Pseudomonas aeruginosa: A Bacterial Platform for Biopharmaceutical Production
Abstract
1. Introduction
2. Pseudomonas aeruginosa, Virulence Factors, and Quorum Sensing
3. Potential of Quorum Sensing Signaling Molecules for Biopharmaceuticals
3.1. N-Acyl-Homoserine-Lactones
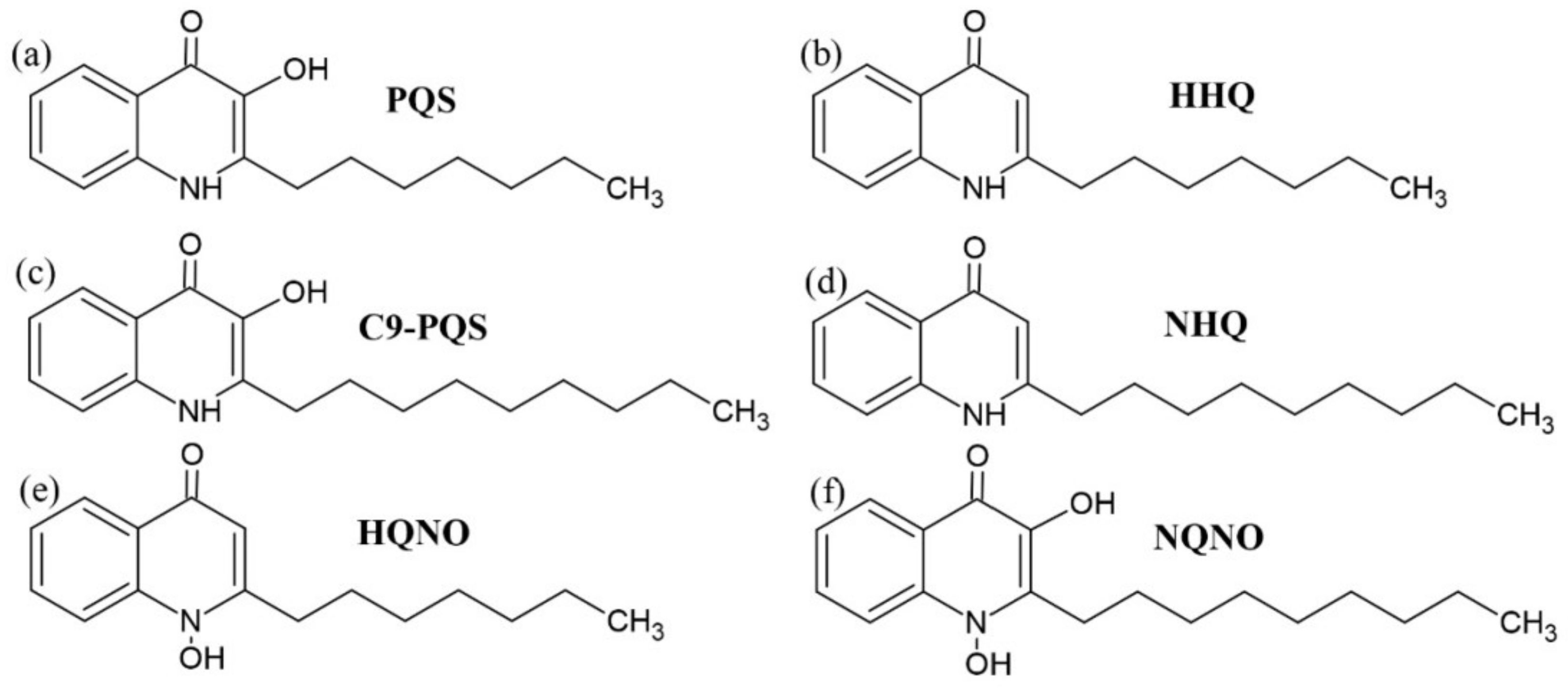
3.2. Alkyl-Quinolones
4. Virulence Factors Molecules as a Potential’s Biopharmaceuticals
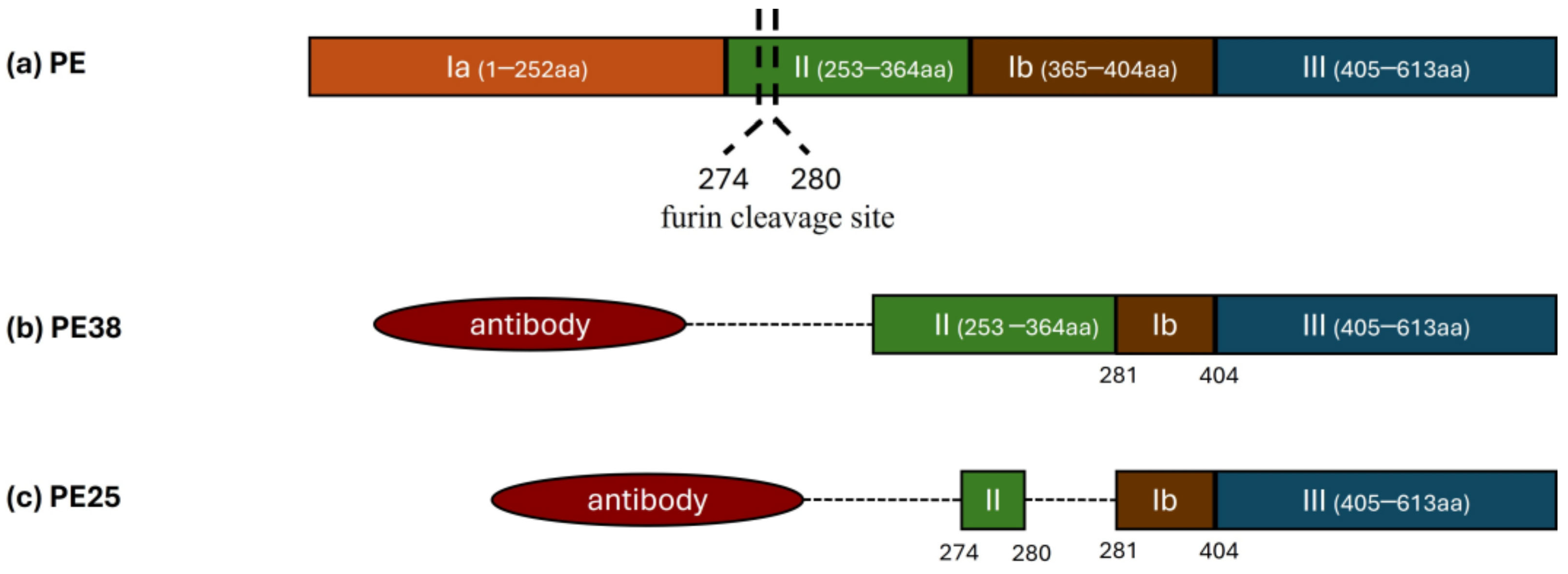
4.1. Exotoxin A
4.2. Phenazines Molecules
| Phenazine | Biological Activities | |||||
|---|---|---|---|---|---|---|
| Antimicrobial | Ref | Anti-Inflammatory | Ref | Antitumoral | Ref | |
| PYO | Staphylococcus aureus, Bacillus cereus, Staphylococcus sciuri, Salmonella paratyphi, Escherichia coli, Klebsiella pneumoniae, Salmonella typhi | [101,102] | Reduction of nitric oxide, TNF-α, and IL-1β. Did not affect leukocyte migration. | [103,104] | Growth inhibition: HepG2, MCF-7, HCT-116, A-549, A54, MDA-MB-231, and Caco-2. | [101,105] |
| PCA | Rhizoctonia solani, Magnaporthe grisea, Fusarium graminearum, Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola, Staphylococcus aureus | [106,107] | Reduces mRNA levels of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 | [108] | Antiproliferative activity in SK-MEL-2 and DU145. | [109,110] |
| 1-OH-PHZ | Salmonella sp., Klebsiella oxytoca, Candida albicans, Aspergillus fumigatus | [108,111] | Reduces mRNA levels of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 | [108] | - | - |
| PCN | Staphylococcus aureus, Rhizoctonia solani, Fusarium oxysporum f. sp. radicis-lycopersici, Rhizoctonia solani, Pythium ultimum, Fusarium oxysporum f. sp. Niveum. | [111,112] | - | Inhibition against lung (A549) and breast (MDA MB-231) cancer cells | [113] | |
| 5-Me-PCA | Staphylococcus aureus, Micrococcus luteus, Bacillus sp., and Candida albicans | [21] | - | Demonstrated selective cytotoxic activity against cancer cells, such as the A549 (lung cancer) and MDA MB-231 (breast cancer) cell lines | [113] | |
4.2.1. Pyocyanin
4.2.2. 1-Hydroxyphenazine
4.2.3. Phenazine-1-Carboxylic Acid
4.2.4. Phenazine-1-Carboxamide
4.2.5. 5-Methyl-Phenazine-1-Carboxylic Acid
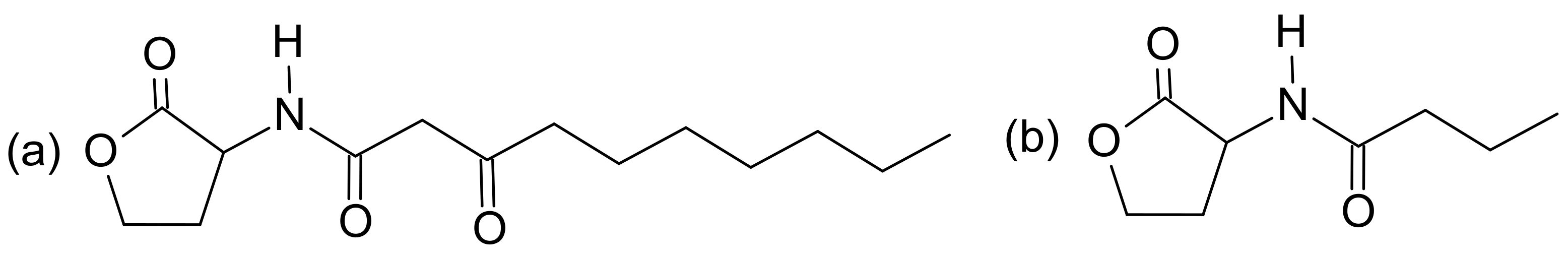
4.2.6. Aeruginosins
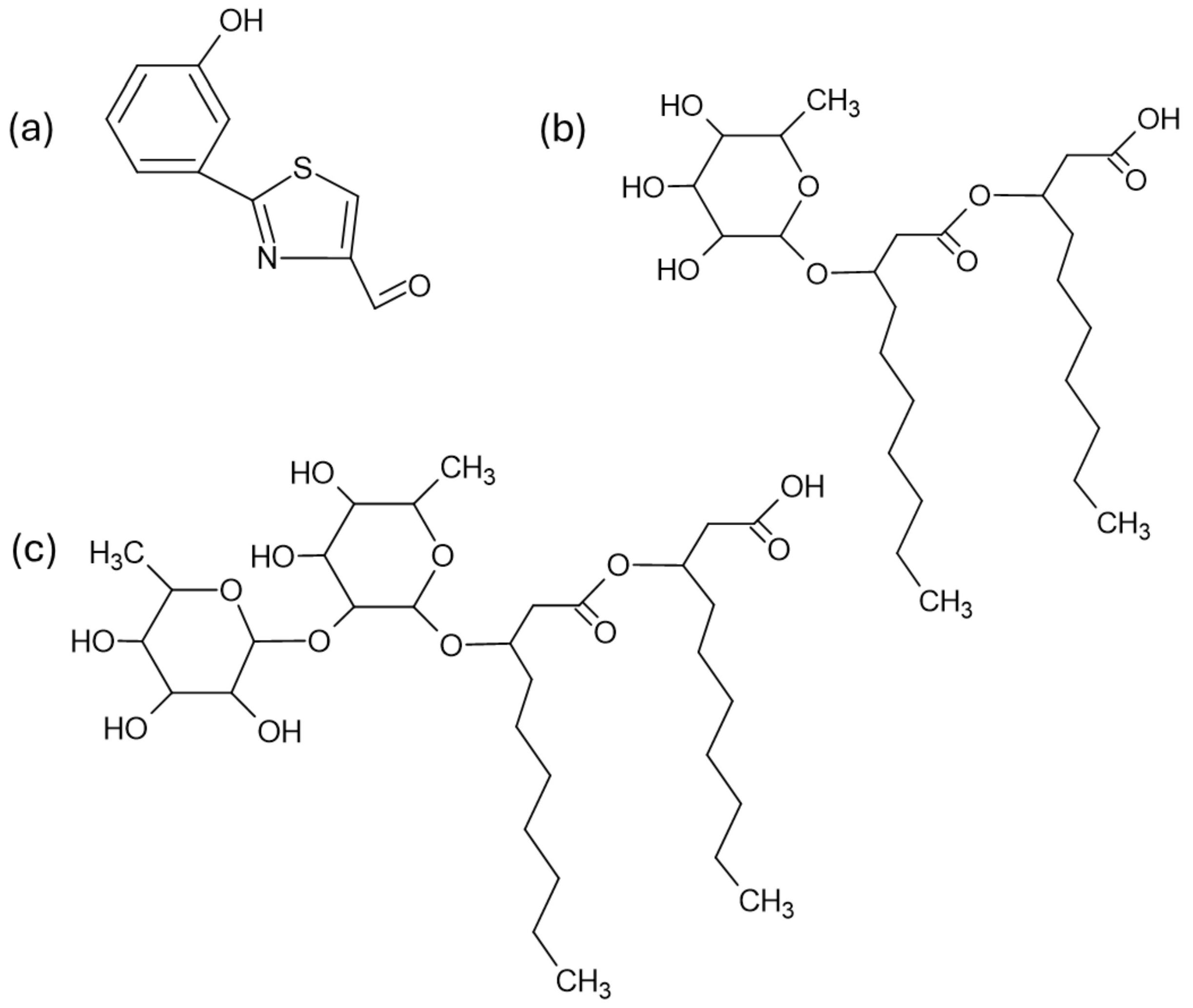
4.3. Aeruginaldehyde
4.4. Rhamnolipids
4.4.1. Rhamnolipids: Antimicrobial Activity
| Congeners # | Microorganism | Strains | Concentration | Results | Ref. |
|---|---|---|---|---|---|
| Mixture of congeners | Gram-negative bacteria | Acinetobacter baumannii | 250–1000 μg/mL | Decreased cell viability. Anti-biofilm action through inhibition of acyl-homoserine lactone. | [156] |
| Mixture of congeners | Gram-positive bacteria | Enterococcus faecium Staphylococcus aureus | 10–1000 μg/mL | Rhamnolipids exhibited inhibitory effects against all bacteria, being more effective than antibiotics (positive control), except for S. aureus and Enterobacter sp. | [157] |
| Gram-negative bacteria | Klebsiella pneumoniae Acinetobacter baumannii Pseudomonas aeruginosa Enterobacter sp. | ||||
| Rha-C10-C10 (54.4%), Rha-Rha-C10-C10 (24.2%), Rha-C10-C8 (7.2%) and other congeners | Gram-positive bacteria | Staphylococcus aureus Listeria monocytogenes Bacillus cereus | 4.9–2500 μg/mL | Gram-negative bacteria were resistant to rhamnolipids, while Gram-positive were sensitive, especially in acidic pH conditions. | [154] |
| Gram-negative bacteria | Escherichia coli Salmonella enterica | ||||
| Rha-C12:1-C10 and Rha-C10-C12:1 (29.8%), Rha-C12-C10 and Rha-C10-C12 (29.8%), Rha-C10-C10, Rha-C8-C12, and Rha-C12-C8 (19.3%), Rha-C14:1-C10, Rha-C12:1-C12, and Rha-C12-C12:1 (5.6%), and other congeners | Gram-positive bacteria | Staphylococcus aureus 6538 Staphylococcus aureus 6538p and clinical isolates: MRSA, MSSA, β-LPSA, QRSA, VRSA, and MLSB | 12.5–50 µg/mL | Inhibition of all strains tested. Anti-biofilm activity with ability to penetrate mature biofilm. | [158] |
| Rha-C10-C10 (54.4%), Rha-Rha-C10-C10 (24.2%), and other congeners | Gram-positive bacteria | Listeria monocytogenes | 4.9–2500 mg/L | Antimicrobial activity was more effective at acidic pH levels. | [155] |
| Mixture of congeners | Gram-positive bacteria | Cutibacterium acnes | 31.3 µg/mL | Bactericidal. | [159] |
| Four rhamnolipid mixtures (rh3774, rh3775, rh3776, and rh3777): Rha-Rha-FA-FA, Rha-FA-FA, Rha-FA, and Rha-Rha-FA | Yeast | Trichosporon cutaneum | 1–1000 mg/L | The four mixtures caused inhibition and reduction of biofilm. | [160] |
| Rha-C12:1 (44.2%), Rha-C18:2 (20%), Rha-C9 (14.5%), Rha-C10 (14.5%), Rha-C14 (12%), Rha-C16 (11%), Rha-C8-C8 (10.1%), Rha-C18:1 (8.2%), Rha-C19 (8.1%), Rha-C8 (8%), Rha-Rha-C10 (7.2%), Rha-C18 (6.1%) Rha-C10-C10 (6.1%), Rha-C10-C12:2 (6.1%), Rha-Rha-C16 (5.5%), and other congeners | Yeast | Candida tropicalis | 1.95–1000 μg/mL | Biofilm disruptive activity. | [161] |
| Mixture of congeners: Rha-C8:2, Rha-C10:2, Rha-C12:2, Rha-C8-C10 or Rha-C10-C8, Rha-C10-C10, Rha-C10-C12:1 or Rha-C12-C10:1 or Rha-C10:1-C12 or Rha-C12:1-C10, Rha-Rha-C10:1-C10:1 or Rha-Rha-C10-C10:2 or Rha-Rha-C10:2-C10 and Rha-Rha-C10-C10 | Yeast | Candida albicans | 25–50.000 µg/mL | Low antifungal activity. | [162] |
| Rha-C8-C10 (44.7%), Rha-C12:2 (35.8%), Rha-C12-C14 (15.4%), Rha-C10:2 (14.4%), Rha-Rha-C8-C10 (12%), Rha-C10:1 (11.4%), Rha-Rha-C12-C12:2 (10.9%), Rha-Rha-C10-C10 (9.4%), Rha-Rha-C10 (7.4%), Rha-C8:2 (6.9%), Rha-C10-C12:2 (6.4%), Rha-C12-C14:1 (5.5%), Rha-C9:2 (5.1%), and other congeners | Filamentous fungus | Trichophyton rubrum Trichophyton mentagrophytes | 0.003–2 mg/mL | Disruptive effect on the biofilms of both fungi and cell death induction. | [163] |
| Mixture of congeners: Rha-C8:2, Rha-C10:2, Rha-C12:2, Rha-C8-C10 or Rha-C10-C8, Rha-C10-C10, Rha-C10-C12:1 or Rha-C12-C10:1 or Rha-C10:1-C12 or Rha-C12:1-C10, Rha-Rha-C10:1-C10:1 or Rha-Rha-C10-C10:2 or Rha-Rha-C10:2-C10 and Rha-Rha-C10-C10 | Enveloped virus | HSV-1, MHV-3, and RSV | 6.25–500 µg/mL | The Rhamnolipids mixture was able to inactivate all enveloped viruses, but not the non-enveloped PV-1 virus. | [162] |
| Non-enveloped virus | PV-1 | ||||
| Rha-C12:1-C10 (19%), Rha-C12-C10 (14%), Rha-C14:1-C10 (13%), Rha-C14-C10 (12%), Rha-C10-C10 (10%), Rha-C12:1-C12:1 (8%), Rha-C8-C10 (5%), Rha-C12:1-C8 (5%), Rha-C12:1-C12 (5%), Rha-C16:1-C10 (5%), and other congeners | Enveloped virus | HSV-1, HSV-2, HCoV-229E, HCoV-OC43, and SARS-CoV-2 | 0.7–50 µg/mL | Strong activity against enveloped viruses and weak activity against the non-enveloped virus PV-1. | [164] |
| Non-enveloped virus | PV-1 |
4.4.2. Rhamnolipids: Anticancer Activity
| Congeners # | Cell Lines | Concentration | Results | Ref. |
|---|---|---|---|---|
| Rha-C10-C10 (74.6%), Rha-C10-C12 (7.6), Rha-C10-C12:1 (5.9%), Rha-C10-C8 (5.6%), and other congeners | HCT-116 and Caco2 Healthy cells: CCD-841-CoN | 10–100 μg/mL | Reduction in viability of both cell lines without significant detrimental effects on the healthy cell. | [171] |
| Di-RLs fraction: Rha-Rha-C10-C10 (major congener) and mono-RL fraction: Rha-C10-C10 (major congener) separately tested | MCF-7 | 0–500 μg/mL | Mono-RLs were more effective than di-RLs in inhibiting cancer cells. | [167] |
| Mixture of congeners | MCF-7, HT-29, and E-143 | 10–250 μg/mL | Significant cytotoxic activity in the three cell lines. | [150] |
| Di-RL mixture: Rha-Rha-C10-C10 (major congener) and mono-RL mixture: Rha-C10-C10 (major congener) separately tested | HepG2, Caco-2 HeLa, and MCF-7 Healthy cells: HK-2 and Hepatocytes | 0–160 mg/L | Cancer cells and healthy cells showed cytotoxicity in the presence of mono- or di-RLs. | [168] |
| RL-1 fraction: Rha-C10-C10 (major congener) RL-2 fraction: Rha-Rha-C10-C10 (major congener) | HL-60, BV-173, SKW-3, and JMSU-1 | 0–250 μM | RL-1 fraction inhibited 50% of cell viability in all cell lines at lower concentrations than the RL-2 fraction. | [172] |
| Mono-RL mixture: Rha-C10-C10 (84.4%), Rha-C10-C12:1/Rha-C12:1-C10 (6.6%), and other congeners Di-RL mixture: Rha-Rha-C10-C10 (58%), Rha-Rha-C10 (23.8%), Rha-Rha-C10-C12/Rha-Rha-C12-C10 (8.7%), and other congeners | SK-MEL-28 Healthy cells: HaCaT | 0–500 μg/mL | Cytotoxic effects were observed in both cell lines, but with more harmful effects in SK-MEL-28. | [166] |
| Mono-RL fraction and Di- RL fraction | MCF-7 and MDA-MB-231 Healthy cells: MCF-10A | 0–10 µg/mL | Both fractions decreased cell viability in the tested cell lines, but a milder effect on healthy MCF-10A cells was observed. Furthermore, the Mono-RLs fraction was more effective than the Di-RL fraction. | [169] |
| Di-RL mixture: Rha-Rha-C10-C10 (major congener) | MCF-7, K-562, HeLa, HOP-62, and HT-29 | 10–80 µg/mL | Inhibition of growth of all cell lines. | [173] |
| Mixture of congeners: Rha-C8:2, Rha-C10:2, Rha-C12:2, Rha-C8-C10 or Rha-C10-C8, Rha-C10-C10, Rha-C10-C12:1 or Rha-C12-C10:1 or Rha-C10:1-C12 or Rha-C12:1-C10, Rha-Rha-C10:1-C10:1 or Rha-Rha-C10-C10:2 or Rha-Rha-C10:2-C10 and Rha-Rha-C10-C10 | HEp-2 and MCF-7 Healthy cells: Vero and L-929 | 1–500 µg/mL | Reduction in tumor cell proliferation, with selective toxicity to MCF-7 cells. | [162] |
4.4.3. Rhamnolipids: Wound Healing and Immunomodulatory Activity
| Congeners # | Biological Model | Concentration | Results | Ref. |
|---|---|---|---|---|
| Mono-RL mixture: Rha-C10-C10 (84.4%), Rha-C10-C12:1/Rha-C12:1-C10 (6.6%), and other congeners Di-RL mixture: Rha-Rha-C10-C10 (58%), Rha-Rha-C10 (23.8%), Rha-Rha-C10-C12/Rha-Rha-C12-C10 (8.7%), and other congeners | HaCaT cell line | 20 μg/mL | Mono-RL showed no effect. Di-RL attenuated IL-8 protein levels and induced IL-1RA production. | [179] |
| Mixture of congeners | Adult Sprague–Dawley rats | Gavage feeding (100 mg/kg of RLs) | Reduction in the levels of pro-inflammatory cytokines: IL-1β, IL-6, and TNF-α. | [30] |
| Mixture of congeners | Broilers (Ross 308) treated with lipopolysaccharide | Feeding with basal diet with 1.000 mg/kg of RLs | Increased levels of anti-inflammatory cytokines IL-4 and IL-10 and reduced levels of pro-inflammatory cytokines: IL-6, IL-1β, and TNF-α. | [28] |
| Mixture of congeners | Linnan Yellow Broilers | Supplementation with 1.000 mg/kg of RLs | Increased IL-1β and IL-6 levels and decreased TNF-α levels. | [29] |
| Rha-C10-C10 (73.3%), Rha-Rha-C10-C10 (24.6%), and other congeners | L929 cell line BALB/c mice with excisional wounds | 5–50 μg/mL (in vitro) 0–10 mg/mL (in vivo) | Increased migration of L929 cells and levels of p-Smad3, α-SMA, and TGF-β1. | [178] |
| Mixture 1: Rha-C12:1 (38.5%), Rha-C10-C10 (23.9%), Rha-C8:2 (15.2%), Rha-Rha-C12:1 (6.2%), and other congeners Mixture 2: Rha-C10 (24%), Rha-C12:1 (24%), Rha-C10-C10 (15.3%), Rha-Rha-C10 (9.1%), Rha-C10:2 (8.1%), Rha-Rha-C12:1 (5%), and other congeners | Wistar rats with excisional wounds infected by Staphylococcus aureus (MTCC 96) | 0.5 mg/mL of mono-RLs or di-RL | Both RL mixtures accelerated healing similarly, showing improved accumulation of dermal cells at the wound site. | [175] |
| Mixture of congeners | Wistar rats with excisional wounds | 5 mg/mL | RLs accelerated the healing process. Increased granulation and decreased inflammation were also observed. | [177] |
| Mixture of congeners | Wistar rats with excisional wounds | 5 mg/mL | RL application increased wound contraction rate, improved synthesis of collagen, and reduced TNF-α. | [176] |
4.4.4. Rhamnolipids: Active Molecules Delivery
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Resko, Z.J.; Suhi, R.F.; Thota, A.V.; Kroken, A.R. Evidence for Intracellular Pseudomonas aeruginosa. J. Bacteriol. 2024, 206, e0010924. [Google Scholar] [CrossRef] [PubMed]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete Genome Sequence of Pseudomonas aeruginosa PAO1, an Opportunistic Pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine Compounds in Fluorescent Pseudomonas spp. Biosynthesis and Regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Bonsall, R.F.; Delaney, S.M.; Soule, M.J.; Phillips, G.; Thomashow, L.S. Functional Analysis of Genes for Biosynthesis of Pyocyanin and Phenazine-1-Carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001, 183, 6454–6465. [Google Scholar] [CrossRef]
- Price-Whelan, A.; Dietrich, L.E.P.; Newman, D.K. Rethinking “secondary” Metabolism: Physiological Roles for Phenazine Antibiotics. Nat. Chem. Biol. 2006, 2, 71–78. [Google Scholar] [CrossRef]
- Vilaplana, L.; Marco, M.-P. Phenazines as Potential Biomarkers of Pseudomonas aeruginosa Infections: Synthesis Regulation, Pathogenesis and Analytical Methods for Their Detection. Anal. Bioanal. Chem. 2020, 412, 5897–5912. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of Structures, Microbial Origins and Roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Exploring the World of Rhamnolipids: A Critical Review of Their Production, Interfacial Properties, and Potential Application. Curr. Opin. Colloid Interface Sci. 2024, 69, 101780. [Google Scholar] [CrossRef]
- Conceição, K.S.; de Alencar Almeida, M.; Sawoniuk, I.C.; Marques, G.D.; de Sousa Faria-Tischer, P.C.; Tischer, C.A.; Vignoli, J.A.; Camilios-Neto, D. Rhamnolipid Production by Pseudomonas aeruginosa Grown on Membranes of Bacterial Cellulose Supplemented with Corn Bran Water Extract. Environ. Sci. Pollut. Res. 2020, 27, 30222–30231. [Google Scholar] [CrossRef] [PubMed]
- Deziel, E.; Lepine, F.; Milot, S.; Villemur, R. RhlA Is Required for the Production of a Novel Biosurfactant Promoting Swarming Motility in Pseudomonas aeruginosa: 3-(3-Hydroxyalkanoyloxy)Alkanoic Acids (HAAs), the Precursors of Rhamnolipids. Microbiology 2003, 149, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Bru, J.-L.; Kasallis, S.J.; Zhuo, Q.; Høyland-Kroghsbo, N.M.; Siryaporn, A. Swarming of P. Aeruginosa: Through the Lens of Biophysics. Biophys. Rev. 2023, 4, 031305. [Google Scholar] [CrossRef]
- Davey, M.E.; Caiazza, N.C.; O’Toole, G.A. Rhamnolipid Surfactant Production Affects Biofilm Architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003, 185, 1027–1036. [Google Scholar] [CrossRef]
- David, A.; Tahrioui, A.; Tareau, A.-S.; Forge, A.; Gonzalez, M.; Bouffartigues, E.; Lesouhaitier, O.; Chevalier, S. Pseudomonas aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production. Antibiotics 2024, 13, 688. [Google Scholar] [CrossRef]
- Noordman, W.H.; Janssen, D.B. Rhamnolipid Stimulates Uptake of Hydrophobic Compounds by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2002, 68, 4502–4508. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Zhong, H.; Xiao, R.; Zeng, G.; Liu, Z.; Cheng, M.; Lai, C.; Zhang, C.; Liu, G.; et al. Mechanisms for Rhamnolipids-Mediated Biodegradation of Hydrophobic Organic Compounds. Sci. Total Environ. 2018, 634, 1–11. [Google Scholar] [CrossRef]
- Abu, E.A.; Su, S.; Sallans, L.; Boissy, R.E.; Greatens, A.; Heineman, W.R.; Hassett, D.J. Cyclic Voltammetric, Fluorescence and Biological Analysis of Purified Aeruginosin A, a Secreted Red Pigment of Pseudomonas aeruginosa PAO1. Microbiology 2013, 159, 1736–1747. [Google Scholar] [CrossRef]
- Hansford, G.S.; Holliman, F.G.; Herbert, R.B. Pigments of Pseudomonas Species. Part IV. in Vitro and in Vivo Conversion of 5-Methylphenazinium-1-Carboxylate into Aeruginosin A. J. Chem. Soc. Perkin 1 1972, 1, 103–105. [Google Scholar] [CrossRef]
- Kennedy, R.K.; Naik, P.R.; Veena, V.; Lakshmi, B.S.; Lakshmi, P.; Krishna, R.; Sakthivel, N. 5-Methyl Phenazine-1-Carboxylic Acid: A Novel Bioactive Metabolite by a Rhizosphere Soil Bacterium That Exhibits Potent Antimicrobial and Anticancer Activities. Chem. Biol. Interact. 2015, 231, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid Biosurfactants as New Players in Animal and Plant Defense against Microbes. Int. J. Mol. Sci. 2010, 11, 5096–5109. [Google Scholar] [CrossRef] [PubMed]
- Varnier, A.-L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.-H.; Kauffmann, S.; Pugin, A.; et al. Bacterial Rhamnolipids Are Novel MAMPs Conferring Resistance to Botrytis Cinerea in Grapevine. Plant Cell Environ. 2009, 32, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Jiang, L.; Shao, H.; You, C.; Zhang, G.; Ding, S.; Bian, T.; Han, C.; Meng, Q. Targeted Killing of Myofibroblasts by Biosurfactant Di-Rhamnolipid Suggests a Therapy against Scar Formation. Sci. Rep. 2016, 6, 37553. [Google Scholar] [CrossRef]
- Gupta, S.; Raghuwanshi, N.; Varshney, R.; Banat, I.M.; Srivastava, A.K.; Pruthi, P.A.; Pruthi, V. Accelerated in Vivo Wound Healing Evaluation of Microbial Glycolipid Containing Ointment as a Transdermal Substitute. Biomed. Pharmacother. 2017, 94, 1186–1196. [Google Scholar] [CrossRef]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced Healing of Full-Thickness Burn Wounds Using Di-Rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, X.; Li, Q.; Cao, G.; Feng, J.; Shen, Y.; Yang, C. Effects of Rhamnolipids on Growth Performance, Immune Function, and Cecal Microflora in Linnan Yellow Broilers Challenged with Lipopolysaccharides. Antibiotics 2021, 10, 905. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Chen, Y.; Liu, J.; Wu, Y.; Xu, Y. Multi-Omics Revealed the Protective Effects of Rhamnolipids in Lipopolysaccharide Challenged Broilers. Front. Immunol. 2022, 13, 824664. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, G.; Zhang, H.; Lan, J.; Yang, C. Effects of Rhamnolipids on Growth Performance and Intestinal Health Parameters in Linnan Yellow Broilers. Poult. Sci. 2021, 100, 810–819. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, S.; Wu, Y.; Zhang, R.; Xu, Y.; Yang, C. Rhamnolipids Regulate Lipid Metabolism, Immune Response, and Gut Microbiota in Rats. Front. Nutr. 2022, 9, 886256. [Google Scholar] [CrossRef]
- Schuster, M.; Greenberg, E.P. A Network of Networks: Quorum-Sensing Gene Regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006, 296, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.-L.; Bassler, B.L. Bacterial Quorum-Sensing Network Architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Diggle, S.P.; Cornelis, P.; Williams, P.; Camara, M. 4-Quinolone Signalling in Pseudomonas aeruginosa: Old Molecules, New Perspectives. Int. J. Med. Microbiol. 2006, 296, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Lostroh, C.P.; Ogi, T.; Greenberg, E.P. Identification, Timing, and Signal Specificity of Pseudomonas aeruginosa Quorum-Controlled Genes: A Transcriptome Analysis. J. Bacteriol. 2003, 185, 2066–2079. [Google Scholar] [CrossRef]
- Hughes, D.T.; Sperandio, V. Inter-Kingdom Signalling: Communication between Bacteria and Their Hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar] [CrossRef]
- Shin, J.; Ahn, S.H.; Kim, S.H.; Oh, D.-J. N-3-Oxododecanoyl Homoserine Lactone Exacerbates Endothelial Cell Death by Inducing Receptor-Interacting Protein Kinase 1-Dependent Apoptosis. Am. J. Physiol. Cell Physiol. 2021, 321, C644–C653. [Google Scholar] [CrossRef]
- Weimann, A.; Dinan, A.M.; Ruis, C.; Bernut, A.; Pont, S.; Brown, K.; Ryan, J.; Santos, L.; Ellison, L.; Ukor, E.; et al. Evolution and Host-Specific Adaptation of Pseudomonas aeruginosa. Science 2024, 385, eadi0908. [Google Scholar] [CrossRef]
- Iglewski, B.H.; Liu, P.V.; Kabat, D. Mechanism of Action of Pseudomonas aeruginosa Exotoxin A: Adenosine Diphosphate-Ribosylation of Mammalian Elongation Factor 2 In Vitro and In Vivo. Infect. Immun. 1977, 15, 138–144. [Google Scholar] [CrossRef]
- Rampioni, G.; Pustelny, C.; Fletcher, M.P.; Wright, V.J.; Bruce, M.; Rumbaugh, K.P.; Heeb, S.; Camara, M.; Williams, P. Transcriptomic Analysis Reveals a Global Alkyl-Quinolone-Independent Regulatory Role for PqsE in Facilitating the Environmental Adaptation of Pseudomonas aeruginosa to Plant and Animal Hosts. Environ. Microbiol. 2010, 12, 1659–1673. [Google Scholar] [CrossRef]
- McGrath, S.; Wade, D.S.; Pesci, E.C. Dueling Quorum Sensing Systems in Pseudomonas aeruginosa Control the Production of the Pseudomonas Quinolone Signal (PQS). FEMS Microbiol. Lett. 2004, 230, 27–34. [Google Scholar] [CrossRef]
- Pustelny, C.; Albers, A.; Bueldt-Karentzopoulos, K.; Parschat, K.; Chhabra, S.R.; Camara, M.; Williams, P.; Fetzner, S. Dioxygenase-Mediated Quenching of Quinolone-Dependent Quorum Sensing in Pseudomonas aeruginosa. Chem. Biol. 2009, 16, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Chan, K.G.; Chang, C.Y. Modulation of Host Biology by Pseudomonas aeruginosa Quorum Sensing Signal Molecules: Messengers or Traitors. Front. Microbiol. 2015, 6, 1226. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Kaufmann, G.F. Exploitation of Host Signaling Pathways by Microbial Quorum Sensing Signals. Curr. Opin. Microbiol. 2012, 15, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hooi, D.S.W.; Bycroft, B.W.; Chhabra, S.R.; Williams, P.; Pritchard, D.I. Differential Immune Modulatory Activity of Pseudomonas aeruginosa Quorum-Sensing Signal Molecules. Infect. Immun. 2004, 72, 6463–6470. [Google Scholar] [CrossRef]
- Ritchie, A.J.; Whittall, C.; Lazenby, J.J.; Chhabra, S.R.; Pritchard, D.I.; Cooley, M.A. The Immunomodulatory Pseudomonas aeruginosa Signalling Molecule N-(3-oxododecanoyl)-L-homoserine Lactone Enters Mammalian Cells in an Unregulated Fashion. Immunol. Cell Biol. 2007, 85, 596–602. [Google Scholar] [CrossRef]
- Jahoor, A.; Patel, R.; Bryan, A.; Do, C.; Krier, J.; Watters, C.; Wahli, W.; Li, G.; Williams, S.C.; Rumbaugh, K.P. Peroxisome Proliferator-Activated Receptors Mediate Host Cell Proinflammatory Responses to Pseudomonas aeruginosa Autoinducer. J. Bacteriol. 2008, 190, 4408–4415. [Google Scholar] [CrossRef]
- Skindersoe, M.E.; Zeuthen, L.H.; Brix, S.; Fink, L.N.; Lazenby, J.; Whittall, C.; Williams, P.; Diggle, S.P.; Froekiaer, H.; Cooley, M.; et al. Pseudomonas aeruginosa Quorum-Sensing Signal Molecules Interfere with Dendritic Cell-Induced T-Cell Proliferation. FEMS Immunol. Med. Microbiol. 2009, 55, 335–345. [Google Scholar] [CrossRef]
- Rowley, A.; Brown, B.S.; Stofega, M.; Hoh, H.; Mathew, R.; Marin, V.; Ding, R.-X.; McClure, R.A.; Bittencourt, F.M.; Chen, J.; et al. Targeting IRAK3 for Degradation to Enhance IL-12 Pro-Inflammatory Cytokine Production. ACS Chem. Biol. 2022, 17, 1315–1320. [Google Scholar] [CrossRef]
- Magram, J.; Connaughton, S.E.; Warrier, R.R.; Carvajal, D.M.; Wu, C.; Ferrante, J.; Stewart, C.; Sarmiento, U.; Faherty, D.A.; Gately, M.K. IL-12-Deficient Mice Are Defective in IFNγ Production and Type 1 Cytokine Responses. Immunity 1996, 4, 471–481. [Google Scholar] [CrossRef]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H. The Role of IL-1β during Human Immunodeficiency Virus Type 1 Infection. Rev. Med. Virol. 2023, 33, e2400. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Chamberlain, T.C.; Mui, A.L.; Little, J.P. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front. Immunol. 2021, 12, 677008. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Meng, J.; Cheng, J.; Fan, Z.; Chen, P.; Ruan, H.; Tu, Z.; Kang, N.; Li, N.; Xu, Y.; et al. Pseudomonas aeruginosa Quorum-Sensing Metabolite Induces Host Immune Cell Death through Cell Surface Lipid Domain Dissolution. Nat. Microbiol. 2018, 4, 97–111. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A Road to Inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Tateda, K.; Ishii, Y.; Horikawa, M.; Matsumoto, T.; Miyairi, S.; Pechere, J.C.; Standiford, T.J.; Ishiguro, M.; Yamaguchi, K. The Pseudomonas aeruginosa Autoinducer N-3-Oxododecanoyl Homoserine Lactone Accelerates Apoptosis in Macrophages and Neutrophils. Infect. Immun. 2003, 71, 5785–5793. [Google Scholar] [CrossRef]
- Saalim, M.; Villegas-Moreno, J.; Clark, B.R. Bacterial Alkyl-4-Quinolones: Discovery, Structural Diversity and Biological Properties. Molecules 2020, 25, 5689. [Google Scholar] [CrossRef]
- Barr, H.L.; Halliday, N.; Cámara, M.; Barrett, D.A.; Williams, P.; Forrester, D.L.; Simms, R.; Smyth, A.R.; Honeybourne, D.; Whitehouse, J.L.; et al. Pseudomonas aeruginosa Quorum Sensing Molecules Correlate with Clinical Status in Cystic Fibrosis. Eur. Respir. J. 2015, 46, 1046–1054. [Google Scholar] [CrossRef]
- Cao, T.; Sweedler, J.V.; Bohn, P.W.; Shrout, J.D. Spatiotemporal Distribution of Pseudomonas aeruginosa Alkyl Quinolones under Metabolic and Competitive Stress. mSphere 2020, 5, 1110–1128. [Google Scholar] [CrossRef]
- Michalet, S.; Allard, P.M.; Commun, C.; Ngoc, V.T.N.; Nouwade, K.; Gioia, B.; Dijoux-Franca, M.G.; Wolfender, J.L.; Doléans-Jordheim, A. Alkyl-Quinolones Derivatives as Potential Biomarkers for Pseudomonas aeruginosa Infection Chronicity in Cystic Fibrosis. Sci. Rep. 2021, 11, 20722. [Google Scholar] [CrossRef]
- Vrla, G.D.; Esposito, M.; Zhang, C.; Kang, Y.; Seyedsayamdost, M.R.; Gitai, Z. Cytotoxic Alkyl-Quinolones Mediate Surface-Induced Virulence in Pseudomonas aeruginosa. PLoS Pathog. 2020, 16, e1008867. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, W.; Saalim, M.; Wei, G.; Zaleta-Pinet, D.A.; Clark, B.R. Isolation of 2-Alkyl-4-Quinolones with Unusual Side Chains from a Chinese Pseudomonas aeruginosa Isolate. J. Nat. Prod. 2020, 83, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Supong, K.; Thawai, C.; Supothina, S.; Auncharoen, P.; Pittayakhajonwut, P. Antimicrobial and Anti-Oxidant Activities of Quinoline Alkaloids from Pseudomonas aeruginosa BCC76810. Phytochem. Lett. 2016, 17, 100–106. [Google Scholar] [CrossRef]
- Ramos, A.F.; Woods, D.F.; Shanahan, R.; Cano, R.; McGlacken, G.P.; Serra, C.; O’gara, F.; Reen, F.J. A Structure-Function Analysis of Interspecies Antagonism by the 2-Heptyl-4-Alkyl-Quinolone Signal Molecule from Pseudomonas aeruginosa. Microbiology 2020, 166, 169–179. [Google Scholar] [CrossRef]
- Wang, Y.; Hoffmann, J.P.; Chou, C.-W.; Höner zu Bentrup, K.; Fuselier, J.A.; Bitoun, J.P.; Wimley, W.C.; Morici, L.A. Burkholderia Thailandensis Outer Membrane Vesicles Exert Antimicrobial Activity against Drug-Resistant and Competitor Microbial Species. J. Microbiol. 2020, 58, 550–562. [Google Scholar] [CrossRef]
- Bultel-Poncé, V.; Berge, J.-P.; Debitus, C.; Nicolas, J.-L.; Le Guyot, M. Metabolites from the Sponge-Associated Bacterium Pseudomonas Species. Mar. Biotechnol. 1999, 1, 384. [Google Scholar] [CrossRef]
- Dubern, J.-F.; Diggle, S.P. Quorum Sensing by 2-Alkyl-4-Quinolones in Pseudomonas aeruginosa and Other Bacterial Species. Mol. Biosyst. 2008, 4, 882. [Google Scholar] [CrossRef]
- Moussa, M.; Ebrahim, W.; Kalscheuer, R.; Liu, Z.; Proksch, P. Co-Culture of the Bacterium Pseudomonas aeruginosa with the Fungus Fusarium Tricinctum Induces Bacterial Antifungal and Quorum Sensing Signaling Molecules. Phytochem. Lett. 2020, 36, 37–41. [Google Scholar] [CrossRef]
- Nazik, H.; Sass, G.; Ansari, S.R.; Ertekin, R.; Haas, H.; Déziel, E.; Stevens, D.A. Novel Intermicrobial Molecular Interaction: Pseudomonas aeruginosa Quinolone Signal (PQS) Modulates Aspergillus Fumigatus Response to Iron. Microbiology 2020, 166, 44–55. [Google Scholar] [CrossRef]
- Rella, A.; Yang, M.W.; Gruber, J.; Montagna, M.T.; Luberto, C.; Zhang, Y.-M.; Del Poeta, M. Pseudomonas aeruginosa Inhibits the Growth of Cryptococcus Species. Mycopathologia 2012, 173, 451–461. [Google Scholar] [CrossRef]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.-C.; Dietl, A.-M.; Visca, P.; Haas, H.; et al. Aspergillus-Pseudomonas Interaction, Relevant to Competition in Airways. Med. Mycol. 2019, 57, S228–S232. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Kethineni, S.; Stevens, D.A. Anti-Fungal (Aspergillus Fumigatus) Activity of Pseudomonas aeruginosa in Cystic Fibrosis Synthetic Sputum. Pathogens 2024, 13, 875. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Yawata, Y.; Toyofuku, M.; Uchiyama, H.; Nomura, N. Interspecies Interaction between Pseudomonas aeruginosa and Other Microorganisms. Microbes Environ. 2013, 28, 13–24. [Google Scholar] [CrossRef]
- Häussler, S.; Becker, T. The Pseudomonas Quinolone Signal (PQS) Balances Life and Death in Pseudomonas aeruginosa Populations. PLoS Pathog. 2008, 4, e1000166. [Google Scholar] [CrossRef]
- Kim, K.; Kim, Y.U.; Koh, B.H.; Hwang, S.S.; Kim, S.H.; Lépine, F.; Cho, Y.H.; Lee, G.R. HHQ and PQS, Two Pseudomonas aeruginosa Quorum-Sensing Molecules, down-Regulate the Innate Immune Responses through the Nuclear Factor-ΚB Pathway. Immunology 2010, 129, 578–588. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.-H.; Lépine, F.; Cho, Y.-H.; Lee, G.R. Global Gene Expression Analysis on the Target Genes of PQS and HHQ in J774A.1 Monocyte/Macrophage Cells. Microb. Pathog. 2010, 49, 174–180. [Google Scholar] [CrossRef]
- Legendre, C.; Reen, F.J.; Mooij, M.J.; McGlacken, G.P.; Adams, C.; O’Gara, F. Pseudomonas aeruginosa Alkyl Quinolones Repress Hypoxia-Inducible Factor 1 (HIF-1) Signaling through HIF-1α Degradation. Infect. Immun. 2012, 80, 3985–3992. [Google Scholar] [CrossRef]
- Feltman, H.; Schulert, G.; Khan, S.; Jain, M.; Peterson, L.; Hauser, A.R. Prevalence of Type III Secretion Genes in Clinical and Environmental Isolates of Pseudomonas aeruginosa. Microbiology 2001, 147, 2659–2669. [Google Scholar] [CrossRef]
- Galle, M.; Jin, S.; Bogaert, P.; Haegman, M.; Vandenabeele, P.; Beyaert, R. The Pseudomonas aeruginosa Type III Secretion System Has an Exotoxin S/T/Y Independent Pathogenic Role during Acute Lung Infection. PLoS ONE 2012, 7, e41547. [Google Scholar] [CrossRef]
- Snell, K.; Holder, I.A.; Leppla, S.A.; Saelingerl, C.B. Role of Exotoxin and Protease as Possible Virulence Factors in Experimental Infections with Pseudomonas aeruginosa. Infect. Immun. 1978, 19, 839–845. [Google Scholar] [CrossRef]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by Evolution for Effective Killing. Front. Microbiol. 2015, 6, 963. [Google Scholar] [CrossRef] [PubMed]
- Andersson, Y.; Juell, S.; Fodstad, Ø. Downregulation of the Antiapoptotic Mcl-1 Protein and Apoptosis in MA-11 Breast Cancer Cells Induced by an Anti-Epidermal Growth Factor Receptor-Pseudomonas Exotoxin a Immunotoxin. Int. J. Cancer 2004, 112, 475–483. [Google Scholar] [CrossRef]
- Hwang, J.; Fitzgerald, D.J.; Adhya, S.; Pastan, I. Functional Domains of Pseudomonas Exotoxin Identified by Deletion Analysis of the Gene Expressed in E. coli. Cell 1987, 48, 129–136. [Google Scholar] [CrossRef]
- Jinno, Y.; Ogata, M.; Chaudhary, V.K.; Willingham, M.C.; Adhya, S.; FitzGerald, D.; Pastan, I. Domain II Mutants of Pseudomonas Exotoxin Deficient in Translocation. J. Biol. Chem. 1989, 264, 15953–15959. [Google Scholar] [CrossRef]
- FDA. FDA Approves Moxetumomab Pasudotox-Tdfk for Hairy Cell Leukemia; FDA: Muntinlupa, Philippines, 2018.
- Kreitman, R.J.; Pastan, I. Antibody Fusion Proteins: Anti-CD22 Recombinant Immunotoxin Moxetumomab Pasudotox. Clin. Cancer Res. 2011, 17, 6398–6405. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Karlin, L.; Robak, T.; Gladstone, D.E.; le Coutre, P.; Dietrich, S.; Gotic, M.; et al. Moxetumomab Pasudotox in Relapsed/Refractory Hairy Cell Leukemia. Leukemia 2018, 32, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Moxetumomab pasudotox in hairy cell leukaemia: A profile of its use. Clin. Drug Investig. 2021, 41, 829–834. [Google Scholar] [CrossRef]
- Dieffenbach, M.; Pastan, I. Mechanisms of Resistance to Immunotoxins Containing Pseudomonas Exotoxin a in Cancer Therapy. Biomolecules 2020, 10, 979. [Google Scholar] [CrossRef]
- Mazor, R.; Pastan, I. Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation. Front. Immunol. 2020, 11, 1261. [Google Scholar] [CrossRef]
- Kaplan, G.; Mazor, R.; Lee, F.; Jang, Y.; Leshem, Y.; Pastan, I. Improving the in Vivo Efficacy of an Anti-Tac (CD25) Immunotoxin by Pseudomonas Exotoxin a Domain II Engineering. Mol. Cancer Ther. 2018, 17, 1486–1493. [Google Scholar] [CrossRef]
- Falgàs, A.; Pallarès, V.; Serna, N.; Sánchez-García, L.; Sierra, J.; Gallardo, A.; Alba-Castellón, L.; Álamo, P.; Unzueta, U.; Villaverde, A.; et al. Selective Delivery of T22-PE24-H6 to CXCR4+ Diffuse Large B-Cell Lymphoma Cells Leads to Wide Therapeutic Index in a Disseminated Mouse Model. Theranostics 2020, 10, 5169–5180. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Lee, Y.; Park, J.; Jeong, B.S.; Jo, M.; Jung, S.T.; Yoo, T.H. Construction of an Immunotoxin via Site-Specific Conjugation of Anti-Her2 IgG and Engineered Pseudomonas Exotoxin A. J. Biol. Eng. 2019, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Yue, Y.; Wang, L.; He, Q.; Xu, S.; Li, J.; Liao, Y.; Chen, Y.; Wang, S.; et al. The Immunotoxin Targeting PRLR Increases Tamoxifen Sensitivity and Enhances the Efficacy of Chemotherapy in Breast Cancer. J. Exp. Clin. Cancer Res. 2024, 43, 173. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, H.; Wang, K.; Ma, X.; Chen, X.; Chen, W.; Wang, X.; Jiang, X.; Feng, M. EGFRvIII-Targeted Immunotoxin Combined with Temozolomide and Bispecific Antibody for the Eradication of Established Glioblastoma. Biomed. Pharmacother. 2022, 155, 113659. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chae, B.-H.; Ko, D.-H.; Lee, S.-G.; Yoon, S.-R.; Kim, D.-S.; Kim, Y.-S. Enhancing the Cytotoxicity of Immunotoxins by Facilitating Their Dissociation from Target Receptors under the Reducing Conditions of the Endocytic Pathway. Int. J. Biol. Macromol. 2024, 278, 134668. [Google Scholar] [CrossRef]
- Huang, W.; Wan, Y.; Zhang, S.; Wang, C.; Zhang, Z.; Su, H.; Xiong, P.; Hou, F. Recent Advances in Phenazine Natural Products: Chemical Structures and Biological Activities. Molecules 2024, 29, 4771. [Google Scholar] [CrossRef]
- Serafim, B.; Bernardino, A.R.; Freitas, F.; Torres, C.A.V. Recent Developments in the Biological Activities, Bioproduction, and Applications of Pseudomonas spp. Phenazines. Molecules 2023, 28, 1368. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Cai, J.; Wang, Y.; Li, D.; Hua, H.; Cao, H. Advances in Phenazines over the Past Decade: Review of Their Pharmacological Activities, Mechanisms of Action, Biosynthetic Pathways and Synthetic Strategies. Mar. Drugs 2021, 19, 610. [Google Scholar] [CrossRef]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas Aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef]
- Marey, M.A.; Abozahra, R.; El-Nikhely, N.A.; Kamal, M.F.; Abdelhamid, S.M.; El-Kholy, M.A. Transforming Microbial Pigment into Therapeutic Revelation: Extraction and Characterization of Pyocyanin from Pseudomonas aeruginosa and Its Therapeutic Potential as an Antibacterial and Anticancer Agent. Microb. Cell Fact. 2024, 23, 174. [Google Scholar] [CrossRef]
- Hamad, M.N.F.; Marrez, D.A.; El-Sherbieny, S.M.R. Toxicity Evaluation and Antimicrobial Activity of Purified Pyocyanin from Pseudomonas aeruginosa. Biointerface Res. Appl. Chem. 2020, 10, 6974–6990. [Google Scholar] [CrossRef]
- Marreiro de Sales-Neto, J.; Lima, A.; Cavalcante-Silva, L.H.A.; Vasconcelos, U.; Rodrigues-Mascarenhas, S. Anti-Inflammatory Potential of Pyocyanin in LPS-Stimulated Murine Macrophages. Immunopharmacol. Immunotoxicol. 2019, 41, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.A.; Kamer, A.M.A.; Al-Monofy, K.B.; Al-Madboly, L.A. Pseudomonas aeruginosa’s Greenish-Blue Pigment Pyocyanin: Its Production and Biological Activities. Microb. Cell Fact. 2023, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.K.; El-Zawhry, Y.A.; Esmaiel, A.A.; Askora, A.A.; Mostafa, M.T. Exploring the Antimicrobial and Anticancer Potential of Pyocyanin Produced by Pseudomonas aeruginosa Strain ONO14782. Preprint 2024. [Google Scholar] [CrossRef]
- Simionato, A.S.; Navarro, M.O.P.; de Jesus, M.L.A.; Barazetti, A.R.; da Silva, C.S.; Simões, G.C.; Balbi-Peña, M.I.; de Mello, J.C.P.; Panagio, L.A.; de Almeida, R.S.C.; et al. The Effect of Phenazine-1-Carboxylic Acid on Mycelial Growth of Botrytis Cinerea Produced by Pseudomonas aeruginosa LV Strain. Front. Microbiol. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, H.X.; Sun, S.; Yang, D.D.; Jin, K.M.; Zhang, W.; He, Y.W. Biotechnological Potential of a Rhizosphere Pseudomonas aeruginosa Strain Producing Phenazine-1-Carboxylic Acid and Phenazine-1-Carboxamide. World J. Microbiol. Biotechnol. 2016, 32, 1–12. [Google Scholar] [CrossRef]
- Xiao, J.; Thwe, A.A.; Liu, T.; Gong, D.; Lin, W.; Shang, C.; Lu, Z.J. Anti-Inflammatory Effects of an Extract from Pseudomonas aeruginosa and Its Purified Product 1-Hydroxyphenazine on RAW264.7 Cells. Curr. Microbiol. 2021, 78, 2762–2773. [Google Scholar] [CrossRef]
- Karuppiah, V.; Alagappan, K.; Sivakumar, K.; Kannan, L. Phenazine-1-Carboxylic Acid-Induced Programmed Cell Death in Human Prostate Cancer Cells Is Mediated by Reactive Oxygen Species Generation and Mitochondrial-Related Apoptotic Pathway. J. Appl. Biomed. 2016, 14, 199–209. [Google Scholar] [CrossRef]
- Patil, S.; Paradeshi, J.; Chaudhari, B. Anti-Melanoma and UV-B Protective Effect of Microbial Pigment Produced by Marine Pseudomonas aeruginosa GS-33. Nat. Prod. Res. 2016, 30, 2835–2839. [Google Scholar] [CrossRef]
- Cardozo, V.F.; Oliveira, A.G.; Nishio, E.K.; Perugini, M.R.E.; Andrade, C.G.T.J.; Silveira, W.D.; Durán, N.; Andrade, G.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial Activity of Extracellular Compounds Produced by a Pseudomonas Strain against Methicillin-Resistant Staphylococcus aureus (MRSA) Strains. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 12. [Google Scholar] [CrossRef]
- Shanmugaiah, V.; Mathivanan, N.; Varghese, B. Purification, Crystal Structure and Antimicrobial Activity of Phenazine-1-Carboxamide Produced by a Growth-Promoting Biocontrol Bacterium, Pseudomonas aeruginosa MML2212. J. Appl. Microbiol. 2010, 108, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.K.; Veena, V.; Naik, P.R.; Lakshmi, P.; Krishna, R.; Sudharani, S.; Sakthivel, N. Phenazine-1-Carboxamide (PCN) from Pseudomonas sp. Strain PUP6 Selectively Induced Apoptosis in Lung (A549) and Breast (MDA MB-231) Cancer Cells by Inhibition of Antiapoptotic Bcl-2 Family Proteins. Apoptosis 2015, 20, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Fordos, M.J. Recherches Sur La Matière Colorante Des Suppurations Bleues: Pyocyanine. CR Acad. Sci. 1860, 51, 215–217. [Google Scholar]
- Ghatak, S.; Hemann, C.; Boslett, J.; Singh, K.; Sharma, A.; El Masry, M.S.; Abouhashem, A.S.; Ghosh, N.; Mathew-Steiner, S.S.; Roy, S.; et al. Bacterial Pyocyanin Inducible Keratin 6A Accelerates Closure of Epithelial Defect under Conditions of Mitochondrial Dysfunction. J. Investig. Dermatol. 2023, 143, 2052–2064.e5. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, J.; Augustyniak, A.; Dubrowska, K.; Rakoczy, R. The Two Faces of Pyocyanin—Why and How to Steer Its Production? World J. Microbiol. Biotechnol. 2023, 39, 103. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Zhao, X.; Shao, B.; Zhu, K. Phenazines Modulate Gastrointestinal Metabolism and Microbiota. Preprint 2021. [Google Scholar] [CrossRef]
- Sudhakar, T.; Karpagam, S.; Premkumar, J. Biosynthesis, Antibacterial Activity of Pyocyanin Pigment Produced by Pseudomonas aeruginosa SU1. J. Chem. Pharm. Res. 2015, 7, 921–924. Available online: www.jocpr.com (accessed on 25 October 2024).
- Farhadi, M.; Momeni, Z.; Abdizadeh Jozam, S. Comparing the Effect of Metronidazole with Pyocyanin Pigment Extracted from Pseudomonas aeruginosa on Trichomonas Vaginalis. J. Microb. World 2023, 16, 89–101. [Google Scholar] [CrossRef]
- Meirelles, L.A.; Newman, D.K. Phenazines and Toxoflavin Act as Interspecies Modulators of Resilience to Diverse Antibiotics. Mol. Microbiol. 2022, 117, 1384–1404. [Google Scholar] [CrossRef]
- Sakhtah, H.; Koyama, L.; Zhang, Y.; Morales, D.K.; Fields, B.L.; Price-Whelan, A.; Hogan, D.A.; Shepard, K.; Dietrich, L.E.P. The Pseudomonas aeruginosa Efflux Pump MexGHI-OpmD Transports a Natural Phenazine That Controls Gene Expression and Biofilm Development. Proc. Natl. Acad. Sci. USA 2016, 113, E3538–E3547. [Google Scholar] [CrossRef]
- Asif, M.Z.; Nocilla, K.A.; Ngo, L.T.; Shah, M.K.; Smadi, Y.; Hafeez, Z.A.; Parnes, M.; Manson, A.; Glushka, J.; Leach, F.E.; et al. Role of Ugt Genes in Detoxification and Glycosylation of 1-Hydroxy Phenazine (1-HP) in Caenorhabditis elegans. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kang, J.; Cho, Y.-H.; Lee, Y. Pyocyanin and 1-Hydroxyphenazine Promote Anaerobic Killing of Pseudomonas aeruginosa via Single-Electron Transfer with Ferrous Iron. Microbiol. Spectr. 2022, 10, e0231222. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Ye, F.C.; Pang, C.P.; Yong, T.Q.; Tang, W.D.; Xiao, J.; Shang, C.H.; Lu, Z.J. Isolation and Identification of Bioactive Substance 1-Hydroxyphenazine from Pseudomonas aeruginosa and Its Antimicrobial Activity. Lett. Appl. Microbiol. 2020, 71, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Huasong, P.; Qingwen, H.; Bilal, M.; Wang, W.; Zhang, X. Kinetics, Mechanism, and Identification of Photodegradation Products of Phenazine-1-Carboxylic Acid. Environ. Technol. 2020, 41, 1848–1856. [Google Scholar] [CrossRef]
- Xun, W.; Gong, B.; Liu, X.; Yang, X.; Zhou, X.; Jin, L. Antifungal Mechanism of Phenazine-1-Carboxylic Acid against Pestalotiopsis Kenyana. Int. J. Mol. Sci. 2023, 24, 11274. [Google Scholar] [CrossRef]
- Huang, H.; Sun, L.; Bi, K.; Zhong, G.; Hu, M. The Effect of Phenazine-1-Carboxylic Acid on the Morphological, Physiological, and Molecular Characteristics of Phellinus Noxius. Molecules 2016, 21, 613. [Google Scholar] [CrossRef]
- Puopolo, G.; Masi, M.; Raio, A.; Andolfi, A.; Zoina, A.; Cimmino, A.; Evidente, A. Insights on the Susceptibility of Plant Pathogenic Fungi to Phenazine-1-Carboxylic Acid and Its Chemical Derivatives. Nat. Prod. Res. 2013, 27, 956–966. [Google Scholar] [CrossRef]
- Song, C.; Yue, S.J.; Liu, W.H.; Zheng, Y.F.; Zhang, C.H.; Feng, T.T.; Hu, H.B.; Wang, W.; Zhang, X.H. Engineering of Glycerol Utilization in Pseudomonas Chlororaphis GP72 for Enhancing Phenazine-1-Carboxylic Acid Production. World J. Microbiol. Biotechnol. 2020, 36, 49. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, P.; Bilal, M.; Wang, W.; Hu, H.; Zhang, X. Enhanced Biosynthesis of Phenazine-1-Carboxamide by Engineered Pseudomonas chlororaphis HT66. Microb. Cell Fact. 2018, 17, 117. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.; Shang, W.; Rao, Y.; Chen, J.; Peng, H.; Huang, J.; Hu, Z.; Zhang, R.; Rao, X. Pyocyanin Biosynthesis Protects Pseudomonas aeruginosa from Nonthermal Plasma Inactivation. Microb. Biotechnol. 2022, 15, 1910–1921. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.; Negi, S. Towards a Better Greener Future—An Alternative Strategy Using Biofertilizers. I: Plant Growth Promoting Bacteria. Plant Gene 2017, 12, 43–49. [Google Scholar] [CrossRef]
- Oziat, J.; Cohu, T.; Elsen, S.; Gougis, M.; Malliaras, G.G.; Mailley, P. Electrochemical Detection of Redox Molecules Secreted by Pseudomonas aeruginosa—Part 1: Electrochemical Signatures of Different Strains. Bioelectrochemistry 2021, 140, 107747. [Google Scholar] [CrossRef] [PubMed]
- El-Fouly, M.Z.; Sharaf, A.M.; Shahin, A.A.M.; El-Bialy, H.A.; Omara, A.M.A. Biosynthesis of Pyocyanin Pigment by Pseudomonas aeruginosa. J. Radiat. Res. Appl. Sci. 2015, 8, 36–48. [Google Scholar] [CrossRef]
- Gibson, J.; Sood, A.; Hogan, D.A. Pseudomonas aeruginosa—Candida Albicans Interactions: Localization and Fungal Toxicity of a Phenazine Derivative. Appl. Environ. Microbiol. 2009, 75, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, W.; Walker, F.; Vogler, B.; Armbruster, W.; Buchenauer, H. Isolation and Identification of N-Mercapto-4-Formylcarbostyril, an Antibiotic Produced by Pseudomonas fluorescens. Phytochemistry 2001, 58, 1297–1303. [Google Scholar] [CrossRef]
- Ye, L.; Cornelis, P.; Guillemyn, K.; Ballet, S.; Christophersen, C.; Hammerich, O. Structure Revision of N-Mercapto-4-Formylcarbostyril Produced by Pseudomonas Fluorescens G308 to 2-(2-Hydroxyphenyl)Thiazole-4-Carbaldehyde [Aeruginaldehyde]. Nat. Prod. Commun. 2014, 9, 789–794. [Google Scholar] [CrossRef]
- Bach, E.; Passaglia, L.M.P.; Jiao, J.; Gross, H. Burkholderia in the Genomic Era: From Taxonomy to the Discovery of New Antimicrobial Secondary Metabolites. Crit. Rev. Microbiol. 2022, 48, 121–160. [Google Scholar] [CrossRef]
- Ni, S.; Li, B.; Xu, Y.; Mao, F.; Li, X.; Lan, L.; Zhu, J.; Li, J. Targeting Virulence Factors as an Antimicrobial Approach: Pigment Inhibitors. Med. Res. Rev. 2020, 40, 293–338. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Y.; Liu, R.; Liang, Y.; Zhang, H.; Shen, N.; Yang, D.; Jiang, M. Antimicrobial Mechanisms and Antifungal Activity of Compounds Generated by Banana Rhizosphere Pseudomonas aeruginosa Gxun-2 against Fusarium oxysporum f. sp. cubense. Front. Microbiol. 2024, 15, 1456847. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Yu, H.; Dela Ahator, S.; Wu, X.; Lv, S.; Zhang, L. Bacterial Quorum-sensing Signal IQS Induces Host Cell Apoptosis by Targeting POT1–P53 Signalling Pathway. Cell Microbiol. 2019, 21, e13076. [Google Scholar] [CrossRef]
- Anjaiah, V.; Koedam, N.; Nowak-Thompson, B.; Loper, J.E.; Höfte, M.; Tambong, J.T.; Cornelis, P. Involvement of Phenazines and Anthranilate in the Antagonism of Pseudomonas aeruginosa PNA1 and Tn 5 Derivatives Toward Fusarium spp. and Pythium spp. Mol. Plant-Microbe Interact. 1998, 11, 847–854. [Google Scholar] [CrossRef]
- Golden, M.M.; Heppe, A.C.; Zaremba, C.L.; Wuest, W.M. Metal Chelation as an Antibacterial Strategy for Pseudomonas aeruginosa and Acinetobacter Baumannii. RSC Chem. Biol. 2024, 5, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Rojas Murcia, N.; Lee, X.; Waridel, P.; Maspoli, A.; Imker, H.J.; Chai, T.; Walsh, C.T.; Reimmann, C. The Pseudomonas aeruginosa Antimetabolite L-2-Amino-4-Methoxy-Trans-3-Butenoic Acid (AMB) Is Made from Glutamate and Two Alanine Residues via a Thiotemplate-Linked Tripeptide Precursor. Front. Microbiol. 2015, 6, 170. [Google Scholar] [CrossRef]
- Azam, M.W.; Khan, A.U. Updates on the Pathogenicity Status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef]
- Ronnebaum, T.A.; McFarlane, J.S.; Prisinzano, T.E.; Booker, S.J.; Lamb, A.L. Stuffed Methyltransferase Catalyzes the Penultimate Step of Pyochelin Biosynthesis. Biochemistry 2019, 58, 665–678. [Google Scholar] [CrossRef]
- Weaver, A.A.; Parmar, D.; Junker, E.A.; Sweedler, J.V.; Shrout, J.D. Differential Spreading of Rhamnolipid Congeners from Pseudomonas aeruginosa. ACS Appl. Bio Mater. 2023, 6, 4914–4921. [Google Scholar] [CrossRef]
- de Santana-Filho, A.P.; Camilios-Neto, D.; de Souza, L.M.; Sassaki, G.L.; Mitchell, D.A.; Krieger, N. Evaluation of the Structural Composition and Surface Properties of Rhamnolipid Mixtures Produced by Pseudomonas aeruginosa UFPEDA 614 in Different Cultivation Periods. Appl. Biochem. Biotechnol. 2014, 175, 988–995. [Google Scholar] [CrossRef]
- Camilios-Neto, D.; Bugay, C.; De Santana-Filho, A.P.; Joslin, T.; De Souza, L.M.; Sassaki, G.L.; Mitchell, D.A.; Krieger, N. Production of Rhamnolipids in Solid-State Cultivation Using a Mixture of Sugarcane Bagasse and Corn Bran Supplemented with Glycerol and Soybean Oil. Appl. Microbiol. Biotechnol. 2011, 89, 1395–1403. [Google Scholar] [CrossRef]
- Sanjivkumar, M.; Deivakumari, M.; Immanuel, G. Investigation on Spectral and Biomedical Characterization of Rhamnolipid from a Marine Associated Bacterium Pseudomonas aeruginosa (DKB1). Arch. Microbiol. 2021, 203, 2297–2314. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Structural and Physicochemical Characterization of Rhamnolipids Produced by Pseudomonas aeruginosa P6. AMB Express 2020, 10, 201. [Google Scholar] [CrossRef]
- Braz, L.M.; Salazar-Bryam, A.M.; Andrade, G.S.S.; Tambourgi, E.B. Optimization and Characterization of Rhamnolipids Produced by Pseudomonas aeruginosa ATCC 9027 Using Molasses as a Substrate. World J. Microbiol. Biotechnol. 2023, 39, 51. [Google Scholar] [CrossRef] [PubMed]
- USEPA Rhamnolipid Biosurfactant. Exemption from the Requirement of a Tolerance. Environ. Prot. Agency Fed. Regist. Rules Regul. 2004, 69, 16796–16800. [Google Scholar]
- de Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The Antibacterial Activity of Rhamnolipid Biosurfactant Is PH Dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Passos, T.F.; Nitschke, M. The Combined Effect of PH and NaCl on the Susceptibility of Listeria Monocytogenes to Rhamnolipids. Food Res. Int. 2024, 192, 114744. [Google Scholar] [CrossRef] [PubMed]
- Firdose, A.; Maeda, T.; Sukri, M.A.M.; Yasin, N.H.M.; Sabturani, N.; Aqma, W.S. Antibacterial Mechanism of Pseudomonas aeruginosa UKMP14T Rhamnolipids against Multidrug Resistant Acinetobacter Baumannii. Microb. Pathog. 2024, 193, 106743. [Google Scholar] [CrossRef]
- Firdose, A.; Chong, N.H.H.; Ramli, R.; Aqma, W.S. Antimicrobial, Antiadhesive, and Antibiofilm Actions of Rhamnolipids on ESKAPE Pathogens. Lett. Appl. Microbiol. 2023, 76, ovad013. [Google Scholar] [CrossRef]
- Buonocore, C.; Giugliano, R.; Della Sala, G.; Palma Esposito, F.; Tedesco, P.; Folliero, V.; Galdiero, M.; Franci, G.; de Pascale, D. Evaluation of Antimicrobial Properties and Potential Applications of Pseudomonas gessardii M15 Rhamnolipids towards Multiresistant Staphylococcus aureus. Pharmaceutics 2023, 15, 700. [Google Scholar] [CrossRef]
- Carra, J.B.; Wessel, K.B.B.; Pereira, G.N.; Oliveira, M.C.; Pattini, P.M.T.; Masquetti, B.L.; Amador, I.R.; Bruschi, M.L.; Casagrande, R.; Georgetti, S.R.; et al. Bioadhesive Polymeric Films Containing Rhamnolipids, An Innovative Antimicrobial Topical Formulation. AAPS PharmSciTech 2024, 25, 177. [Google Scholar] [CrossRef]
- Maťátková, O.; Kolouchová, I.; Lokočová, K.; Michailidu, J.; Jaroš, P.; Kulišová, M.; Řezanka, T.; Masák, J. Rhamnolipids as a Tool for Eradication of Trichosporon Cutaneum Biofilm. Biomolecules 2021, 11, 1727. [Google Scholar] [CrossRef]
- Borah, S.N.; Sen, S.; Goswami, L.; Bora, A.; Pakshirajan, K.; Deka, S. Rice Based Distillers Dried Grains with Solubles as a Low Cost Substrate for the Production of a Novel Rhamnolipid Biosurfactant Having Anti-Biofilm Activity against Candida Tropicalis. Colloids Surf. B Biointerfaces 2019, 182, 110358. [Google Scholar] [CrossRef]
- Cerqueira dos Santos, S.; Araújo Torquato, C.; de Alexandria Santos, D.; Orsato, A.; Leite, K.; Serpeloni, J.M.; Losi-Guembarovski, R.; Romão Pereira, E.; Dyna, A.L.; Lopes Barboza, M.G.; et al. Production and Characterization of Rhamnolipids by Pseudomonas aeruginosa Isolated in the Amazon Region, and Potential Antiviral, Antitumor, and Antimicrobial Activity. Sci. Rep. 2024, 14, 4629. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Borah, S.N.; Bora, A.; Deka, S. Rhamnolipid Exhibits Anti-Biofilm Activity against the Dermatophytic Fungi Trichophyton Rubrum and Trichophyton Mentagrophytes. Biotechnol. Rep. 2020, 27, e00516. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.; Buonocore, C.; Zannella, C.; Chianese, A.; Esposito, F.P.; Tedesco, P.; De Filippis, A.; Galdiero, M.; Franci, G.; de Pascale, D. Antiviral Activity of the Rhamnolipids Mixture from the Antarctic Bacterium Pseudomonas gessardii M15 against Herpes Simplex Viruses and Coronaviruses. Pharmaceutics 2021, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, I.; Petkovic, M.; Jovanovic, M.; Milivojevic, D.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Anti-Biofilm Properties of Bacterial Di-Rhamnolipids and Their Semi-Synthetic Amide Derivatives. Front. Microbiol. 2017, 8, 2454. [Google Scholar] [CrossRef]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics 2022, 14, 360. [Google Scholar] [CrossRef]
- Rahimi, K.; Lotfabad, T.B.; Jabeen, F.; Mohammad Ganji, S. Cytotoxic Effects of Mono- and Di-Rhamnolipids from Pseudomonas aeruginosa MR01 on MCF-7 Human Breast Cancer Cells. Colloids Surf. B Biointerfaces 2019, 181, 943–952. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, C.; Long, X.; Zhang, G.; Meng, Q. Rhamnolipids Elicit the Same Cytotoxic Sensitivity between Cancer Cell and Normal Cell by Reducing Surface Tension of Culture Medium. Appl. Microbiol. Biotechnol. 2014, 98, 10187–10196. [Google Scholar] [CrossRef]
- Semkova, S.; Antov, G.; Iliev, I.; Tsoneva, I.; Lefterov, P.; Christova, N.; Nacheva, L.; Stoineva, I.; Kabaivanova, L.; Staneva, G.; et al. Rhamnolipid Biosurfactants—Possible Natural Anticancer Agents and Autophagy Inhibitors. Separations 2021, 8, 92. [Google Scholar] [CrossRef]
- Madsen, J.K.; Pihl, R.; Møller, A.H.; Madsen, A.T.; Otzen, D.E.; Andersen, K.K. The Anionic Biosurfactant Rhamnolipid Does Not Denature Industrial Enzymes. Front. Microbiol. 2015, 6, 292. [Google Scholar] [CrossRef]
- Twigg, M.S.; Adu, S.A.; Sugiyama, S.; Marchant, R.; Banat, I.M. Mono-Rhamnolipid Biosurfactants Synthesized by Pseudomonas aeruginosa Detrimentally Affect Colorectal Cancer Cells. Pharmaceutics 2022, 14, 2799. [Google Scholar] [CrossRef]
- Christova, N.; Tuleva, B.; Kril, A.; Georgieva, M.; Konstantinov, S.; Terziyski, I.; Nikolova, B.; Stoineva, I. Chemical Structure and In Vitro Antitumor Activity of Rhamnolipids from Pseudomonas aeruginosa BN10. Appl. Biochem. Biotechnol. 2013, 170, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Ankulkar, R.; Chavan, S.; Aphale, D.; Chavan, M.; Mirza, Y. Cytotoxicity of Di-Rhamnolipids Produced by Pseudomonas aeruginosa RA5 against Human Cancerous Cell Lines. 3 Biotech 2022, 12, 323. [Google Scholar] [CrossRef]
- Ali, A.M.; Hill, H.J.; Elkhouly, G.E.; Bakkar, M.R.; Raya, N.R.; Stamataki, Z.; Abo-zeid, Y. Rhamnolipid Nano-Micelles Inhibit SARS-CoV-2 Infection and Have No Dermal or Eye Toxic Effects in Rabbits. Antibiotics 2022, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Malakar, C.; Kashyap, B.; Bhattacharjee, S.; Kalita, M.C.; Mukherjee, A.K.; Deka, S. Antibiofilm and Wound Healing Efficacy of Rhamnolipid Biosurfactant against Pathogenic Bacterium Staphylococcus aureus. Microb. Pathog. 2024, 195, 106855. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.; Datta, S.; Biswas, D.; Auddy, B.; Gupta, M.; Chattopadhyay, H. Excision Wound Healing Activity of a Common Biosurfactant Produced by Pseudomonas sp. Wound Med. 2018, 23, 47–52. [Google Scholar] [CrossRef]
- Alghazal, A.M.; Hamed, R.S.; Deleme, Z.H. Impact of Rhamnolipid on Skin Wound Regeneration in Rats. Al-Rafidain Dent. J. 2024, 24, 220–230. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, S.; Kumar, R.; Zhang, Z.; Zhang, Z.; Wang, Y.; Jiang, X.; Lin, K.; Kaur, G.; Yung, K.K.L. Rhamnolipids from Non-Pathogenic Acinetobacter Calcoaceticus: Bioreactor-Scale Production, Characterization and Wound Healing Potency. N. Biotechnol. 2022, 67, 23–31. [Google Scholar] [CrossRef]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Characterisation of Cytotoxicity and Immunomodulatory Effects of Glycolipid Biosurfactants on Human Keratinocytes. Appl. Microbiol. Biotechnol. 2023, 107, 137–152. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An Evolving Chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Liu, J.; Zeng, X.; Wu, Y.; Yang, C. Rhamnolipids Enhance Growth Performance by Improving the Immunity, Intestinal Barrier Function, and Metabolome Composition in Broilers. J. Sci. Food Agric. 2022, 102, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine to Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Nunes, I.P.F.; de Jesus, R.S.; Almeida, J.A.; Costa, W.L.R.; