 46). | IHC | EZR expression was highly expressed in the cell membrane. EZR appears to be involved in the progress from in situ carcinoma in the tongue into squamous cell carcinoma. |
| Assao et al. [58] | Oral squamous cell carcinoma | No | Yes, samples of 91 patients with primary squamous cell carcinoma of the lower lip. | IHC | EZR expression in squamous cell carcinoma of the lip suggests the participation of this protein in cell movement and invasion. |
| Wang et al. [68] | Oral squamous cell carcinoma | Yes, SCC9 and SCC25 | Yes, primary tongue carcinomas collected from 63 patients. | IHC | EZR may be a therapeutic target to reverse EMT in tongue cancers and prevent TSCC progression. EZR activation was associated with metastasis and poor patient survival in TSCCs. |
| Köbel et al. [70] | Ovarian cancer | Yes, SKOV-3 | Yes, ovarian carcinoma samples from 105 patients. | IHC | EZR was overexpressed in 49% of the samples. EZR expression correlated with reduced overall survival and appeared as an independent risk factor. |
| Li et al. [71] | Ovarian cancer | Yes, SKOV3 and CaOV3 | No. | Western blotting, qPCR | EZR was overexpressed in either CaOV3 or SKOV3. |
| Horwitz et al. [72]. | Ovarian cancer | Yes, ES2 and OVCAR3 | Yes, 93 effusions (76 peritoneal, 17 pleural) from 93 patients diagnosed with high-grade serous carcinoma (HGSC). | qRT-PCR | High expression of EZR was found in tumors. EZR expression in effusions was unrelated to clinical outcome. |
| Zhou et al. [73] | Pancreatic cancer | Yes, SW1990 | Yes, 19 samples were obtained from patients undergoing surgical resection. | IHC, plasmid transfection, target gene expression | The expression of phosphorylated EZR proteins was related to pancreatic cancer’s clinical and pathological features. Phosphorylated EZR induced a positive regulatory role in growth, adhesion, and invasion. |
| Liprei da Silva et al. [77] | Pancreatic cancer | No | Yes, the cohort was deposited in the TCGA. | Bioinformatics | High EZR expression predicted worse survival outcomes in the pancreatic adenocarcinoma cohort. |
| Valdman et al. [78] | Prostate cancer | No | Yes, 103 radical prostatectomy specimens. | IHC | EZR immunoreactivity in prostate cancers was moderate or strong in 70% of specimens. EZR expression was correlated with Gleason score and seminal vesicle invasion. |
| Pang et al. [79] | Prostate cancer | No | Yes, 19 high-grade prostatic intraepithelial neoplasia (HGPIN) samples obtained from radical prostatectomy specimens. | IHC | Immunoreactivity for EZR was absent or weak in benign prostatic epithelial cells. The immunostaining was moderate or strong in all HGPIN samples. |
| Chen et al. [81] | Prostate cancer | Yes, 22RV1 and PC-3 | Yes, samples from 80 prostate cancer patients. | qRT-PCR and IHC | Ezrin was significantly increased in prostate cancer tissues and 22RV1 and PC-3 cell samples. EZR expression in CTCs from patients with prostate cancer was significantly increased with metastatic grade. |
| Publication | Cancer Type | Cell Lines | Approach | Activity | Notes |
|---|---|---|---|---|---|
| Li et al. [19] | Breast cancer | MCF-7, MDA-MB-453, MDA-MB-435s, and MDA-MB-231. | shRNA | EZR downregulation | Decreased EZR expression reversed metastatic behaviors of human breast cancer cells by inducing c-SRC-mediated E-cadherin expression. |
| Federici et al. [22] | Melanoma | MM1, MM2, MM3, and HeLa. | Stable transfection | EZR deletion mutant | Expression of EZR deletion mutant comprising 146 N-terminal amino acids abolished metastatic dissemination. |
| Zhang et al. [24] | Melanoma | A375. | MiR-183 overexpression and knockdown | EZR downregulation | miR-183 inhibits A375 human melanoma cell migration and invasion, possibly through the downregulation of EZR. |
| Kong et al. [25] | Cervical carcinoma | HeLa, SiHa, C33A, and CaSki. | RNAi | EZR downregulation | EZR silencing inhibited the proliferation, migration, and invasion of uterine cervical cancer cells through epithelial–mesenchymal transition inhibition. |
| Li et al. [30] | Colorectal cancer | SW480 and SW116. | siRNA and shRNA | EZR downregulation | Inhibition of EZR reduced EGF-induced epithelial–mesenchymal transition and lung metastasis of colorectal cancer. |
| Leiphrakpam et al. [3] | Colorectal cancer | GEO, GEORI, CBS, HCT166, and HCT166b. | NSC668394 and NSC305787 | Pharmacological inhibition of EZR phosphorylation | EZR inhibitors were effective in dephosphorylating EZR at T567 and decreased XIAP levels in metastatic colorectal cancer cells. |
| Ohtani et al. [34]. | Endometrial cancer | Ishikawa and mEIIL. | ePONs | EZR downregulation | Inhibitory effect of ePONs on invasiveness results from EZR suppression. |
