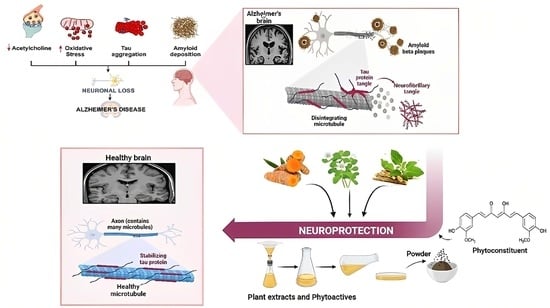
Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights
Abstract
:1. Introduction
2. Methodology
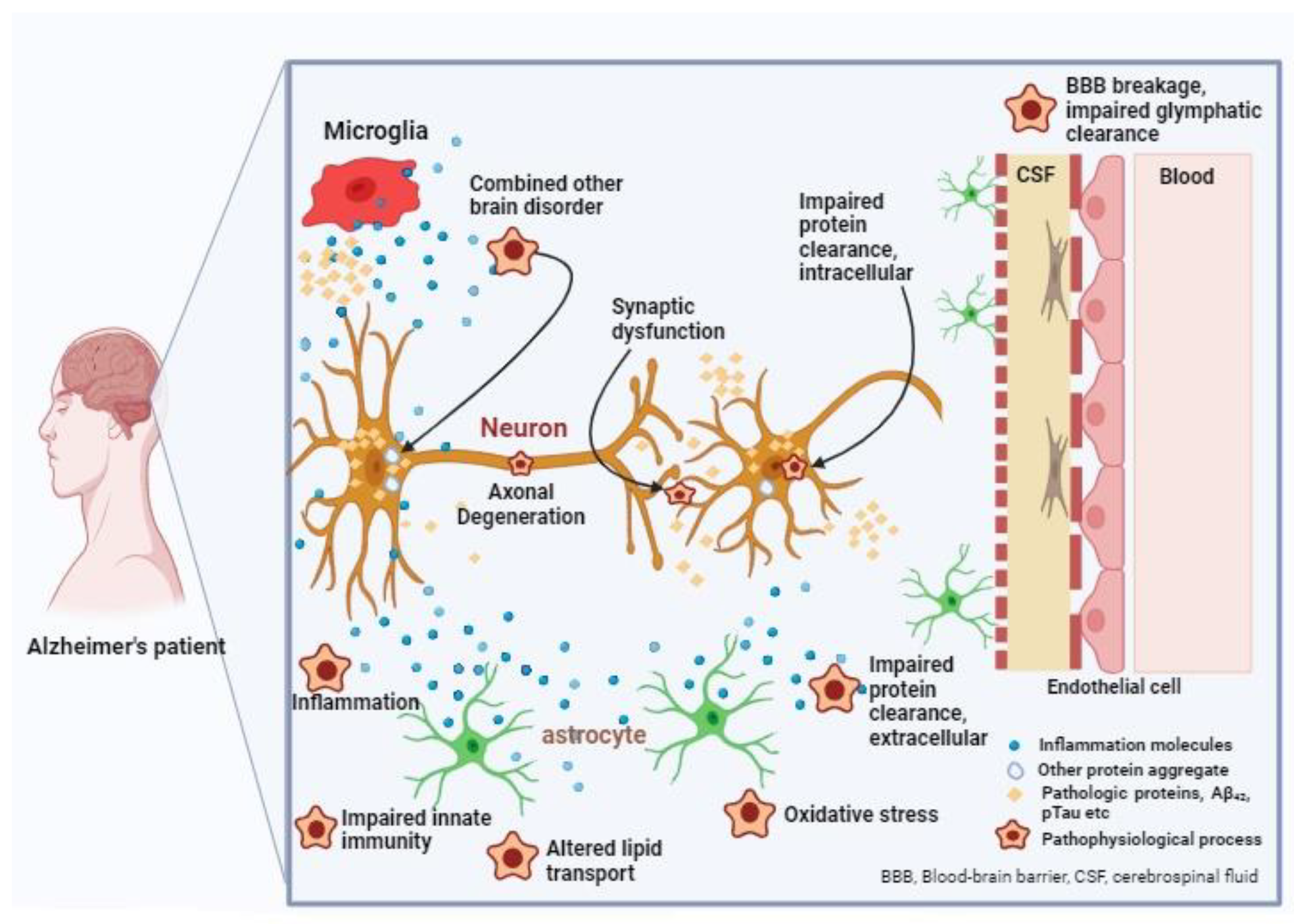
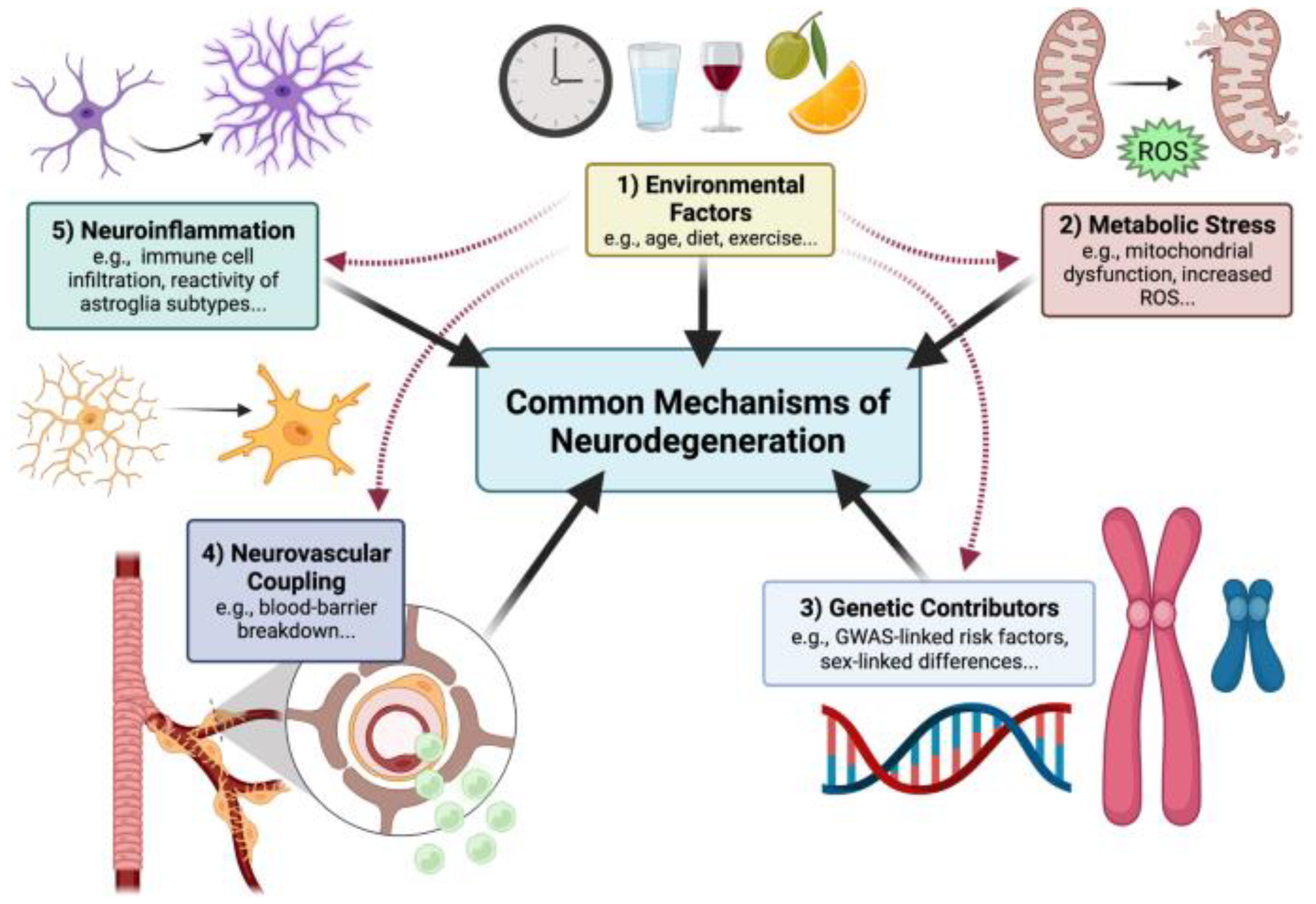
3. Pathophysiology of Alzheimer’s Disease
3.1. Amyloid Cascade Hypothesis
3.2. Tau Hypothesis
3.3. Cholinergic Hypothesis
3.4. Autophagy and Neuroinflammation
3.5. Oxidative Stress
3.6. Tau Truncation
4. Neuroprotective Potential of Medicinal Plants against Alzheimer’s Disease
4.1. Panax ginseng
4.2. Curcuma longa
4.3. Ginkgo biloba
4.4. Centella asiatica
4.5. Glycyrrhiza Species
4.6. Bacopa monnieri
4.7. Tinospora cordifolia
4.8. Convolvulus pluricaulis
4.9. Withania somnifera
4.10. Celastrus paniculatus
5. Clinical Trials
6. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Xiong, H.; Zhang, B.; Lee, I.; Xie, J.; Li, M.; Zhang, H.; Kim, J.S. Photodynamic Alzheimer’s disease therapy: From molecular catalysis to photo-nanomedicine. Coord. Chem. Rev. 2022, 470, 214726. [Google Scholar] [CrossRef]
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen. Res. 2017, 12, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, R.; Knapp, M.; Hu, B.; Comas-Herrera, A.; King, D.; Rehill, A.; Shi, C.; Banerjee, S.; Patel, A.; Jagger, C.; et al. The costs of dementia in England. Int. J. Geriatr. Psychiatry 2019, 34, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Duara, R.; Barker, W. Heterogeneity in Alzheimer’s Disease Diagnosis and Progression Rates: Implications for Therapeutic Trials. Neurotherapeutics 2022, 19, 8–25. [Google Scholar] [CrossRef]
- Paramanick, D.; Singh, V.D.; Singh, V.K. Neuroprotective effect of phytoconstituents via nanotechnology for treatment of Alzheimer diseases. J. Control. Release 2022, 351, 638–655. [Google Scholar] [CrossRef] [PubMed]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Green, R.C.; Harvey, D.; Jack, C.R.; Jagust, W.; Morris, J.C.; et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Dement. 2019, 15, 106–152. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Challenges and hopes for Alzheimer’s disease. Drug Discov. Today 2022, 27, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Pedrini, S.; Ashton, N.J.; Tegg, M.; Goozee, K.; Singh, A.K.; Karikari, T.K.; Simrén, J.; Vanmechelen, E.; Armstrong, N.J.; et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Kaduszkiewicz, H.; van den Bussche, H. Acetylcholinesterase Inhibitors and Alzheimer’s Disease. In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 9–13. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet. Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; DeCarli, C.; Green, R.C.; Harvey, D.; Clifford, R.J., Jr.; Jagust, W.; Landau, S.M.; et al. Using the Alzheimer’s Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 824–857. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Albratty, M. An update on the novel and approved drugs for Alzheimer disease. Saudi Pharm. J. 2022, 30, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Kothari, S.L.; Kachhwaha, S. Nootropic medicinal plants: Therapeutic alternatives for Alzheimer’s disease. J. Herb. Med. 2019, 17–18, 100291. [Google Scholar] [CrossRef]
- John, O.O.; Amarachi, I.S.; Chinazom, A.P.; Adaeze, E.; Kale, M.B.; Umare, M.D.; Upaganlawar, A.B. Phytotherapy: A promising approach for the treatment of Alzheimer’s disease. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Embaye, K.S.; Huang, F.; Li, L.; Zhu, F.; Wang, J.Z.; Liu, R.; Feng, J.; Wang, X. Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing Res. Rev. 2022, 74, 101544. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Barroso, M.F.; De Simone, A.; Emriková, E.; Dias-Teixeira, M.; Pereira, J.P.; Chlebek, J.; Fernandes, V.C.; Rodrigues, F.; Andrisano, V.; et al. Multi-target neuroprotective effects of herbal medicines for Alzheimer’s disease. J. Ethnopharmacol. 2022, 290, 2021. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 3954. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, S.; Jin, C.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H. Traditional East Asian Herbal Medicine Treatment for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Pharmaceuticals 2022, 15, 174. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, P.; Pandey, A.; Khanna, V.K.; Pant, A.B. Phytomedicine: A Potential Alternative Medicine in Controlling Neurological Disorders; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Majidazar, R.; Rezazadeh-Gavgani, E.; Sadigh-Eteghad, S.; Naseri, A. Pharmacotherapy of Alzheimer’s disease: An overview of systematic reviews. Eur. J. Clin. Pharmacol. 2022, 78, 1567–1587. [Google Scholar] [CrossRef]
- Peng, Y.; Tao, H.; Wang, S.; Xiao, J.; Wang, Y.; Su, H. Dietary intervention with edible medicinal plants and derived products for prevention of Alzheimer’s disease: A compendium of time-tested strategy. J. Funct. Foods 2021, 81, 104463. [Google Scholar] [CrossRef]
- Zieneldien, T.; Kim, J.; Cao, C. The Multifaceted Role of Neuroprotective Plants in Alzheimer’s Disease Treatment. Geriatrics 2022, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Okem, A.; Henstra, C.; Lambert, M.; Hayeshi, R. Medicine in Drug Discovery A review of the pharmacodynamic effect of chemo-herbal drug combinations therapy for cancer treatment. Med. Drug Discov. 2023, 17, 100147. [Google Scholar] [CrossRef]
- Samim, K.S.; Khatik, G.L.; Datusalia, A.K. Strategies for treatment of disease-associated dementia beyond Alzheimer disease: An update. Curr. Neuropharmacol. 2022, 21, 309–339. [Google Scholar] [CrossRef]
- Yarns, B.C.; Holiday, K.A.; Carlson, D.M.; Cosgrove, C.K.; Melrose, R.J. Pathophysiology of Alzheimer’s Disease. Psychiatr. Clin. N. Am. 2022, 45, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.R.; Ghasemnejad-Berenji, M. The Role of Caspases in Alzheimer’s Disease: Pathophysiology Implications and Pharmacologic Modulation. J. Alzheimer’s Dis. 2022, 91, 71–90. [Google Scholar] [CrossRef]
- Jansen, W.J.; Janssen, O.; Tijms, B.M.; Vos, S.J.B.; Ossenkoppele, R.; Visser, P.J.; Aarsland, D.; Alcolea, D.; Altomare, D.; von Arnim, C.; et al. Prevalence Estimates of Amyloid Abnormality Across the Alzheimer Disease Clinical Spectrum. JAMA Neurol. 2022, 79, 228–243. [Google Scholar] [CrossRef]
- Festa, B.; Barbosa, A.D.; Rob, M.; Rubinsztein, D.C. The pleiotropic roles of autophagy in Alzheimer’s disease: From pathophysiology to therapy. Curr. Opin. Pharmacol. 2021, 60, 149–157. [Google Scholar] [CrossRef]
- Sriram, S.; Mehkri, Y.; Quintin, S.; Lucke-Wold, B. Shared pathophysiology: Understanding stroke and Alzheimer’s disease. Clin. Neurol. Neurosurg. 2022, 218, 107306. [Google Scholar] [CrossRef]
- Levin, J.; Vöglein, J.; Quiroz, Y.T.; Bateman, R.J.; Ghisays, V.; Lopera, F.; McDade, E.; Reiman, E.; Tariot, P.N.; Morris, J.C. Testing the amyloid cascade hypothesis: Prevention trials in autosomal dominant Alzheimer disease. Alzheimer’s Dement. 2022, 18, 2687–2698. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W. The role of APP and BACE1 trafficking in APP processing and amyloid-β generation. Alzheimer’s Res. Ther. 2013, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Alawode, D.O.T.; Fox, N.C.; Zetterberg, H.; Heslegrave, A.J. Alzheimer’s Disease Biomarkers Revisited from the Amyloid Cascade Hypothesis Standpoint. Front. Neurosci. 2022, 16, 837390. [Google Scholar] [CrossRef]
- Phuna, Z.X.; Madhavan, P. A reappraisal on amyloid cascade hypothesis: The role of chronic infection in Alzheimer’s disease. Int. J. Neurosci. 2022, 133, 1071–1089. [Google Scholar] [CrossRef] [PubMed]
- Arreola, F.; Salazar, B.; Martinez, A. Fitting Contralateral Neuroanatomical Asymmetry into the Amyloid Cascade Hypothesis. Healthcare 2022, 10, 1643. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Tomé, S.O. The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef]
- Schwab, E.D.; Queiroz, R.; Fiebrantz, A.K.B.; Bastos, M.; Bonini, J.S.; da Silva, W.C.F.N. Hypothesis on ontogenesis and pathophysiology of Alzheimer’s disease. Einstein 2022, 20, eRW0170. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Chiriac, S.I.B.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Siri, M.; Dastghaib, S.; Zamani, M.; Rahmani-Kukia, N.; Geraylow, K.R.; Fakher, S.; Keshvarzi, F.; Mehrbod, P.; Ahmadi, M.; Mokarram, P.; et al. Autophagy, unfolded protein response, and neuropilin-1 cross-talk in SARS-COV-2 infection: What can be learned from other coronaviruses. Int. J. Mol. Sci. 2021, 22, 5992. [Google Scholar] [CrossRef]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Moreau, K.; Fleming, A.; Imarisio, S.; Ramirez, A.L.; Mercer, J.L.; Jimenez-Sanchez, M.; Bento, C.F.; Puri, C.; Zavodszky, E.; Siddiqi, F.; et al. PICALM modulates autophagy activity and tau accumulation. Nat. Commun. 2014, 5, 4998. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: Impact on the aging process. Ageing Res. Rev. 2013, 12, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Watzlawik, J.O.; Fiesel, F.C.; Springer, W. Autophagy in Parkinson’s disease. J. Mol. Biol. 2020, 432, 2651–2672. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, S.D.; Ferreira, A.C.; Sousa, J.C.; Correia-Neves, M.; Sousa, N.; Marques, F. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci. Biobehav. Rev. 2016, 68, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. NeuroMolecular Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Guillozet-Bongaarts, A.L.; Garcia-Sierra, F.; Reynolds, M.R.; Horowitz, P.M.; Fu, Y.; Wang, T.; Cahill, M.E.; Bigio, E.H.; Berry, R.W.; Binder, L.I. Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol. Aging 2005, 26, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Latina, V.; Corsetti, V.; Calissano, P. N-terminal tau truncation in the pathogenesis of Alzheimer’s disease (AD): Developing a novel diagnostic and therapeutic approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165584. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, E.A.; Gong, Y.; Yuan, H.; Hu, Y.; Bai, X.; Liao, X. Medicinal Plants for Anti-neurodegenerative diseases in West Africa. J. Ethnopharmacol. 2022, 285, 114468. [Google Scholar] [CrossRef]
- Klose, J.; Griehl, C.; Roßner, S.; Schilling, S. Natural Products from Plants and Algae for Treatment of Alzheimer’s Disease: A Review. Biomolecules 2022, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Reiss, A.B.; Pinkhasov, A.; Kasselman, L.J. Plants, Plants, and More Plants: Plant-Derived Nutrients and Their Protective Roles in Cognitive Function, Alzheimer’s Disease, and Other Dementias. Medicina 2022, 58, 1025. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Calfío, C.; González, A.; Lüttges, V. Novel Nutraceutical Compounds in Alzheimer Prevention. Biomolecules 2022, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- De Oliveira Zanuso, B.; de Oliveira dos Santos, A.R.; Miola, V.F.B.; Campos, L.M.G.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef]
