Current Methods and Advances in the Immunotherapy Treatment of Non-Ovarian Gynaecological Cancers
Abstract
:1. Introduction
2. Methodology
3. Current Standard of Care
4. Genomic Alterations in Gynaecological Cancers
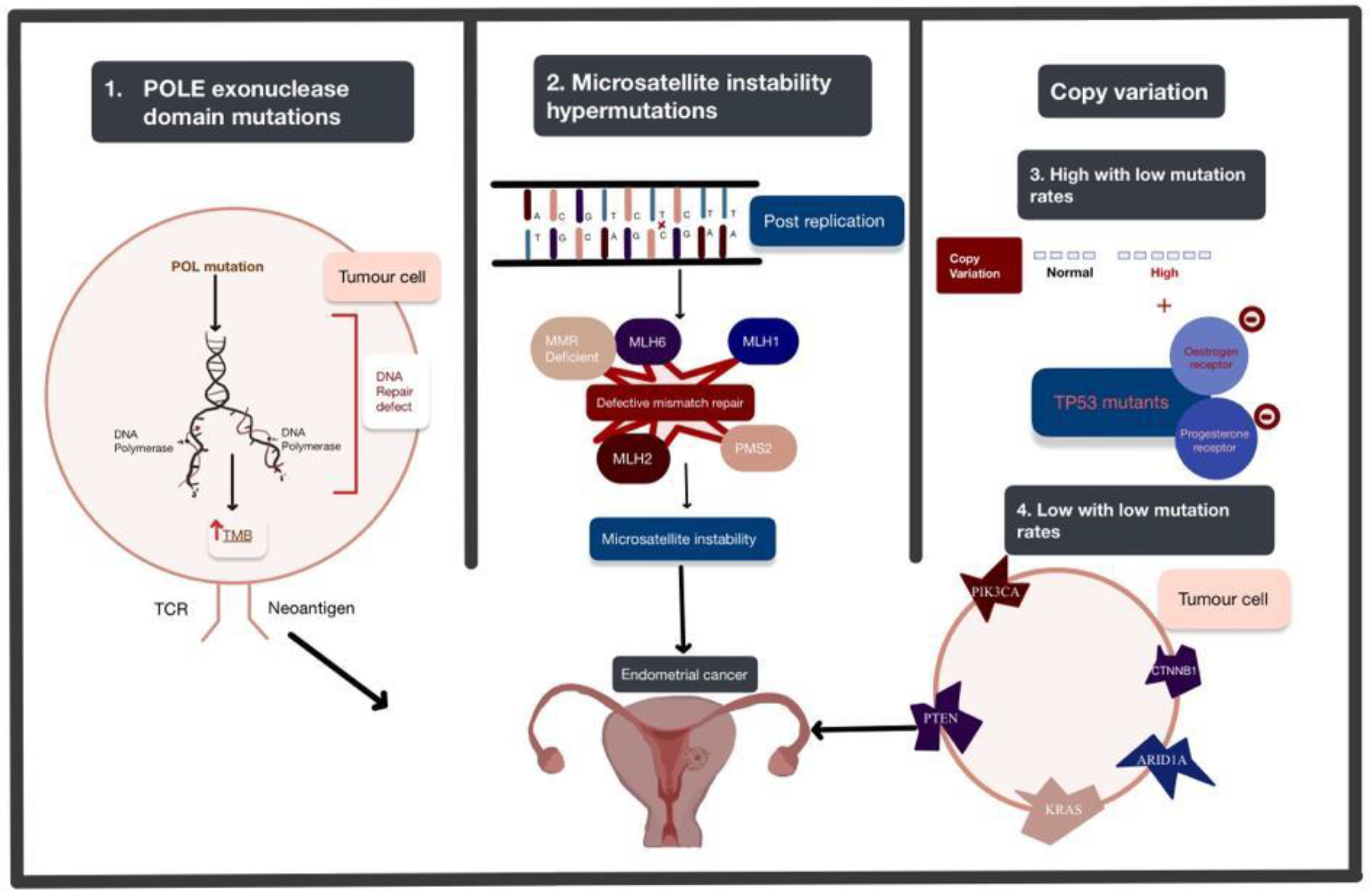
4.1. Endometrial Cancer
4.2. Cervical Cancer
4.3. Vulval Cancer
5. Rationale for Immunotherapy in Gynaecological Cancers
5.1. Endometrial Cancer
5.2. Cervical Cancer
5.3. Vulvar Cancer
6. Current Checkpoints in Clinical Practice
6.1. Endometrial Cancer
6.2. Cervical Cancer
6.3. Vulvar Cancer
7. Checkpoints in Development (Human Trials) and in Pre-clinical Development (Animal Studies)
7.1. Adoptive T-Cell Therapy
7.2. Cancer Vaccines
7.3. Novel Immune-Based Immunotherapy
7.4. Intra-Tumoural Oncolytic Viral Therapy
8. Future Directions
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levinson, K.; Dorigo, O.; Rubin, K.; Moore, K. Immunotherapy in Gynecologic Cancers: What We Know Now and Where We Are Headed. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e126–e140. [Google Scholar] [CrossRef] [PubMed]
- Olawaiye, A.B.; Cuello, M.A.; Rogers, L.J. Cancer of the vulva: 2021 update. Int. J. Gynaecol. Obs. 2021, 155 (Suppl. S1), 7–18. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Slomovitz, B.; Baldwin, P.J.W.; van Doorn, H.C.; van der Velden, J.; de Hullu, J.A.; Gaarenstroom, K.N.; Slangen, B.F.M.; Vergote, I.; Brännström, M.; et al. Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients with Micrometastases in the Sentinel Node: Results of GROINSS-V II. J. Clin. Oncol. 2021, 39, 3623–3632. [Google Scholar] [CrossRef]
- Gaunt, E.; Pounds, R.; Yap, J. Vulval cancer in pregnancy: Two case reports. Case Rep. Women’s Health 2021, 33, e00374. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Giorda, G.; Viel, A.; Fornasarig, M.; Zdjelar, A.; Segatto, E.; Sorio, R.; Corsetti, S.; Scalone, S.; Nicoloso, M.S.; et al. An Exceptional Response to Dostarlimab in Mismatch Repair Deficient, Microsatellite Instability-High and Platinum Refractory Endometrial Cancer. Curr. Oncol. 2022, 29, 5209–5212. [Google Scholar] [CrossRef]
- Lee, N.K.; Cheung, M.K.; Shin, J.Y.; Husain, A.; Teng, N.N.; Berek, J.S.; Kapp, D.S.; Osann, K.; Chan, J.K. Prognostic Factors for Uterine Cancer in Reproductive-Aged Women. Obstet. Gynecol. 2007, 109, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; et al. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. JNCI J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Capuano, A.; Bellastella, G.; Maiorino, M.I.; Giugliano, D. Metabolic syndrome and endometrial cancer: A meta-analysis. Endocrine 2014, 45, 28–36. [Google Scholar] [CrossRef]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T. Reproductive Factors and the Risk of Endometrial Cancer. Int. J. Gynecol. Cancer 2014, 24, 384. [Google Scholar] [CrossRef] [PubMed]
- Di Dio, C.; Bogani, G.; Di Donato, V.; Cuccu, I.; Muzii, L.; Musacchio, L.; Scambia, G.; Lorusso, D. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol. Oncol. 2023, 169, 27–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Saleh, M.; Virarkar, M.; Javadi, S.; Elsherif, S.B.; de Castro Faria, S.; Bhosale, P. Cervical Cancer: 2018 Revised International Federation of Gynecology and Obstetrics Staging System and the Role of Imaging. Am. J. Roentgenol. 2020, 214, 1182–1195. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Director-General Calls for All Countries to Take Action to Help End the Suffering Caused by Cervical Cancer. Available online: https://www.who.int/news/item/18-05-2018-who-dg-calls-for-all-countries-to-take-action-to-help-end-the-suffering-caused-by-cervical-cancer (accessed on 2 January 2023).
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.C.; et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef]
- Canfell, K.; Kim, J.J.; Brisson, M.; Keane, A.; Simms, K.T.; Caruana, M.; Burger, E.A.; Martin, D.; Nguyen, D.T.N.; Bénard, É.; et al. Mortality impact of achieving WHO cervical cancer elimination targets: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Cervical Screening: What Is the NHS Cervical Screening Programme? Available online: https://cks.nice.org.uk/topics/cervical-screening/background-information/the-cervical-screening-programme/ (accessed on 29 January 2023).
- Petry, K.U.; Liebrich, C.; Luyten, A.; Zander, M.; Iftner, T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 2017, 4, 85–89. [Google Scholar] [CrossRef]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Front. Oncol. 2020, 10, 606335. [Google Scholar] [CrossRef] [PubMed]
- Pilch, H.; Günzel, S.; Schäffer, U.; Tanner, B.; Brockerhoff, P.; Maeurer, M.; Höckel, M.; Hommel, G.; Knapstein, P.G. The presence of HPV DNA in cervical cancer: Correlation with clinico-pathologic parameters and prognostic significance: 10 years experience at the Department of Obstetrics and Gynecology of the Mainz University. Int. J. Gynecol. Cancer 2001, 11, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tan, Y.; Zhu, L.X.; Zhou, L.N.; Zeng, P.; Liu, Q.; Chen, M.B.; Tian, Y. Prognostic value of HPV DNA status in cervical cancer before treatment: A systematic review and meta-analysis. Oncotarget 2017, 8, 66352–66359. [Google Scholar] [CrossRef]
- Borella, F.; Preti, M.; Bertero, L.; Collemi, G.; Castellano, I.; Cassoni, P.; Cosma, S.; Carosso, A.R.; Bevilacqua, F.; Gallio, N.; et al. Is There a Place for Immune Checkpoint Inhibitors in Vulvar Neoplasms? A State of the Art Review. Int. J. Mol. Sci. 2020, 22, 190. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Vulvar Cancer. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 2 January 2023).
- de Sanjosé, S.; Alemany, L.; Ordi, J.; Tous, S.; Alejo, M.; Bigby, S.M.; Joura, E.A.; Maldonado, P.; Laco, J.; Bravo, I.G.; et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur. J. Cancer 2013, 49, 3450–3461. [Google Scholar] [CrossRef]
- Kang, Y.J.; Smith, M.; Barlow, E.; Coffey, K.; Hacker, N.; Canfell, K. Vulvar cancer in high-income countries: Increasing burden of disease. Int. J. Cancer 2017, 141, 2174–2186. [Google Scholar] [CrossRef]
- Woelber, L.; Mathey, S.; Prieske, K.; Kuerti, S.; Hillen, C.; Burandt, E.; Coym, A.; Mueller, V.; Schmalfeldt, B.; Jaeger, A. Targeted Therapeutic Approaches in Vulvar Squamous Cell Cancer (VSCC): Case Series and Review of the Literature. Oncol. Res. 2021, 28, 645–659. [Google Scholar] [CrossRef]
- Aravantinou-Fatorou, A.; Andrikopoulou, A.; Liontos, M.; Fiste, O.; Georgakopoulou, V.E.; Dimopoulos, M.A.; Gavriatopoulou, M.; Zagouri, F. Pembrolizumab in endometrial cancer: Where we stand now. Oncol. Lett. 2021, 22, 821. [Google Scholar] [CrossRef]
- Mauricio, D.; Zeybek, B.; Tymon-Rosario, J.; Harold, J.; Santin, A.D. Immunotherapy in Cervical Cancer. Curr. Oncol. Rep. 2021, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Biliatis, I.; Morice, P.; Reed, N.; Mangler, M.; Kesic, V.; Denschlag, D. European Society of Gynecological Oncology Task Force for Fertility Preservation: Clinical Recommendations for Fertility-Sparing Management in Young Endometrial Cancer Patients. Int. J. Gynecol. Cancer 2015, 25, 1258. [Google Scholar] [CrossRef]
- Gallos, I.D.; Yap, J.; Rajkhowa, M.; Luesley, D.M.; Coomarasamy, A.; Gupta, J.K. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2012, 207, e261–e266. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; Manusirivithaya, S.; Hanprasertpong, J. Fertility-Sparing in Endometrial Cancer. Gynecol. Obstet. Investig. 2009, 67, 250–268. [Google Scholar] [CrossRef]
- Podzielinski, I.; Randall, M.E.; Breheny, P.J.; Escobar, P.F.; Cohn, D.E.; Quick, A.M.; Chino, J.P.; Lopez-Acevedo, M.; Seitz, J.L.; Zook, J.E.; et al. Primary radiation therapy for medically inoperable patients with clinical stage I and II endometrial carcinoma. Gynecol. Oncol. 2012, 124, 36–41. [Google Scholar] [CrossRef]
- Fishman, D.A.; Roberts, K.B.; Chambers, J.T.; Kohorn, E.I.; Schwartz, P.E.; Chambers, S.K. Radiation Therapy as Exclusive Treatment for Medically Inoperable Patients with Stage I and II Endometrioid Carcinoma of the Endometrium. Gynecol. Oncol. 1996, 61, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Practice, B.B. Endometrial Cancer—Treatment Algorithm. Available online: https://bestpractice.bmj.com/topics/en-gb/266/treatment-algorithm (accessed on 5 January 2023).
- Hishida, T.; Masai, K.; Kaseda, K.; Asakura, K.; Asamura, H. Debulking surgery for malignant tumors: The current status, evidence and future perspectives. Jpn. J. Clin. Oncol. 2021, 51, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.; Balega, J.; Barwick, T.; Buckley, L.; Burton, K.; Eminowicz, G.; Forrest, J.; Ganesan, R.; Harrand, R.; Holland, C.; et al. British Gynaecological Cancer Society (BGCS) cervical cancer guidelines: Recommendations for practice. Eur. J. Obs. Gynecol. Reprod. Biol. 2021, 256, 433–465. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Cross, P.; Nayar, A.; Mayadevi, S.; Lopes, A.; Godfrey, K.; Hatem, H. Conservative surgical management of small-volume stage IB1 cervical cancer. BJOG: Int. J. Obstet. Gynaecol. 2007, 114, 958–963. [Google Scholar] [CrossRef]
- O’Donnell, R.; Naik, R. Conservative (non-radical) surgery for stage IB1 cervical cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021, 75, 54–64. [Google Scholar] [CrossRef]
- Rose, P.G.; Ali, S.; Watkins, E.; Thigpen, J.T.; Deppe, G.; Clarke-Pearson, D.L.; Insalaco, S. Long-Term Follow-Up of a Randomized Trial Comparing Concurrent Single Agent Cisplatin, Cisplatin-Based Combination Chemotherapy, or Hydroxyurea During Pelvic Irradiation for Locally Advanced Cervical Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 2804–2810. [Google Scholar] [CrossRef]
- Neoadjuvant Chemotherapy for Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: A systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur. J. Cancer 2003, 39, 2470–2486. [Google Scholar] [CrossRef]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L., 3rd; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C., Jr.; Clarke-Pearson, D.L.; Liao, S.Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Sill, M.W.; McMeekin, D.S.; Cohn, D.E.; Ramondetta, L.M.; Boardman, C.H.; Benda, J.; Cella, D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J. Clin. Oncol. 2009, 27, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Baldwin, P.; Buckley, L.; Cogswell, L.; Edey, K.; Faruqi, A.; Ganesan, R.; Hall, M.; Hillaby, K.; Reed, N.; et al. British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: Recommendations for practice. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 252, 502–525. [Google Scholar] [CrossRef] [PubMed]
- RCOG; BCGS. Guidelines for the Diagnosis and Management of Vulval Carcinoma-British Gynaecological Cancer Society & Royal College of Obstetricians & Gynaecologists. Available online: https://www.rcog.org.uk/media/dqwnw2a1/vulvalcancerguideline.pdf (accessed on 2 March 2023).
- BGCS. British Gynaecological Cancer Society Vulval Cancer Guidelines: Recommendations for Practice. Available online: https://www.bgcs.org.uk/wp-content/uploads/2021/07/BGCS-vulval-guidelines-v22.pdf (accessed on 2 March 2023).
- Levine, D.A.; Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M.; Kandoth, C.; Dooling, D.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Simon, M.; Giot, L.; Faye, G. The 3′ to 5′ exonuclease activity located in the DNA polymerase delta subunit of Saccharomyces cerevisiae is required for accurate replication. Embo J. 1991, 10, 2165–2170. [Google Scholar] [CrossRef]
- Talhouk, A.; McAlpine, J.N. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol. Oncol. Res. Pract. 2016, 3, 14. [Google Scholar] [CrossRef]
- León-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Modrich, P.; Lahue, R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu. Rev. Biochem. 1996, 65, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Tiraby, J.G.; Fox, M.S. Marker discrimination in transformation and mutation of pneumococcus. Proc. Natl. Acad. Sci. USA 1973, 70, 3541–3545. [Google Scholar] [CrossRef]
- O’Hara, A.J.; Bell, D.W. The genomics and genetics of endometrial cancer. Adv. Genom. Genet. 2012, 2012, 33–47. [Google Scholar] [CrossRef]
- McConechy, M.K.; Ding, J.; Senz, J.; Yang, W.; Melnyk, N.; Tone, A.A.; Prentice, L.M.; Wiegand, K.C.; McAlpine, J.N.; Shah, S.P.; et al. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod. Pathol. 2014, 27, 128–134. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Zerdes, I.; Matikas, A.; Bergh, J.; Rassidakis, G.Z.; Foukakis, T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: Biology and clinical correlations. Oncogene 2018, 37, 4639–4661. [Google Scholar] [CrossRef]
- Hong, A.M.; Ferguson, P.; Dodds, T.; Jones, D.; Li, M.; Yang, J.; Scolyer, R.A. Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer. Oral Oncol. 2019, 92, 33–39. [Google Scholar] [CrossRef]
- Lyu, X.; Zhang, M.; Li, G.; Jiang, Y.; Qiao, Q. PD-1 and PD-L1 Expression Predicts Radiosensitivity and Clinical Outcomes in Head and Neck Cancer and is Associated with HPV Infection. J. Cancer 2019, 10, 937–948. [Google Scholar] [CrossRef]
- Costa, F.F. Non-coding RNAs, epigenetics and complexity. Gene 2008, 410, 9–17. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Godinho, M.; Meijer, D.; Setyono-Han, B.; Dorssers, L.C.J.; van Agthoven, T. Characterization of BCAR4, a novel oncogene causing endocrine resistance in human breast cancer cells. J. Cell. Physiol. 2011, 226, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Z.; Fang, X.; Li, N.; Fang, J. Long noncoding RNA Breast cancer antiestrogen resistance 4 is associated with cancer progression and its significant prognostic value. J. Cell. Physiol. 2019, 234, 12956–12963. [Google Scholar] [CrossRef]
- Zou, R.; Chen, X.; Jin, X.; Li, S.; Ou, R.; Xue, J.; Yan, X.; Chen, L.; Hu, Y.; Zhu, H. Up-regulated BCAR4 contributes to proliferation and migration of cervical cancer cells. Surg. Oncol. 2018, 27, 306–313. [Google Scholar] [CrossRef]
- Smith, J.S.; Backes, D.M.; Hoots, B.E.; Kurman, R.J.; Pimenta, J.M. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obs. Gynecol. 2009, 113, 917–924. [Google Scholar] [CrossRef]
- van der Avoort, I.A.; Shirango, H.; Hoevenaars, B.M.; Grefte, J.M.; de Hullu, J.A.; de Wilde, P.C.; Bulten, J.; Melchers, W.J.; Massuger, L.F. Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways. Int. J. Gynecol. Pathol. 2006, 25, 22–29. [Google Scholar] [CrossRef]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Nigro, J.M.; Baker, S.J.; Preisinger, A.C.; Jessup, J.M.; Hostetter, R.; Cleary, K.; Bigner, S.H.; Davidson, N.; Baylin, S.; Devilee, P.; et al. Mutations in the p53 gene occur in diverse human tumour types. Nature 1989, 342, 705–708. [Google Scholar] [CrossRef]
- Riley, T.; Sontag, E.; Chen, P.; Levine, A. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell. Biol. 2008, 9, 402–412. [Google Scholar] [CrossRef]
- Vazquez, A.; Bond, E.E.; Levine, A.J.; Bond, G.L. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat. Rev. Drug Discov. 2008, 7, 979–987. [Google Scholar] [CrossRef]
- Thangarajah, F.; Morgenstern, B.; Pahmeyer, C.; Schiffmann, L.M.; Puppe, J.; Mallmann, P.; Hamacher, S.; Buettner, R.; Alidousty, C.; Holz, B.; et al. Clinical impact of PD-L1 and PD-1 expression in squamous cell cancer of the vulva. J. Cancer Res. Clin. Oncol. 2019, 145, 1651–1660. [Google Scholar] [CrossRef]
- Franzin, R.; Netti, G.S.; Spadaccino, F.; Porta, C.; Gesualdo, L.; Stallone, G.; Castellano, G.; Ranieri, E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front. Immunol. 2020, 11, 574271. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Retifanlimab: First Approval. Drugs 2023, 83, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Imanishi, N.; Ichiki, Y.; Tanaka, F. Immune Checkpoint Inhibitors (ICIs) in Non-Small Cell Lung Cancer (NSCLC). J. Uoeh 2018, 40, 173–189. [Google Scholar] [CrossRef]
- Schepisi, G.; Casadei, C.; Toma, I.; Poti, G.; Iaia, M.L.; Farolfi, A.; Conteduca, V.; Lolli, C.; Ravaglia, G.; Brighi, N.; et al. Immunotherapy and Its Development for Gynecological (Ovarian, Endometrial and Cervical) Tumors: From Immune Checkpoint Inhibitors to Chimeric Antigen Receptor (CAR)-T Cell Therapy. Cancers 2021, 13, 840. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, L.; Boccia, S.M.; Caruso, G.; Santangelo, G.; Fischetti, M.; Tomao, F.; Perniola, G.; Palaia, I.; Muzii, L.; Pignata, S.; et al. Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients? J. Clin. Med. 2020, 9, 1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fan, W.; Ye, M.; Tian, C.; Zhao, L.; Wang, J.; Han, W.; Yang, W.; Gu, C.; Li, M.; et al. Molecular profiles and tumor mutational burden analysis in Chinese patients with gynecologic cancers. Sci. Rep. 2018, 8, 8990. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L.; et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Investig 2016, 126, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Westin, S.N.; Coleman, R.L. Microsatellite instability in endometrial cancer: New purpose for an old test. Cancer 2019, 125, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Longoria, T.C.; Eskander, R.N. Immunotherapy in endometrial cancer—An evolving therapeutic paradigm. Gynecol. Oncol. Res. Pract 2015, 2, 11. [Google Scholar] [CrossRef]
- An, H.J.; Kim, K.I.; Kim, J.Y.; Shim, J.Y.; Kang, H.; Kim, T.H.; Kim, J.K.; Jeong, J.K.; Lee, S.Y.; Kim, S.J. Microsatellite instability in endometrioid type endometrial adenocarcinoma is associated with poor prognostic indicators. Am. J. Surg. Pathol. 2007, 31, 846–853. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Vanderstraeten, A.; Luyten, C.; Verbist, G.; Tuyaerts, S.; Amant, F. Mapping the immunosuppressive environment in uterine tumors: Implications for immunotherapy. Cancer Immunol. Immunother. 2014, 63, 545–557. [Google Scholar] [CrossRef]
- Cao, W.; Ma, X.; Fischer, J.V.; Sun, C.; Kong, B.; Zhang, Q. Immunotherapy in endometrial cancer: Rationale, practice and perspectives. Biomark. Res. 2021, 9, 49. [Google Scholar] [CrossRef]
- Turinetto, M.; Valsecchi, A.A.; Tuninetti, V.; Scotto, G.; Borella, F.; Valabrega, G. Immunotherapy for Cervical Cancer: Are We Ready for Prime Time? Int. J. Mol. Sci. 2022, 23, 3559. [Google Scholar] [CrossRef]
- Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 7 January 2023).
- Adefuye, A.; Sales, K. Regulation of Inflammatory Pathways in Cancer and Infectious Disease of the Cervix. Scientifica 2012, 2012, 548150. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Wild, C.M.; Mittelberger, J.; Dobler, F.; Schneider, M.; Ansorge, N.; Köpke, M.; Strieder, A.; Ditsch, N.; Jeschke, U.; et al. The Role of Chemokines in Cervical Cancers. Medicina 2021, 57, 1141. [Google Scholar] [CrossRef] [PubMed]
- López-Ocejo, O.; Viloria-Petit, A.; Bequet-Romero, M.; Mukhopadhyay, D.; Rak, J.; Kerbel, R.S. Oncogenes and tumor angiogenesis: The HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene 2000, 19, 4611–4620. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, L.; Zhao, J.; Tan, G.; Zhang, W.; Zhang, N.; Tian, J.; Qu, P. The value of cytokine levels in triage and risk prediction for women with persistent high-risk human papilloma virus infection of the cervix. Infect. Agent. Cancer 2019, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, I.; Otter, S.J.; Bharathan, R.; Stewart, A. Vascular endothelial growth factor (VEGF) inhibitors for the treatment of metastatic and recurrent cervical cancer. Cochrane Database Syst. Rev. 2020, 8, CD013605. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Monk, B.J.; Penson, R.T.; Long, H.J., 3rd; Poveda, A.; Landrum, L.M.; Leitao, M.M.; Brown, J.; Reid, T.J.; et al. Prospective Validation of Pooled Prognostic Factors in Women with Advanced Cervical Cancer Treated with Chemotherapy with/without Bevacizumab: NRG Oncology/GOG Study. Clin. Cancer Res. 2015, 21, 5480–5487. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Long, H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved Survival with Bevacizumab in Advanced Cervical Cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef]
- Gyawali, B.; Iddawela, M. Bevacizumab in Advanced Cervical Cancer: Issues and Challenges for Low- and Middle-Income Countries. J. Glob. Oncol. 2017, 3, 93–97. [Google Scholar] [CrossRef]
- Ota, N.; Yoshimoto, Y.; Darwis, N.D.M.; Sato, H.; Ando, K.; Oike, T.; Ohno, T. High tumor mutational burden predicts worse prognosis for cervical cancer treated with radiotherapy. Jpn. J. Radiol. 2022, 40, 534–541. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Omenai, S.A.; Ajani, M.A.; Okolo, C.A. Programme death ligand 1 expressions as a surrogate for determining immunotherapy in cervical carcinoma patients. PLoS ONE 2022, 17, e0263615. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liang, H.; Hu, J.; Liu, S.; Hao, X.; Wong, M.S.K.; Li, X.; Hu, L. PD-L1 Expression Correlates with Tumor Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Cervical Cancer. J. Cancer 2018, 9, 2938–2945. [Google Scholar] [CrossRef]
- Praiss, A.; Navitski, A.; Cohen, S.; Tessier-Cloutier, B.; Broach, V.; O’Cearbhaill, R.E. Immunotherapy for recurrent or metastatic vulvar carcinoma: A case report and review of current guidelines. Gynecol. Oncol. Rep. 2022, 41, 100982. [Google Scholar] [CrossRef]
- Choschzick, M.; Gut, A.; Fink, D. PD-L1 receptor expression in vulvar carcinomas is HPV-independent. Virchows Arch. 2018, 473, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, G.; Manai, M.; YaÔche, R.; Charfi, L.; Ghalleb, M.; Douik, H.; Mrad, K.; Doghri, R. Evaluation of PD-L1 expression in vulvar cancer. F1000Research 2022, 11, 1225. [Google Scholar] [CrossRef]
- Hecking, T.; Thiesler, T.; Schiller, C.; Lunkenheimer, J.M.; Ayub, T.H.; Rohr, A.; Condic, M.; Keyver-Paik, M.D.; Fimmers, R.; Kirfel, J.; et al. Tumoral PD-L1 expression defines a subgroup of poor-prognosis vulvar carcinomas with non-viral etiology. Oncotarget 2017, 8, 92890–92903. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, L.; Xie, X.; Qin, Y.; Xie, Z.; Ouyang, M.; Zhou, C. Prognostic Biomarker TP53 Mutations for Immune Checkpoint Blockade Therapy and Its Association with Tumor Microenvironment of Lung Adenocarcinoma. Front. Mol. Biosci. 2020, 7, 602328. [Google Scholar] [CrossRef]
- Liu, S.; Geng, S.; Shi, N.; Zhang, L.; Xue, W.; Li, Y.; Jiang, K. Survival Prediction of Patients Treated with Immune Checkpoint Inhibitors via KRAS/TP53/EGFR-Single Gene Mutation. Front. Pharm. 2022, 13, 878540. [Google Scholar] [CrossRef]
- Choschzick, M.; Hantaredja, W.; Tennstedt, P.; Gieseking, F.; Wölber, L.; Simon, R. Role of TP53 mutations in vulvar carcinomas. Int. J. Gynecol. Pathol. 2011, 30, 497–504. [Google Scholar] [CrossRef]
- Pinto, A.P.; Miron, A.; Yassin, Y.; Monte, N.; Woo, T.Y.; Mehra, K.K.; Medeiros, F.; Crum, C.P. Differentiated vulvar intraepithelial neoplasia contains Tp53 mutations and is genetically linked to vulvar squamous cell carcinoma. Mod. Pathol. 2010, 23, 404–412. [Google Scholar] [CrossRef]
- Rakislova, N.; Alemany, L.; Clavero, O.; Saco, A.; Torné, A.; Del Pino, M.; Munmany, M.; Rodrigo-Calvo, M.T.; Guerrero, J.; Marimon, L.; et al. p53 Immunohistochemical Patterns in HPV-Independent Squamous Cell Carcinomas of the Vulva and the Associated Skin Lesions: A Study of 779 Cases. Int. J. Mol. Sci. 2020, 21, 8091. [Google Scholar] [CrossRef]
- Sand, F.L.; Nielsen, D.M.B.; Frederiksen, M.H.; Rasmussen, C.L.; Kjaer, S.K. The prognostic value of p16 and p53 expression for survival after vulvar cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2019, 152, 208–217. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Muro, K.; Chung, H.C.; Shankaran, V.; Geva, R.; Catenacci, D.; Gupta, S.; Eder, J.P.; Golan, T.; Le, D.T.; Burtness, B.; et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016, 17, 717–726. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Barber, E.L.; Chen, S.; Pineda, M.J.; Robertson, S.E.; Hill, E.K.; Teoh, D.; Schilder, J.; O’Shea, K.L.; Kocherginsky, M.; Zhang, B.; et al. Clinical and Biological Activity of Chemoimmunotherapy in Advanced Endometrial Adenocarcinoma: A Phase II Trial of the Big Ten Cancer Research Consortium. Cancer Res. Commun. 2022, 2, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.-P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti–Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients with Recurrent or Advanced Mismatch Repair–Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- Costa, B.; Vale, N. Dostarlimab: A Review. Biomolecules 2022, 12, 1031. [Google Scholar] [CrossRef]
- Mirza, M.R.; Coleman, R.L.; Hanker, L.C.; Slomovitz, B.M.; Valabrega, G.; Im, E.; Walker, M.; Guo, W.; Powell, M.A. ENGOT-EN6/NSGO-RUBY: A phase III, randomized, double-blind, multicenter study of dostarlimab + carboplatin-paclitaxel versus placebo + carboplatin-paclitaxel in recurrent or primary advanced endometrial cancer (EC). J. Clin. Oncol. 2020, 38, TPS6107. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.F.; Emens, L.A.; Eder, J.P.; Hamilton, E.P.; Liu, J.F.; Liu, B.; Molinero, L.; Fasso, M.; O’Hear, C.; Braiteh, F.S. Clinical activity, safety and biomarker results from a phase Ia study of atezolizumab (atezo) in advanced/recurrent endometrial cancer (rEC). J. Clin. Oncol. 2017, 35, 5585. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II Study of Avelumab in Patients with Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Antill, Y.C.; Kok, P.S.; Robledo, K.; Barnes, E.; Friedlander, M.; Baron-Hay, S.E.; Shannon, C.M.; Coward, J.; Beale, P.J.; Goss, G.; et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). J. Clin. Oncol. 2019, 37, 5501. [Google Scholar] [CrossRef]
- Frenel, J.S.; Le Tourneau, C.; O’Neil, B.; Ott, P.A.; Piha-Paul, S.A.; Gomez-Roca, C.; van Brummelen, E.M.J.; Rugo, H.S.; Thomas, S.; Saraf, S.; et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J. Clin. Oncol. 2017, 35, 4035–4041. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Study of Chemoradiotherapy with or without Pembrolizumab (MK-3475) for the Treatment of Locally Advanced Cervical Cancer (MK-3475-A18/KEYNOTE-A18/ENGOT-cx11/GOG-3047). Available online: https://clinicaltrials.gov/ct2/show/NCT04221945 (accessed on 3 January 2023).
- Youn, J.W.; Hur, S.-Y.; Woo, J.W.; Kim, Y.-M.; Lim, M.C.; Park, S.Y.; Seo, S.S.; No, J.H.; Kim, B.-G.; Lee, J.-K.; et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: Interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1653–1660. [Google Scholar] [CrossRef]
- Santin, A.D.; Deng, W.; Frumovitz, M.; Buza, N.; Bellone, S.; Huh, W.; Khleif, S.; Lankes, H.A.; Ratner, E.S.; O’Cearbhaill, R.E.; et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol. Oncol. 2020, 157, 161–166. [Google Scholar] [CrossRef]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.P.; Delord, J.P.; Evans, T.R.J.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Aghbash, P.S.; Hemmat, N.; Fathi, H.; Baghi, H.B. Monoclonal antibodies in cervical malignancy-related HPV. Front. Oncol. 2022, 12, 904790. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.-S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G. PD-1/PD-L1 blockade in cervical cancer: Current studies and perspectives. Front. Med. 2019, 13, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.P.; Cassier, P.; Loirat, D.; Tavernaro, A.; Bastien, B.; Bendjama, K. 1210P—Phase Ib/II trial of TG4001 (Tipapkinogene sovacivec), a therapeutic HPV-vaccine, and Avelumab in patients with recurrent/metastatic (R/M) HPV-16+ cancers. Ann. Oncol. 2019, 30, v494–v495. [Google Scholar] [CrossRef]
- Avelumab, Utomilumab, Anti-OX40 Antibody PF-04518600, and Radiation Therapy in Treating Patients with Advanced Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03217747 (accessed on 4 January 2023).
- Cohen, E.E.; Moore, K.N.; Slomovitz, B.M.; Chung, C.H.; Anderson, M.L.; Morris, S.R.; Mauro, D.; Burtness, B. Phase I/II study of ADXS11-001 or MEDI4736 immunotherapies alone and in combination, in patients with recurrent/metastatic cervical or human papillomavirus (HPV)-positive head and neck cancer. J. Immunother. Cancer 2015, 3 (Suppl. S2), P147. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Bean, S.; Bradley, K.; Campos, S.M.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 660–666. [Google Scholar] [CrossRef]
- Han, X.; Chang, W.W.; Xia, X. Immune checkpoint inhibitors in advanced and recurrent/metastatic cervical cancer. Front. Oncol. 2022, 12, 996495. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Enserro, D.M.; Mayadev, J.S.; Skeate, J.G.; Matsuo, K.; Pham, H.Q.; Lankes, H.A.; Moxley, K.M.; Ghamande, S.A.; Lin, Y.G.; et al. Immune Activation in Patients with Locally Advanced Cervical Cancer Treated with Ipilimumab Following Definitive Chemoradiation (GOG-9929). Clin. Cancer Res. 2020, 26, 5621–5630. [Google Scholar] [CrossRef]
- Lheureux, S.; Butler, M.O.; Clarke, B.; Cristea, M.C.; Martin, L.P.; Tonkin, K.; Fleming, G.F.; Tinker, A.V.; Hirte, H.W.; Tsoref, D.; et al. Association of Ipilimumab with Safety and Antitumor Activity in Women with Metastatic or Recurrent Human Papillomavirus–Related Cervical Carcinoma. JAMA Oncol. 2018, 4, e173776. [Google Scholar] [CrossRef] [PubMed]
- AdvanTIG-202: Anti-PD-1 Monoclonal Antibody Tislelizumab (BGB-A317) Combined with or without Anti-TIGIT Monoclonal Antibody Ociperlimab (BGB-A1217) in Participants with Previously Treated Recurrent or Metastatic Cervical Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04693234 (accessed on 3 January 2023).
- Liu, L.; Wang, A.; Liu, X.; Han, S.; Sun, Y.; Zhang, J.; Guo, L.; Zhang, Y. Blocking TIGIT/CD155 signalling reverses CD8(+) T cell exhaustion and enhances the antitumor activity in cervical cancer. J. Transl. Med. 2022, 20, 280. [Google Scholar] [CrossRef]
- Rotte, A.; Sahasranaman, S.; Budha, N. Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. Biomedicines 2021, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated with Pembrolizumab Across 20 Cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yeku, O.; Russo, A.L.; Lee, H.; Spriggs, D. A phase 2 study of combined chemo-immunotherapy with cisplatin-pembrolizumab and radiation for unresectable vulvar squamous cell carcinoma. J. Transl. Med. 2020, 18, 350. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Van Morris, K.; Vo, H.H.; Eck, S.; Lin, Y.-F.; Rivas, J.M.; Andersson, B.S. T-cell receptor-based therapy: An innovative therapeutic approach for solid tumors. J. Hematol. Oncol. 2021, 14, 102. [Google Scholar] [CrossRef]
- Doran, S.L.; Stevanović, S.; Adhikary, S.; Gartner, J.J.; Jia, L.; Kwong, M.L.M.; Faquin, W.C.; Hewitt, S.M.; Sherry, R.M.; Yang, J.C.; et al. T-Cell Receptor Gene Therapy for Human Papillomavirus–Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef]
- Malla, R.; Kamal, M.A. E6 and E7 Oncoproteins: Potential Targets of Cervical Cancer. Curr. Med. Chem. 2021, 28, 8163–8181. [Google Scholar] [CrossRef]
- Son, J.; George, G.C.; Nardo, M.; Krause, K.J.; Jazaeri, A.A.; Biter, A.B.; Hong, D.S. Adoptive cell therapy in gynecologic cancers: A systematic review and meta-analysis. Gynecol. Oncol. 2022, 165, 664–670. [Google Scholar] [CrossRef]
- Draper, L.M.; Kwong, M.L.M.; Gros, A.; Stevanović, S.; Tran, E.; Kerkar, S.; Raffeld, M.; Rosenberg, S.A.; Hinrichs, C.S. Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6. Clin. Cancer Res. 2015, 21, 4431–4439. [Google Scholar] [CrossRef]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus-associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.R.; Rodrigues, K.B.; Pelegrin, G.F.; Sales, N.S.; Muramatsu, H.; Silva, M.D.O.; Porchia, B.F.M.M.; Moreno, A.C.R.; Aps, L.R.M.M.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef]
- de Sousa, L.G.; Rajapakshe, K.; Canales, J.R.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and nivolumab for HPV-16+ cancer: Updated clinical efficacy and immune correlates of response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef]
- Brown, T.A.; Byrd, K.; Vreeland, T.J.; Clifton, G.T.; Jackson, D.O.; Hale, D.F.; Herbert, G.S.; Myers, J.W.; Greene, J.M.; Berry, J.S.; et al. Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients. Cancer Med. 2019, 8, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Huh, J.W.; Shin, J.K.; Park, Y.A.; Cho, Y.B.; Kim, H.C.; Yun, S.H.; Lee, W.Y. Prognostic Factors and Treatment of Recurrence after Local Excision of Rectal Cancer. Yonsei Med. J. 2021, 62, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.M.; Skeate, J.G.; Chavez-Juan, E.; Lühen, K.P.; Wu, J.M.; Wu, C.M.; Kast, W.M.; Hwang, K. Therapeutic efficacy of a human papillomavirus type 16 E7 bacterial exotoxin fusion protein adjuvanted with CpG or GPI-0100 in a preclinical mouse model for HPV-associated disease. Vaccine 2019, 37, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Carmona, T.; Arango-Bravo, E.; Serrano-Olvera, J.A.; Flores-de La Torre, C.; Cruz-Esquivel, I.; Villalobos-Valencia, R.; Morán-Mendoza, A.; Castro-Eguiluz, D.; Cetina-Pérez, L. ADXS11-001 LM-LLO as specific immunotherapy in cervical cancer. Hum. Vaccin. Immunother. 2021, 17, 2617–2625. [Google Scholar] [CrossRef]
- Karpel, H.; Slomovitz, B.; Coleman, R.L.; Pothuri, B. Biomarker-driven therapy in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 343. [Google Scholar] [CrossRef]
- Friedman, L.A.; Ring, K.L.; Mills, A.M. LAG-3 and GAL-3 in Endometrial Carcinoma: Emerging Candidates for Immunotherapy. Int. J. Gynecol. Pathol. 2020, 39, 203–212. [Google Scholar] [CrossRef]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tezlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. LAG-3 and PD-1 blockade raises the bar for melanoma. Nat. Cancer 2021, 2, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.; Chelariu-Raicu, A.; Slomovitz, B.M. Immunotherapy in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 351. [Google Scholar] [CrossRef] [PubMed]
- Lauder, S.N.; Vanhaesebroeck, B.; Gallimore, A. Sequential targeting of PI3Kδ and LAG3 as an effective anti-cancer approach. Br. J. Cancer 2021, 125, 467–469. [Google Scholar] [CrossRef]
- Pagliano, O.; Morrison, R.M.; Chauvin, J.M.; Banerjee, H.; Davar, D.; Ding, Q.; Tanegashima, T.; Gao, W.; Chakka, S.R.; DeBlasio, R.; et al. Tim-3 mediates T cell trogocytosis to limit antitumor immunity. J. Clin. Investig 2022, 132, 9. [Google Scholar] [CrossRef]
- NIH. Safety and Efficacy of Retifanlimab (INCMGA00012) Alone or in Combination with Other Therapies in Participants with Advanced or Metastatic Endometrial Cancer Who Have Progressed on or after Platinum-Based Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT04463771 (accessed on 9 March 2023).
- Gourd, K. ESMO World Congress on Gastrointestinal Cancer 2022. Lancet Oncol. 2022, 23, 988. [Google Scholar] [CrossRef]
- Hamid, O.; Ismail, R.; Puzanov, I. Intratumoral Immunotherapy-Update 2019. Oncologist 2020, 25, e423–e438. [Google Scholar] [CrossRef]
- Kagabu, M.; Yoshino, N.; Murakami, K.; Kawamura, H.; Sasaki, Y.; Muraki, Y.; Baba, T. Treatment of HPV-Related Uterine Cervical Cancer with a Third-Generation Oncolytic Herpes Simplex Virus in Combination with an Immune Checkpoint Inhibitor. Int. J. Mol. Sci. 2023, 24, 1988. [Google Scholar] [CrossRef]
- Moore, M.; Ring, K.L.; Mills, A.M. TIM-3 in endometrial carcinomas: An immunotherapeutic target expressed by mismatch repair-deficient and intact cancers. Mod. Pathol. 2019, 32, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, X.; Huang, X.; Li, Q.; Gao, L.; Jiang, L.; Huang, M.; Zhou, J. Tim-3 Expression in Cervical Cancer Promotes Tumor Metastasis. PLoS ONE 2013, 8, e53834. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Komatsu, M.; Chiwaki, F.; Komatsuzaki, R.; Nakamura, K.; Tsuji, K.; Kobayashi, Y.; Tominaga, E.; Ono, M.; Banno, K.; et al. Upregulation of IGF2R evades lysosomal dysfunction-induced apoptosis of cervical cancer cells via transport of cathepsins. Cell. Death Dis. 2019, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- ICR. ATARI: ATr Inhibitor in Combination with Olaparib in Gynaecological Cancers with ARId1A Loss or No Loss. Available online: https://www.icr.ac.uk/our-research/centres-and-collaborations/centres-at-the-icr/clinical-trials-and-statistics-unit/our-research/clinical-trials/atari (accessed on 5 March 2023).
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Sanofi. Positive Phase 3 Libtayo® (cemiplimab) Results in Advanced Cervical Cancer Presented at ESMO Virtual Plenary—Sanofi. 2023. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-05-12-17-35-00-2228565 (accessed on 5 March 2023).
- Shields, L.B.E.; Gordinier, M.E. Pembrolizumab in Recurrent Squamous Cell Carcinoma of the Vulva: Case Report and Review of the Literature. Gynecol. Obs. Investig 2019, 84, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Shapira-Frommer, R.; Mileshkin, L.; Manzyuk, L.; Penel, N.; Burge, M.; Piha-Paul, S.A.; Girda, E.; Lopez Martin, J.A.; van Dongen, M.G.J.; Italiano, A.; et al. Efficacy and safety of pembrolizumab for patients with previously treated advanced vulvar squamous cell carcinoma: Results from the phase 2 KEYNOTE-158 study. Gynecol. Oncol. 2022, 166, 211–218. [Google Scholar] [CrossRef] [PubMed]



| Cancer Type | Immunotherapy Agent | Treatment Recommendations |
|---|---|---|
| Endometrial Cancer | Pembrolizumab | Pembrolizumab is approved for use as a second-line treatment in unresectable or metastatic MSI/MMRd EC. It has also been shown to have an increased clinical benefit as a combination therapy over standard chemotherapy treatment (carboplatin/paclitaxel) when used as a second-line systemic treatment for microsatellite-stable carcinomas [8,36]. |
| Dostarlimab | Dostarlimab is an anti-PD-1 inhibitor approved by the FDA as another second-line immunotherapy agent for patients with advanced or recurrent dMMR- MSI-H EC following its significant response rates in the phase I GARNET study [137]. As a combination therapy with carboplatin/paclitaxel, evidence has been generated of a decreased rate of disease-free progression compared to chemotherapy alone in patients with recurrent or advanced disease [139]. | |
| Cervical Cancer | Pembrolizumab | Pembrolizumab is a second-line treatment in PD-L1-positive recurrent or metastatic CC. In clinical trials, it has shown a greater overall response rate when used in patients with PD-L1-positive tumours compared to platinum-based therapy [145]. Its efficacy as a combination therapy is currently being studied in trials [146,147]. |
| Ipilimumab | Although ipilimumab has not shown significant response as a monotherapy in metastatic disease, durable responses have been observed when it has been used as an adjuvant therapy with definitive CRT in locally advanced CC [159]. | |
| Vulvar Cancer | Pembrolizumab | Pembrolizumab with cisplatin-sensitised radiation therapy is currently being evaluated as a combination therapy for unresectable, local or metastatic VC in a phase II trial (NCT04430699) [165]. |
| Nivolumab | Although nivolumab has been shown to have a notable efficacy and a tolerable safety profile in recurrent or metastatic VC, the study sample size was small [149]. Given the lack of sufficient evidence of the efficacy of ICIs in VC, clinicians should exercise clinical judgment when considering immunotherapy agents in the treatment of recurrent or metastatic VC. |
| Therapy | Summary of Novel Treatments | |
|---|---|---|
| Adoptive T cell therapy | E6 and E7 oncoprotein-based therapies | HPV-positive tumours have been evaluated as potential targets for E6 and E7 oncoprotein-based T cell therapy in CC and VC. Recent studies have shown promising results for their use in cervical cancer, which may elicit further interest in their development [171,172]. |
| Cancer vaccines | GDE7 mRNA-based vaccines | Preclinical studies have found that GDE7 mRNA-based vaccines may be effective in HPV-16-associated cancers, such as CC and VC, in controlling tumour proliferation and the effectiveness of CD8+ tumours [173]. |
| ISA101 | ISA101 is a novel peptide protein developed for HPV-associated cancers. It has been successful when used in combination with nivolumab to prevent cancer recurrence, with a notable response occurring in EC despite the lack of expression of folate binding proteins in EC. | |
| Novel immune-based immunotherapy | LAG-3 | LAG-3 has been found to be expressed mainly in mismatch repair-deficient tumours and in nonmethylated mismatch repair-deficient EC [180]. Dual therapies with PD-1 and LAG-3 have already been shown to be more effective in mediating tumour regression in other cancers and enhancing the anti-tumoural response [183,184]. |
| GAL-3 | GAL-3 has also been shown to have an increased expression in mismatch repair-deficient tumours and nonmethylated mismatch repair-deficient EC [180], and it is likely that specific therapies will be developed utilising its potential as a novel target. | |
| PI3Kδ | PI3Kδ can facilitate tumour cell proliferation when used in combination with an anti-LAG3 antibody therapy; however, an increased CD8:Treg ratio is required to see an increased response from this dual therapy in non-ovarian gynaecological cancers [185]. | |
| TIM-3 | TIM-3 can decrease tumour mutational burden by disrupting cellular trogocytosis of CD8+ TILs, and an ongoing phase II study is comparing the efficacy and safety of retifanlimab (anti-PD-1 therapy) when used as a monotherapy or in combination with other novel targets in advanced EC. | |
| Bispecific monoclonal antibodies | Bispecific antibodies are currently being investigated in a phase II trial on advanced gynaecological cancers. If found to have sufficient efficacy, they could be used to exploit dual targets, such as PD-1 and CTLA-4 [187]. | |
| Intra-tumoural oncolytic viral therapy | Oncolytic herpes simplex virus | Oncolytic herpes simplex viral therapy can stimulate a reduction in tumour growth and increase the number of CD8+ T cells in CC [189]. However, the statistical significance of the results (p = 0.03) is insufficient to attract significant interest in it as an alternative immunotherapy agent to existing treatments [189]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeleke, S.; Gao, Y.; Okoli, S.; Choi, S.; Ding, H.; Galante, J.R.; Mikropoulos, C. Current Methods and Advances in the Immunotherapy Treatment of Non-Ovarian Gynaecological Cancers. Future Pharmacol. 2023, 3, 488-514. https://doi.org/10.3390/futurepharmacol3020031
Adeleke S, Gao Y, Okoli S, Choi S, Ding H, Galante JR, Mikropoulos C. Current Methods and Advances in the Immunotherapy Treatment of Non-Ovarian Gynaecological Cancers. Future Pharmacology. 2023; 3(2):488-514. https://doi.org/10.3390/futurepharmacol3020031
Chicago/Turabian StyleAdeleke, Sola, Yujia Gao, Somto Okoli, Sunyoung Choi, Hao Ding, Joao R. Galante, and Christos Mikropoulos. 2023. "Current Methods and Advances in the Immunotherapy Treatment of Non-Ovarian Gynaecological Cancers" Future Pharmacology 3, no. 2: 488-514. https://doi.org/10.3390/futurepharmacol3020031
APA StyleAdeleke, S., Gao, Y., Okoli, S., Choi, S., Ding, H., Galante, J. R., & Mikropoulos, C. (2023). Current Methods and Advances in the Immunotherapy Treatment of Non-Ovarian Gynaecological Cancers. Future Pharmacology, 3(2), 488-514. https://doi.org/10.3390/futurepharmacol3020031