| Lipreri da Silva et al. [55] | Acute myeloid leukemia | Kasumi-1 and MOLM-13. | NSC305787 and NSC668394 | Pharmacological inhibition of EZR phosphorylation | EZR inhibition reduced cell viability, clonogenicity, phosphorylation of the AKT signaling pathway and increased apoptosis in acute myeloid leukemia cell lines. |
| Lipreri da Silva et al. [56] | Chronic lymphocytic leukemia | MEC-1 and primary patients’ cells. | NSC305787 | Pharmacological inhibition of EZR phosphorylation | NSC305787 reduced viability, clonogenicity, and cell cycle progression and induced apoptosis in chronic lymphocytic leukemia cells. Pharmacological EZR inhibition also attenuated ERK, S6RP, and NFκB activation. |
| Saito et al. [64] | Oral squamous cell carcinoma | HSC-3. | RNAi | EZR downregulation | EZR depletion significantly reduced cell proliferation, migration, and invasiveness and disturbed actin reorganization during podia formation. |
| Noi et al. [5] | Oral squamous cell carcinoma | Yes, HSC-3 and HSC-4 (3D culture). | siRNA | EZR downregulation | No marked morphological differences were observed upon EZR silencing. |
| Wang et al. [68]. | Oral squamous cell carcinoma | SCC9 and SCC25. | siRNA and lentivirus-mediated shRNA | EZR downregulation | EZR silencing reduced the invasion and migration of SCC9 and SCC25 cells. Downregulation of EZR also inhibited EGF-induced EMT in tongue squamous carcinoma cells. |
| Song et al. [69] | Ovarian cancer | SKOV3 and DOV13. | Estradiol treatment | Estrogen-induced EZR overexpression | Estrogen induced EZR over-expression and the invasiveness of OVCA cells in culture. |
| Köbel et al. [70] | Ovarian cancer | SKOV-3. | siRNA | EZR downregulation | EZR silencing reduced cell invasion in vitro. |
| Li et al. [71] | Ovarian cancer | SKOV3 and CaOV3. | siRNA and FLAG-EZR overexpression plasmid | EZR downregulation and EZR overexpression | EZR ectopic expression increased cell proliferation, invasiveness, and epithelial–mesenchymal transition. EZR knockdown prevented cell proliferation, invasiveness, and epithelial–mesenchymal transition. |
| Horwitz et al. [72]. | Ovarian cancer | ES2 and OVCAR3. | Lentivirus-mediated shRNA | EZR downregulation | Reduced EZR expression impaired ovarian cancer cells’ invasion ability and branching capacity. |
| Zhou et al. [73]. | Pancreatic cancer | SW1990. | Recombinant plasmids | EZR overexpression | Overexpression of T567D ezrin (p-EZR), a mutant that mimics permanent phosphorylation, in SW1990 cells increased proliferative capacity and growth rate. |
| Liprei da Silva et al. [77] | Pancreatic cancer | PANC-1, AsPC-1, and MIA PaCa-2. | NSC305787 | Pharmacological inhibition of EZR phosphorylation | Inhibition of EZR favors a molecular network that reduces proliferation and induces apoptosis in pancreatic cancer cells. |
| Chuan et al. [80] | Prostate cancer | LNCaP-FGC, PC-3 and PCa. | Androgen treatment, siRNA and VSV-G-tagged human wild-type EZR and Y353F-EZR mutant | Androgen-induced EZR expression, EZR downregulation, and EZR overexpression | Androgen treatment induces EZR expression and phosphorylation of ezrin in T567 and Y353. EZR inhibition function using short interference RNA or the overexpression of T567A and Y353F-EZR mutants significantly reduces androgen-induced Matrigel invasion. Androgens regulate EZR at transcriptional and posttranscriptional levels. |
| Chen et al. [81] | Prostate cancer | 22RV1 and PC-3. | EZR overexpression and si-EZR plasmids | EZR downregulation and EZR overexpression | EZR overexpression promoted the migratory and invasive abilities of 22RV1 and PC-3 cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipreri da Silva, J.C.; Vicari, H.P.; Machado-Neto, J.A. Perspectives for Targeting Ezrin in Cancer Development and Progression. Future Pharmacol. 2023, 3, 61-79. https://doi.org/10.3390/futurepharmacol3010005
Lipreri da Silva JC, Vicari HP, Machado-Neto JA. Perspectives for Targeting Ezrin in Cancer Development and Progression. Future Pharmacology. 2023; 3(1):61-79. https://doi.org/10.3390/futurepharmacol3010005
Chicago/Turabian StyleLipreri da Silva, Jean Carlos, Hugo Passos Vicari, and João Agostinho Machado-Neto. 2023. "Perspectives for Targeting Ezrin in Cancer Development and Progression" Future Pharmacology 3, no. 1: 61-79. https://doi.org/10.3390/futurepharmacol3010005
APA StyleLipreri da Silva, J. C., Vicari, H. P., & Machado-Neto, J. A. (2023). Perspectives for Targeting Ezrin in Cancer Development and Progression. Future Pharmacology, 3(1), 61-79. https://doi.org/10.3390/futurepharmacol3010005