- González-Burgos, E.; Fernandez-Moriano, C.; Gómez-Serranillos, M. Potential neuroprotective activity of Ginseng in Parkinson’s disease: A review. J. Neuroimmune Pharmacol. 2015, 10, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; Liu, S.; Song, F.; Jin, Y. Studies on the mechanism of Panax Ginseng in the treatment of deficiency of vital energy dementia rats based on urine metabolomics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1191, 123115. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, X.; Hu, Y.; Fan, X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105263. [Google Scholar] [CrossRef]
- Choi, R.J.; Roy, A.; Jung, H.J.; Ali, Y.; Min, B.S.; Park, C.H.; Yokozawa, T.; Fan, T.P.; Choi, J.S.; Jung, H.A. BACE1 molecular docking and anti-Alzheimer’s disease activities of ginsenosides. J. Ethnopharmacol. 2016, 190, 219–230. [Google Scholar] [CrossRef]
- Guan, P.P.; Cao, L.L.; Wang, P. Elevating the levels of calcium ions exacerbate Alzheimer’s disease via inducing the production and aggregation of β-amyloid protein and phosphorylated tau. Int. J. Mol. Sci. 2021, 22, 5900. [Google Scholar] [CrossRef] [PubMed]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, T.; Qi, B.; Kong, L.; Jiao, Y.; Cao, Y.; Zhang, J.; Yang, J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 179, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Go, E.A.; Uddin, M.S.; Jo, S.H.; Choi, D.K. Biological evidence of gintonin efficacy in memory disorders. Pharmacol. Res. 2021, 163, 105221. [Google Scholar] [CrossRef] [PubMed]
- Nadh, A.G.; Revikumar, A.; Sudhakaran, R.; Nair, A.S. Identification of potential lead compounds against BACE1 through in-silico screening of phytochemicals of Medhya rasayana plants for Alzheimer’s disease management. Comput. Biol. Med. 2022, 145, 105422. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, N.E.; Cho, H.J.; Lee, R.M.; Rhim, H.; Kim, H.C.; Han, M.; Lee, E.H.; Park, J.; Kim, J.N.; et al. Gintonin facilitates brain delivery of donepezil, a therapeutic drug for Alzheimer disease, through lysophosphatidic acid 1/3 and vascular endothelial growth factor receptors. J. Ginseng Res. 2021, 45, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, S.; Kim, W. Drug repositioning for cancer therapy based on large-scale drug-induced transcriptional signatures. PLoS ONE 2016, 11, e0150460. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, S.W.; Kim, S.Y.; Cho, I.H.; Kim, H.C.; Rhim, H.; Kim, M.; Nah, S.Y. Panax ginseng as an adjuvant treatment for Alzheimer’s disease. J. Ginseng Res. 2018, 42, 401–411. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Kim, M.J.; Kwon, S.H.; Jeon, J.; Shin, S.J.; Park, S.; Chang, S.; Kim, H.U.; Lee, Y.Y.; et al. Proteomic analysis for the effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on Alzheimer’s disease in a mouse model. J. Ginseng Res. 2022, 47, 302–310. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.Y.; Kim, Y.; Jeon, S.; Cha, M.S.; Kim, Y.J.; Yoon, H.G. Beneficial Effects of a Combination of Curcuma longa L. and Citrus junos Against Beta-Amyloid Peptide-Induced Neurodegeneration in Mice. J. Med. Food 2022, 25, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Gilani, A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-β in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef] [PubMed]
- Goozee, K.G.; Shah, T.M.; Sohrabi, H.R.; Rainey-Smith, S.R.; Brown, B.; Verdile, G.; Martins, R.N. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- Bland, A.R.; Ashton, J.C.; Kamal, M.A.; Sahebkar, A. The Current State of Evidence for the Therapeutic Role of Curcumin in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2022, 22, 318–320. [Google Scholar] [CrossRef]
- HYu, H.; Yamashita, T.; Hu, X.; Bian, Z.; Hu, X.; Feng, T.; Tadokoro, K.; Morihara, R.; Abe, K. Protective and anti-oxidative effects of curcumin and resveratrol on Aβ-oligomer-induced damage in the SH-SY5Y cell line. J. Neurol. Sci. 2022, 441, 120356. [Google Scholar] [CrossRef]
- Don, T.M.; Chang, W.J.; Jheng, R.; Huang, Y.C.; Chuang, E.Y. Curcumin-laden dual-targeting fucoidan/chitosan nanocarriers for inhibiting brain inflammation via intranasal delivery. Int. J. Biol. Macromol. 2021, 181, 835–846. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Bin Sulaimee, N.H.; Pareek, T.; Cheong, W.F.; Wenk, M.R.; Pant, H.C.; Frautschy, S.A.; Low, C.M.; Kesavapany, S. Curcumin Ameliorates Neuroinflammation, Neurodegeneration, and Memory Deficits in 25 Transgenic Mouse Model that Bears Hallmarks of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Utomo, R.Y.; Sugie, A.; Okada, S.; Miura, K.; Nakamura, H. Detoxification of amyloid β fibrils by curcumin derivatives and their verification in a Drosophila Alzheimer’s model†. Chem. Commun. 2022, 58, 2576–2579. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Hosseini, M.J.; Rostamizadeh, K.; Anoush, M. Investigation of therapeutic effect of curcumin α and β glucoside anomers against Alzheimer’s disease by the nose to brain drug delivery. Brain Res. 2021, 1766, 147517. [Google Scholar] [CrossRef]
- Liu, X.G.; Lu, X.; Gao, W.; Yang, H. Structure, synthesis, biosynthesis, and activity of the characteristic compounds from Ginkgo biloba L. Nat. Prod. Rep. 2022, 39, 474–511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, H.; Yu, J.; Lei, Y.; Huang, G.; Huang, H. Extraction and properties of Ginkgo biloba leaf polysaccharide and its phosphorylated derivative. Ind. Crops Prod. 2022, 189, 115822. [Google Scholar] [CrossRef]
- Nguyen, T.; Alzahrani, T. Ginkgo Biloba; StatPearls [Internet]: Treasure Island, FL, USA, 2022.
- Liu, Y.; Xin, H.; Zhang, Y.; Che, F.; Shen, N.; Cui, Y. Leaves, seeds and exocarp of Ginkgo biloba L. (Ginkgoaceae): A Comprehensive Review of Traditional Uses, phytochemistry, pharmacology, resource utilization and toxicity. J. Ethnopharmacol. 2022, 298, 115645. [Google Scholar] [CrossRef]
- Shareena, G.; Kumar, D. Traversing through half a century research timeline on Ginkgo biloba, in transforming a botanical rarity into an active functional food ingredient. Biomed. Pharmacother. 2022, 153, 113299. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Wang, G.; Cao, F. Growth and flavonol accumulation of Ginkgo biloba leaves affected by red and blue light. Ind. Crops Prod. 2022, 187, 115488. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhang, J.; Gao, Y.; Li, S.; Chang, C.; Yu, D.; Yang, G. Ginkgolide B inhibits NLRP3 inflammasome activation and promotes microglial M2 polarization in Aβ1-42-induced microglia cells. Neurosci. Lett. 2021, 764, 136206. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Tang, C.; Wang, Z. Neuroprotective effect of ginkgetin in experimental cerebral ischemia/reperfusion via apoptosis inhibition and PI3K/Akt/mTOR signaling pathway activation. J. Cell. Biochem. 2019, 120, 18487–18495. [Google Scholar] [CrossRef] [PubMed]
- Usuki, T.; Yoshimoto, Y.; Sato, M.; Takenaka, T.; Takezawa, R.; Yoshida, Y.; Satake, M.; Suzuki, N.; Hashizume, D.; Dzyuba, S.V. Bilobalide and PC12 cells: A structure activity relationship study. Bioorganic Med. Chem. 2020, 28, 115251. [Google Scholar] [CrossRef]
- Liu, X.; Hao, W.; Qin, Y.; Decker, Y.; Wang, X.; Burkart, M.; Schötz, K.; Menger, M.D.; Fassbender, K.; Liu, Y. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain. Behav. Immun. 2015, 46, 121–131. [Google Scholar] [CrossRef]
- Hassan, I.; Ibrahim, W.N.W.; Yusuf, F.M.; Ahmad, S.A.; Ahmad, S. Biochemical Constituent of Ginkgo biloba (Seed) 80% Methanol Extract Inhibits Cholinesterase Enzymes in Javanese Medaka (Oryzias javanicus) Model. J. Toxicol. 2020, 2020, 815313. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, H.; Li, W.; Shen, N.; Cui, Y. Ginkgo biloba endopleura: An unexplored industrial waste as a potential source of flavonoids, lipids and anti-lung cancer compounds. Ind. Crops Prod. 2022, 189, 115851. [Google Scholar] [CrossRef]
- Vinutha, K.; Divakara, M.; Veena, K.; Kumar, M.A.; Ravikumar, C.; Santosh, M.; Nagaswarupa, H.; Avinash, B.; Gurushantha, K. Centella asiatica and its carbonaceous composites as novel materials for photocatalytic and electrochemical applications. Mater. Today Proc. 2020, 46, 5936–5941. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Cusido, R.M.; Moyano, E.; Palazon, J.; Bonfill, M. Metabolic gene expression and centelloside production in elicited Centella asiatica hairy root cultures. Ind. Crops Prod. 2022, 184, 114988. [Google Scholar] [CrossRef]
- Kumar, R.; Arora, R.; Sarangi, S.C.; Shankar Ganeshan, N.; Agarwal, A.; Kaleekal, T.; Gupta, Y.K. Pharmacodynamic and pharmacokinetic interactions of hydroalcoholic leaf extract of Centella asiatica with valproate and phenytoin in experimental models of epilepsy in rats. J. Ethnopharmacol. 2021, 270, 113784. [Google Scholar] [CrossRef] [PubMed]
- Jantwal, A.; Durgapal, S.; Upadhyay, J.; Rana, M.; Tariq, M.; Dhariwal, A.; Joshi, T. Centella asiatica. In Naturally Occurring Chemicals Against Alzheimer’s Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Gray, N.E.; Zweig, J.A.; Caruso, M.; Zhu, J.Y.; Wright, K.M.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in β-amyloid overexpressing mice. Mol. Cell. Neurosci. 2018, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Zweig, J.A.; Murchison, C.; Caruso, M.; Matthews, D.G.; Kawamoto, C.; Harris, C.J.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates Aβ-induced neurodegenerative spine loss and dendritic simplification. Neurosci. Lett. 2017, 646, 24–29. [Google Scholar] [CrossRef]
- Doulah, A.; Mahmoodi, G.; Borujeni, M.P. Evaluation of the pre-treatment effect of Centella asiatica medicinal plants on long-term potentiation (LTP) in rat model of Alzheimer’s disease. Neurosci. Lett. 2019, 729, 135026. [Google Scholar] [CrossRef] [PubMed]
- Witter, S.; Witter, R.; Vilu, R.; Samoson, A. Medical Plants and Nutraceuticals for Amyloid-β Fibrillation Inhibition. J. Alzheimer’s Dis. Rep. 2018, 2, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Tsai, W.H.; Chen, C.J.; Pan, T.M. Centella asiatica extract protects against amyloid β1–40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J. Tradit. Complement. Med. 2016, 6, 362–369. [Google Scholar] [CrossRef]
- Song, D.; Jiang, X.; Liu, Y.; Sun, Y.; Cao, S.; Zhang, Z. Asiaticoside attenuates cell growth inhibition and apoptosis induced by Aβ1–42 via inhibiting the TLR4/NF-κB signaling pathway in human brain microvascular endothelial cells. Front. Pharmacol. 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Thenmozhi, A.J.; Manivasagam, T.; Bharathi, M.D.; Essa, M.M.; Guillemin, G.J. Neuroprotective role of asiatic acid in aluminium chloride induced rat model of Alzheimer’s disease. Front. Biosci. Sch. 2018, 10, 262–275. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, W.; Wang, P.; Chu, J. Asiatic acid protects differentiated PC12 cells from Aβ25–35-induced apoptosis and tau hyperphosphorylation via regulating PI3K/Akt/GSK-3β signaling. Life Sci. 2018, 208, 96–101. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Niu, Z.; Zhong, K.; Liu, T.; Yang, M.; Ji, L.; Hu, W. A review of pharmacokinetic and pharmacological properties of asiaticoside, a major active constituent of Centella asiatica (L.) Urb. J. Ethnopharmacol. 2023, 302, 115865. [Google Scholar] [CrossRef] [PubMed]
- Simayi, Z.; Aierken, W.; Rozi, P.; Ababaikeri, G.; Bo, C.; Chenglin, Z.; Askar, G.; Xiaojun, Y. Optimization of ultrasound-assisted extraction, structural, functional, and antioxidant properties of Glycyrrhiza uralensis seed protein. Process Biochem. 2022, 124, 1–12. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Dhiman, A.; Handa, M.; Ruwali, M.; Singh, D.; Kesharwani, P.; Shukla, R. Recent trends of natural based therapeutics for mitochondria targeting in Alzheimer’s disease. Mitochondrion 2022, 64, 112–124. [Google Scholar] [CrossRef]
- Hasan, A.; Zhang, M.; Shang, Z.P.; Yi, Y.; Kuang, Y.; Yu, R.; Fan, J.J.; Huang, Y.X.; Nijat, D.; Qiao, X.; et al. Bioactive prenylated phenolic compounds from the aerial parts of Glycyrrhiza uralensis. Phytochemistry 2022, 201, 113284. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, Z.; Zhang, L.; Sui, R.; Khan, S. Exploring the inhibitory effects of liquiritigenin against tau fibrillation and related neurotoxicity as a model of preventive care in Alzheimer’s disease. Int. J. Biol. Macromol. 2021, 183, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.Y.; Park, H.K.; Kim, S.K. Effect of glycyrrhizic acid on scopolamine-induced cognitive impairment in mice. Int. Neurourol. J. 2020, 13, S48–S55. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, W.; Huang, Y.; Zeng, X. Licochalcone B, a chalcone derivative from Glycyrrhiza inflata, as a multifunctional agent for the treatment of Alzheimer’s disease. Nat. Prod. Res. 2020, 34, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Yadav, Y.C.; Singh, A.; Kannaujia, S.K.; Yadav, R. Neuroprotective effect of Citrus limon juice against scopolamine induced amnesia in Wistar rats: Role of cholinergic neurotransmission monitoring and beta-actin signaling. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100191. [Google Scholar] [CrossRef]
- Saran, L.; Damor, H.I.; Lodaya, D.H.; Suthar, M.K.; Kalariya, K.A.; Roy, S. Identification of potential accessions of Bacopa monnieri L. for herbage yield and bacosides A content. Ind. Crops Prod. 2022, 176, 114348. [Google Scholar] [CrossRef]
- Dubey, T.; Chinnathambi, S. Brahmi (Bacopa monnieri): An ayurvedic herb against the Alzheimer’s disease. Arch. Biochem. Biophys. 2019, 676, 108153. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.S. Comprehensive review of Alzheimer’s disease drugs (conventional, newer, and plant-derived) with focus on Bacopa monnieri. In Nutraceuticals in Brain Health and Beyond; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Mehla, J.; Gupta, P.; Pahuja, M.; Diwan, D.; Diksha, D. Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment. Brain Sci. 2020, 10, 964. [Google Scholar] [CrossRef]
- Malishev, R.; Nandi, S.; Kolusheva, S.; Shaham-Niv, S.; Gazit, E.; Jelinek, R. Bacoside-A, an anti-amyloid natural substance, inhibits membrane disruption by the amyloidogenic determinant of prion protein through accelerating fibril formation. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Plaingam, W.; Tencomnao, T. Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. J. Tradit. Complement. Med. 2020, 10, 460–470. [Google Scholar] [CrossRef]
- Bai, Q.K.; Zhao, Z.G. Isolation and neuronal apoptosis inhibitory property of bacoside-A3 via downregulation of β-amyloid induced inflammatory response. Biotechnol. Appl. Biochem. 2022, 69, 726–734. [Google Scholar] [CrossRef]
- Payyappallimana, U.; Ravikumar, K.; Venkatasubramanian, P. Can Guduchi (Tinospora cordifolia), a well-known ayurvedic hepato-protectant cause liver damage? J. Ayurveda Integr. Med. 2022, 14, 100658. [Google Scholar] [CrossRef]
- Sharma, A.; Bajaj, P.; Bhandari, A.; Kaur, G. From ayurvedic folk medicine to preclinical neurotherapeutic role of a miraculous herb, Tinospora cordifolia. Neurochem. Int. 2020, 141, 104891. [Google Scholar] [CrossRef]
- Adib, M.; Islam, R.; Ahsan, M.; Rahman, A.; Hossain, M.; Rahman, M.; Alshehri, S.M.; Kazi, M.; Mazid, A. Cholinesterase inhibitory activity of tinosporide and 8-hydroxytinosporide isolated from Tinospora cordifolia: In vitro and in silico studies targeting management of Alzheimer’s disease. Saudi J. Biol. Sci. 2021, 28, 3893–3900. [Google Scholar] [CrossRef]
- Arunachalam, K.; Yang, X.; San, T.T. Tinospora cordifolia (Willd.) Miers: Protection mechanisms and strategies against oxidative stress-related diseases. J. Ethnopharmacol. 2022, 283, 114540. [Google Scholar] [CrossRef] [PubMed]
- Malabadi, R.B. Biosciences and Plant Biology Recent updates on the role of herbal medicine for Alzheimer’s disease. Dementia 2021, 8, 14–45. [Google Scholar]
- Reddi, K.K.; Tetali, S.D. Dry leaf extracts of Tinospora cordifolia (Willd.) Miers attenuate oxidative stress and inflammatory condition in human monocytic (THP-1) cells. Phytomedicine 2019, 61, 152831. [Google Scholar] [CrossRef]
- Vig, R.; Bhadra, F.; Gupta, S.K.; Krishnamurthy, S.; Vasundhara, M. Neuroprotective effects of quercetin produced by an endophytic fungus Nigrospora oryzae isolated from Tinospora cordifolia. J. Appl. Microbiol. 2021, 132, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Somarathinam, K.; Gunalan, S.; Sailapathi, A.; Dharani, A.M.A.; Pannerselvam, B.; Mohanasundari, M.; Balaraman, A.K.; Kothandan, G. Antihypertensive effects of pentacyclic triterpenoid from Convolvulus pluricaulis and its plausible mechanism of action hypothesizing its selectivity targeting Mineralocorticoid receptor of RAAS. Phytomedicine Plus 2023, 3, 100408. [Google Scholar] [CrossRef]
- Sharma, R.; Singla, R.K.; Banerjee, S.; Sinha, B.; Shen, B.; Sharma, R. Role of Shankhpushpi (Convolvulus pluricaulis) in neurological disorders: An umbrella review covering evidence from ethnopharmacology to clinical studies. Neurosci. Biobehav. Rev. 2022, 140, 104795. [Google Scholar] [CrossRef] [PubMed]
- Rachitha, P.; Krupashree, K.; Jayashree, G.; Kandikattu, H.K.; Amruta, N.; Gopalan, N.; Rao, M.; Khanum, F. Chemical composition, antioxidant potential, macromolecule damage and neuroprotective activity of Convolvulus pluricaulis. J. Tradit. Complement. Med. 2018, 8, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, W. Prophylactic actions of Medhya rasayana drug—Shankhapushpi (Convolvulus pluricaulis Chois) on Drosophila (common fruit fly) model of Alzheimer’s diseases. J. Ayurveda Integr. Med. 2018, 9, S4. [Google Scholar] [CrossRef]
- Gupta, G.L.; Fernandes, J. Protective effect of Convolvulus pluricaulis against neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Biomed. Pharmacother. 2019, 109, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, F.; Abdullah; Shahzadi, I.; Ahmed, I.; Waheed, M.T.; Mirza, B. Characterization of Withania somnifera chloroplast genome and its comparison with other selected species of Solanaceae. Genomics 2020, 112, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, R.; Garg, N. Withania somnifera—A magic plant targeting multiple pathways in cancer related inflammation. Phytomedicine 2022, 101, 154137. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Wongtrakul, J.; Thongtan, T.; Kumrapich, B.; Saisawang, C.; Ketterman, A.J. Neuroprotective Effects of Withania Somnifera In The Sh-Sy5y Parkinson Cell Model. Heliyon 2021, 7, e08172. [Google Scholar] [CrossRef]
- Singh, S.K.; Valicherla, G.R.; Bikkasani, A.K.; Cheruvu, S.H.; Hossain, Z.; Taneja, I.; Ahmad, H.; Raju, K.S.; Sangwan, N.S.; Singh, S.K.; et al. Elucidation of plasma protein binding, blood partitioning, permeability, CYP phenotyping and CYP inhibition studies of Withanone using validated UPLC method: An active constituent of neuroprotective herb Ashwagandha. J. Ethnopharmacol. 2021, 270, 113819. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and Safety of Ashwagandha (Withania somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Dar, N.J.; Muzamil, A. Neurodegenerative diseases and Withania somnifera (L.): An update. J. Ethnopharmacol. 2020, 256, 112769. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Kallubai, M.; Subramanyam, R. Improving the inhibition of β-amyloid aggregation by withanolide and withanoside derivatives. Int. J. Biol. Macromol. 2021, 173, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Godkar, P.; Gordon, R.K.; Ravindran, A.; Doctor, B.P. Celastrus paniculatus seed water soluble extracts protect cultured rat forebrain neuronal cells from hydrogen peroxide-induced oxidative injury. Fitoterapia 2003, 74, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Godkar, B.; Gordon, R.K.; Ravindran, A.; Doctor, B.P. Celastrus paniculatus seed oil and organic extracts attenuate hydrogen peroxide- and glutamate-induced injury in embryonic rat forebrain neuronal cells. Phytomedicine 2006, 13, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bhanumathy, M.; Harish, M.S.; Shivaprasad, H.N.; Sushma, G. Nootropic activity of Celastrus paniculatus seed. Pharm. Biol. 2010, 48, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.H.V.; Gupta, Y.K. Antioxidant property of Celastrus paniculatus Willd.: A possible mechanism in enhancing cognition. Phytomedicine 2002, 9, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Katekhaye, S.; Duggal, S.; Singh, A.P. An Inside Preview of Nutritional and Pharmacological Profile of Celastrus paniculatus. Int. J. Recent. Adv. Pharm. Res. 2015, 1, 1069. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Alkhelaif, A.S. In vitro antimicrobial potency of Elettaria cardamomum ethanolic extract against multidrug resistant of food poisoning bacterial strains. J. King Saud. Univ. Sci. 2022, 34, 102167. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kumar, S. Alpha-terpinyl acetate: A natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. J. Funct. Foods 2020, 68, 103892. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Hashim, A.; Ahmed, M.G.; Rahiman, N.B.A.; Manikkoth, S.; Pramod, K.L. Evaluation of the neuroprotective activity of amarus in attenuating arsenic-induced neurotoxicity—An in vivo study. Phytomedicine Plus 2022, 2, 100316. [Google Scholar] [CrossRef]
- Tan, S.; Tan, E.N.Y.; Lim, Q.Y.; Nafiah, M.A. Phyllanthus acidus (L.) Skeels: A review of its traditional uses, phytochemistry, and pharmacological properties. J. Ethnopharmacol. 2020, 253, 112610. [Google Scholar] [CrossRef]
- Rauf, A.; Patel, S.; Uddin, G.; Siddiqui, B.S.; Ahmad, B.; Muhammad, N.; Mabkhot, Y.N.; Ben Hadda, T. Phytochemical, ethnomedicinal uses and pharmacological profile of genus Pistacia. Biomed. Pharmacother. 2017, 86, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Bahukhandi, A.; Joshi, T.; Kumar, A. Lepidium meyenii. In Naturally Occurring Chemicals Against Alzheimer’s Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, H.; Lu, W.; Yu, B.; Liu, L.; Jia, W.; Lin, Z.; Wang, H.; Chen, S. Four new honokiol derivatives from the stem bark of Magnolia officinalis and their anticholinesterase activities. Phytochem. Lett. 2019, 29, 195–198. [Google Scholar] [CrossRef]
- Xu, K.L.; Ma, J.; Li, C.; Li, C.J.; Yu, Y.; Chen, X.Y.; Wang, X.L.; Zhang, D.M. P-menthane-based meroterpenoids with neuroprotective effects from the bark of Magnolia officinalis var. biloba. Tetrahedron 2022, 123, 132964. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, N.; Verma, D.; Rathi, P.; Sharma, V.; Kuca, K.; Prajapati, P.K. Indian medicinal plants as drug leads in neurodegenerative disorders. In Nutraceuticals in Brain Health and Beyond; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Ramesh, B.; Karuna, R.; Sreenivasa, R.S.; Haritha, K.; Sai, M.D.; Sasis, B.R.B.; Saralakumari, D. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac. J. TroBiomed. 2012, 2, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Chin, V.K.; Ismail, N.; Ooi, D.J.; Khong, N.M.H.; Esa, N.M. Biological activities and therapeutic effects of Celastrus paniculatus seed oil. In Multiple Biological Activities of Unconventional Seed Oils; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Anaduaka, E.G.; Okagu, I.U.; Uchendu, N.O.; Ezeanyika, L.U.S.; Nwanguma, B.C. Hepato-renal toxicity of Myristica fragrans Houtt. (Myristicaceae) seed extracts in rats. J. King Saud. Univ. Sci. 2022, 34, 101694. [Google Scholar] [CrossRef]
- Shen, H.; Zheng, Y.; Chen, R.; Huang, X.; Shi, G. Neuroprotective effects of quercetin 3-O-sophoroside from Hibiscus rosa-sinensis Linn. on scopolamine-induced amnesia in mice. J. Funct. Foods 2021, 76, 104291. [Google Scholar] [CrossRef]
- Rengarajan, S.; Melanathuru, V.; Govindasamy, C.; Chinnadurai, V.; Elsadek, M.F. Antioxidant activity of flavonoid compounds isolated from the petals of Hibiscus rosa sinensis. J. King Saud. Univ. Sci. 2020, 32, 2236–2242. [Google Scholar] [CrossRef]
- Wu, M.; Liu, M.; Wang, F.; Cai, J.; Luo, Q.; Li, S.; Zhu, J.; Tang, Z.; Fang, Z.; Wang, C.; et al. The inhibition mechanism of polyphenols from Phyllanthus emblica Linn. fruit on acetylcholinesterase: A interaction, kinetic, spectroscopic, and molecular simulation study. Food Res. Int. 2022, 158, 111497. [Google Scholar] [CrossRef]
- Cha, J.M.; Yoon, D.H.; Kim, S.Y.; Kim, C.S.; Lee, K.R. Neurotrophic and anti-neuroinflammatory constituents from the aerial parts of Coriandrum sativum. Bioorg. Chem. 2020, 105, 104443. [Google Scholar] [CrossRef] [PubMed]
- Khojah, H.; Edrada-Ebel, R. P43 The Isolation and Purification of Bioactive Metabolites from Ficus carica and Their Neuroprotective Effects in Alzheimer’s Disease. Biochem. Pharmacol. 2017, 139, 140. [Google Scholar] [CrossRef]
- Hajam, T.A.; Saleem, H. Phytochemistry, biological activities, industrial and traditional uses of fig (Ficus carica): A review. Chem. Biol. Interact. 2022, 368, 110237. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Hatcher, D.; Good, A. A randomised controlled trial of Lavender (Lavandula Angustifolia) and Lemon Balm (Melissa Officinalis) essential oils for the treatment of agitated behaviour in older people with and without dementia. Complement. Ther. Med. 2019, 42, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Parveen, B.; Parveen, R.; Ahmad, S. Challenges and guidelines for clinical trial of herbal drugs. J. Pharm. Bioallied Sci. 2015, 7, 329–333. [Google Scholar] [CrossRef]
- Soheili, M.; Karimian, M.; Hamidi, G.; Salami, M. Alzheimer’s disease treatment: The share of herbal medicines. Iran. J. Basic. Med. Sci. 2021, 24, 123–135. [Google Scholar] [CrossRef]
- Baghel, M.; Yadav, P.; Badwaik, H.; Nakhate, K. Phyto-complexed systems as a versatile tool for the delivery of plant-based drugs. In Phytopharmaceuticals and Herbal Drugs; Academic Press: Cambridge, MA, USA, 2023; pp. 115–137. [Google Scholar] [CrossRef]
- Nakhate, K.; Mangrulkar, S.; Badwaik, H.; Choudhary, R.; Baghel, M.; Goyal, S. Impact of phytomedicines and their novel delivery systems as an alternative for the treatment of neurodegenerative disorders. In Phytopharmaceuticals and Herbal Drugs; Academic Press: Cambridge, MA, USA, 2023; pp. 403–431. [Google Scholar] [CrossRef]
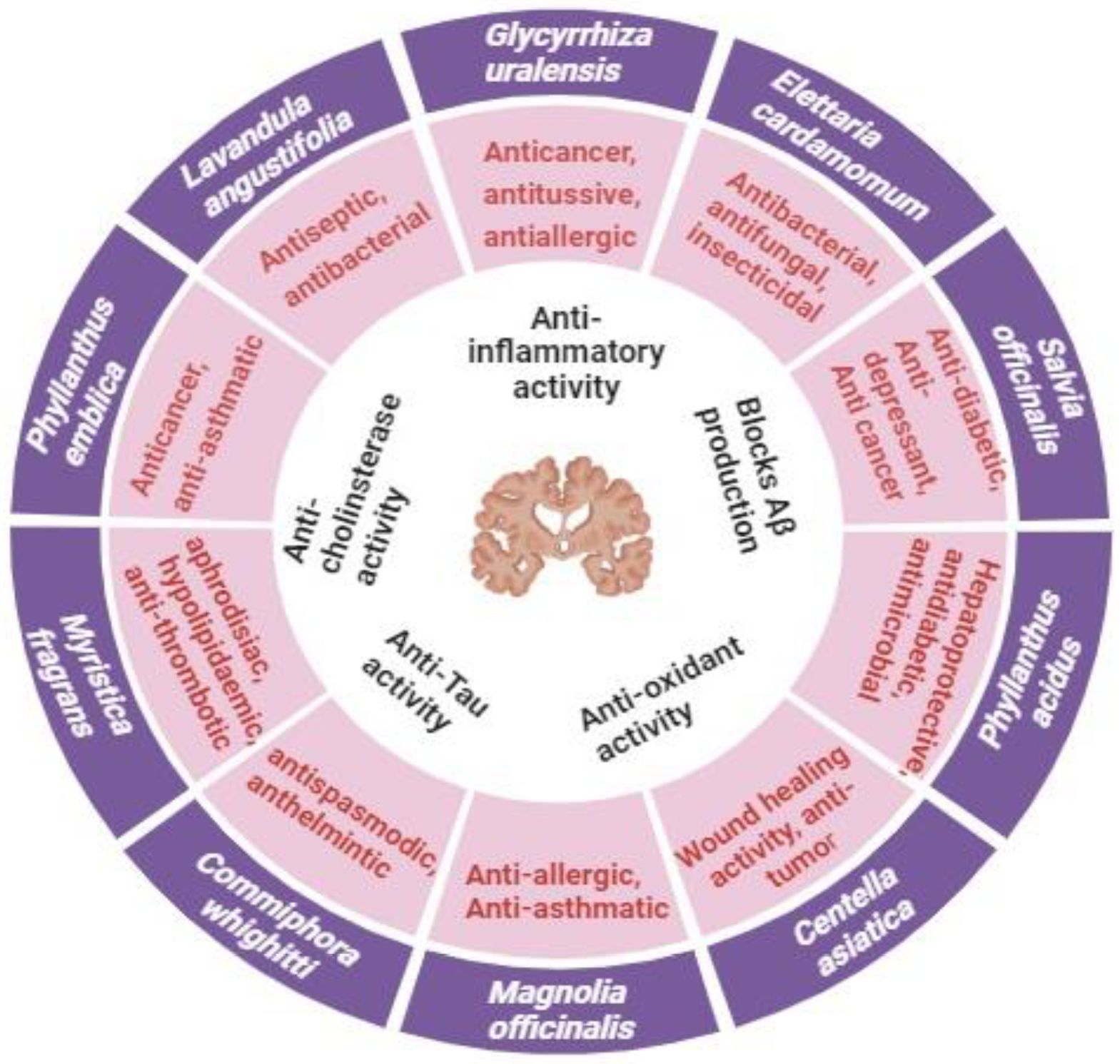
| S. No. | Plant/Herb | Family | Part Studied | Chemical Active Compounds | Mode of Anti-Alzheimer’s Action | Reference |
|---|---|---|---|---|---|---|
| 1 | Citrus limon | Rutaceae | Fresh lemon juice | Flavonoids, vitamin C, poly phenol, folic acid, potassium, pectin | Improve the cognitive performance, Citrus limon juice improved cholinergic neurotransmission and enhanced the antioxidant system | [122] |
| 2 | Elettaria cardamomum | Zingiberaceae | Ethanolic extract of E. cardamomum (seeds) | Alpha-terpinyl acetate, phenolic compounds, flavonoids, and tannins | AChE enzyme inhibition, BuChE enzyme inhibition, decrease Aβ-induced neurotoxicity, reduced oxidative stress induced by hydrogen peroxide, antioxidant activity, and anti-amyloidogenic activity | [155,156] |
| 3 | Salvia officinalis | Lamiaceae | Essential oil, ethanolic extract | Flavonoids, terpenoids, and essential oil | AChE inhibitory activity and pathogenesis of dementia | [157] |
| 4 | Phyllanthus acidus | Phyllanthaceae | Ethanolic extract of leaves | Triterpene, diterpene, sesquiterpene, and glycosides | The ethanolic extract significantly decreased lipid peroxidase and increased super oxidase dismutase and brain catalase activities against arsenic-induced neurotoxicity, AChE inhibitory potential | [158,159] |
| 5 | Pistacia vera | Anacardiaceae | Fruit extract | Flavonoids, phenolic, essential oil | Ameliorates cognitive process in scopolamine-induced Swiss albino mice, anti-Aβ aggregation, anti-neuroinflammatory properties, and AChE inhibitory activity | [15,160] |
| 6 | Lepidium meyenii | Brassicaceae | Dried hypocotyls aqueous and hydroalcoholic extracts | Polysaccharides, alkaloids, and polyphenols | Ameliorates the scopolamine-induced memory deficit, inhibits AChE activity | [161] |
| 7 | Magnolia officinalis | Magnoliaceae | Extract of stem bark | Honokiol derivatives, meroterpenoids, lignans, glycosides, alkaloids | AChE and BChE inhibitory activity | [162,163] |
| 8 | Commiphora whighitti | Burseraceae | Resin | Guggulsterone, guggulipid | AChE inhibition | [164,165] |
| 9 | Celastrus paniculatus | Celastraceae | Seed oil | Triterpenoids and sesquiterpenes | Improves ACh level | [164,166] |
| 10 | Myristica fragrans | Myristicaceae | Seed | Myristicin, elemicin, safrole, myristic acid, alpha-pinene | Improves memory deficit | [164,167] |
| 11 | Hibiscus rosa-sinensis | Malvaceae | Buds and flowers ethanolic extract | Flavonoids, glycosides, quercetin 3-O-sophoroside | Reversed the scopolamine-induced decrease in ChAT expression, increased AChE expression, and decreased ACh | [168,169] |
| 12 | Phyllanthus emblica | Euphorbiaceae | Fruit | Polyphenols, myricetin, quercetin, fisetin, and gallic acid | Inhibition effect on AChE, improves memory | [170] |
| 13 | Coriandrum sativum | Apiaceae | MeOH extract of the aerial parts | Glycosides, α-terpinene, linalool, a-pinene | Anti-neuroinflammatory activity, potent NGF secretion activity | [171] |
| 14 | Ficus carica | Moraceae | Crude extract of mesocarp | γ-sitosterol, umbelliferone, rutin, anthocyanin, coumarins | Reduce oxidative stress | [172,173] |
| 15 | Lavandula angustifolia | Lamiaceae | Leaves | Linalool, 1,8-cineole, linalyl acetate, lavandulyl acetate | Improves memory deficit | [174] |
| NCT Number | Title | Status | Interventions | Phase | Population | Sponsor | Result |
|---|---|---|---|---|---|---|---|
| NCT00391833 | Effect of Panax Ginseng on the Cognitive Performance in AD | Completed | Panax ginseng | Phase 1 Phase 2 | Enrollment: 97 Age: 40 years to 83 years (adult, older adult) | Seoul National University Hospital, Republic of Korea | Enhanced cognitive metrics observed in Alzheimer’s patients. Improvement in memory retention and recall demonstrated significant potential for Panax ginseng as a therapeutic agent in cognitive decline associated with AD. |
| NCT03221894 | A Retrospective Study to Investigate the Additive Effectiveness of Chinese Herbal Medicine in AD | Completed | Dietary supplement: GRAPE granules | Not available | Enrollment: 120 Age: 50 years to 85 years (adult, older adult) | Dongzhimen Hospital, Beijing, China; Beijing Hospital, China; Chinese PLA General Hospital, China; Peking University Third Hospital, China | Chinese herbal medicine, specifically GRAPE granules, exhibits supplementary efficacy in mitigating cognitive decline in Alzheimer’s patients. Notable improvements in memory consolidation and attentional functions were observed. |
| NCT04570644 | Randomized I/II Phase Study of ALZT-OP1 Combination Therapy in AD and Normal Healthy Volunteers | Completed | ALZT-OP1 (cromolyn and ibuprofen); ALZT-OP1a (cromolyn) and ALZT-OP1b (ibuprofen) | Phase 1 | Enrollment: 56 Age: 55 years to 79 years (adult, older adult) | AZ Therapies, Inc., Boston, MA, USA | ALZT-OP1 combination therapy exhibited promising results in both Alzheimer’s patients and healthy volunteers. Reduction in neuroinflammatory markers and cognitive enhancement were observed, indicating a potential breakthrough in disease-modifying interventions. |
| NCT04149860 | Study with Lu AF87908 in Healthy Participants and Participants with AD | Recruiting | Lu AF87908; Placebo | Phase 1 | Enrollment: 88 Age: 18 years to 65 years (adult, older adult) | H. Lundbeck A/S, Copenhagen, Den-mark | Ongoing trial to assess the safety and preliminary efficacy of Lu AF87908 in both healthy individuals and those afflicted with AD. Early indications suggest a potential for cognitive enhancement, warranting further investigation. |
| NCT02547818 | Safety and Efficacy Study of ALZT-OP1 in Subjects with Evidence of Early AD | Completed | ALZT-OP1a; ALZT-OP1b; Placebo ALZT-OP1a; Placebo ALZT-OP1b | Phase 3 | Enrollment: 620 Age: 55 years to 79 years (adult, older adult) | AZ Therapies, Inc., Boston, MA, USA | ALZT-OP1 demonstrates safety and substantial efficacy in subjects with early-stage AD. Robust cognitive preservation and a significant reduction in disease progression were observed, supporting its potential as a disease-modifying therapy. |
| NCT04300569 | A Study to Determine the Signs and Symptoms that Impact Daily Life of Participants with Irregular Sleep-Wake Rhythm Disorder | Completed | Non-interventional | Not available | Enrollment: 37 Age: 18 years to 90 years (adult, older adult) | Eisai Inc., Tokyo, Japan | The study delineated the significant impact of irregular sleep–wake rhythm disorder on participants’ daily lives. Key indicators affecting cognitive function were identified, shedding light on potential therapeutic avenues. |
| NCT05591027 | Safety and Target Engagement of Centella Asiatica in Cognitive Impairment | Not yet recruiting | Centella asiatica product; Placebo | Phase 1 | Enrollment: 48 Age: 65 years to 85 years (older adult) | Oregon Health and Science University Alzheimer’s Association, USA | Anticipated study aims to ascertain safety and efficacy of Centella asiatica in cognitive impairment. Preliminary data suggest promising neuroprotective effects, warranting further investigation. |
| NCT05269173 | Efficacy and Safety of Flos Gossypii Flavonoids Tablet in the Treatment of AD | Recruiting | Flos gossypii flavonoids tablet | Phase 2 | Enrollment: 240 Age: 50 years to 85 years (adult, older adult) | Capital Medical University, China; Xinjiang Uygur Pharmaceutical Co., Ltd., Wuhan, China | Ongoing investigation into the potential of Flos Gossypii flavonoids tablet in treating AD. Preliminary data suggest a favorable impact on cognitive function and disease progression. |
| NCT03286608 | Polyphenols and Risk of Dementia | Completed | Observational study (no intervention) | Not available | Enrollment: 1329 Age: 65 years and older (older adult) | Jean-François Dartigues, France; University of Bordeaux, France | Observational study elucidating the relationship between polyphenols and dementia risk. Data underscore a potential protective effect, warranting deeper mechanistic exploration. |
| NCT00205179 | AD: Potential Benefit of Isoflavones | Completed | Novasoy; Placebo | Phase 2 | Enrollment: 72 Age: 55 years and older (adult, older adult) | University of Wisconsin, Madison, USA; National Institutes of Health (NIH), USA; National Institute on Aging (NIA), USA | Isoflavones, specifically novasoy, exhibit potential benefits in AD. Significant improvements in cognitive metrics were observed, suggesting a role in disease management. |
| NCT00500500 | Effect of EGb 761® on Patients with Mild to Moderate AD | Terminated | EGb 761® (Tanakan®) | Phase 2 | 50 years to 85 years (older adult) | Ipsen, Paris, France | Trial exploring the effect of EGb 761® on patients with mild-to-moderate AD. Study discontinued prematurely, with limited conclusive data available. |
| NCT00164749 | A Pilot Study of Curcumin and Ginkgo for Treating AD | Completed | Placebo and ginkgo extract; Curcumin and ginkgo extract | Phase 1 Phase 2 | 50 years and older | Chinese University of Hong Kong; BUPA Foundation, Hong Kong; Kwong Wah Hospital, Hong Kong | Pilot study investigating the potential benefits of curcumin and ginkgo in AD. Preliminary data suggest a positive impact on cognitive function, warranting further exploration. |
| NCT00276510 | A Study of EGb 761® (Tanakan®) in Dementia of Alzheimer’s-Type Onset in Patients Suffering From Memory Complaints | Completed | EGb 761® (Tanakan®); Placebo | Phase 4 | 70 years and older | Ipsen, Paris, France | Study assessing the impact of EGb 761® in patients with dementia of Alzheimer’s-type onset. Data indicate potential cognitive benefits, suggesting a role in early intervention. |
| NCT00010803 | Ginkgo biloba Prevention Trial in Older Individuals | Completed | Ginkgo biloba Placebo | Phase 3 | 75 years and older | National Center for Complementary and Integrative Health (NCCIH), USA; Office of Dietary Supplements (ODS); National Institute of Neurological Disorders and Stroke (NINDS), USA; National Institute on Aging (NIA), USA; National Heart, Lung, and Blood Institute (NHLBI), USA | Comprehensive trial investigating the preventive potential of Ginkgo biloba in older individuals. No significant cognitive benefits were observed, necessitating further research into alternative interventions. |
| NCT03090516 | Clinical Efficacy of Ginkgo Biloba Extract in the Treatment of AD | Unknown status | Ginkgo biloba dispersible tablets; Donepezil; Ginkgo biloba dispersible tablets and donepezil | Phase 2 Phase 3 | 50 years to 85 years | The First Affiliated Hospital with Nanjing Medical University, China | Ongoing investigation into the clinical efficacy of ginkgo biloba extract in AD. Preliminary data are pending, with outcomes yet to be determined. |
| NCT01001637 | Efficacy and Safety of Curcumin Formulation in AD | Unknown status | Dietary supplement: Curcumin formulation; Dietary supplement: Placebo | Phase 2 | Enrollment: 26 Age: 50 years to 80 years (adult, older adult) | Jaslok Hospital and Research Centre, Mumbai, India; Pharmanza Herbal Pvt Ltd., Gujarat, India; Verdure Sciences; University of California, Los Angeles, USA | Study evaluating the efficacy and safety of curcumin formulation in AD. Preliminary data are pending, with outcomes yet to be determined. |
| NCT00099710 | Curcumin in Patients with Mild to Moderate AD | Completed | Dietary supplement: Curcumin C3 complex | Phase 2 | Enrollment: 33 Age: 50 years and older (adult, older adult) | John Douglas French Alzheimer’s Foundation, USA; Institute for the study of Aging (ISOA), USA; National Institute on Aging (NIA), USA | Investigation into the potential benefits of curcumin C3 complex in patients with mild-to-moderate AD. Preliminary data suggest positive cognitive effects, warranting further exploration. |
| NCT01811381 | Curcumin and Yoga Therapy for Those at Risk for AD | Unknown status | Curcumin; Behavioral: Aerobic yoga; Behavioral: Non-aerobic yoga; Dietary supplement: Placebo | Phase 2 | Enrollment: 80 Age: 50 years to 90 years (adult, older adult) | VA Office of Research and Development, USA | Ongoing trial assessing the combined impact of curcumin and yoga therapy in individuals at risk for AD. Preliminary data are pending, with outcomes yet to be determined. |
| NCT01716637 | Short Term Efficacy and Safety of Perispinal Administration of Etanercept in Mild to Moderate AD | Completed | Etanercept; Dietary supplement: Curcumin, Luteol, Theaflavin, Lipoic Acid, Fish Oil, Quercetin, Resveratrol | Phase 1 | Enrollment: 12 Age: 60 years to 85 years (adult, older adult) | Life Extension Foundation Inc., Fort Lauderdale, FL, USA | Study investigating the short-term efficacy and safety of perispinal administration of etanercept in mild-to-moderate AD. Preliminary data indicate a potential for cognitive improvement, warranting further exploration. |
| NCT04606420 | Lifestyle Changes can Reverse Early-Stage AD | Active, not recruiting | Behavioral: Lifestyle medicine | No data available | Enrollment: 51 Age: 45 years to 90 years (adult, older adult) | Preventive Medicine Research Institute; University of California, San Francisco, USA; Harvard Medical School (HMS and HSDM), USA; University of California, SanDiego, USA; The Cleveland Clinic; Renown Health, USA | Ongoing investigation into the potential of lifestyle changes in reversing early-stage AD. Preliminary data are pending, with outcomes yet to be determined. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagori, K.; Nakhate, K.T.; Yadav, K.; Ajazuddin; Pradhan, M. Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights. Future Pharmacol. 2023, 3, 877-907. https://doi.org/10.3390/futurepharmacol3040053
Nagori K, Nakhate KT, Yadav K, Ajazuddin, Pradhan M. Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights. Future Pharmacology. 2023; 3(4):877-907. https://doi.org/10.3390/futurepharmacol3040053
Chicago/Turabian StyleNagori, Kushagra, Kartik T. Nakhate, Krishna Yadav, Ajazuddin, and Madhulika Pradhan. 2023. "Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights" Future Pharmacology 3, no. 4: 877-907. https://doi.org/10.3390/futurepharmacol3040053
APA StyleNagori, K., Nakhate, K. T., Yadav, K., Ajazuddin, & Pradhan, M. (2023). Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights. Future Pharmacology, 3(4), 877-907. https://doi.org/10.3390/futurepharmacol3040053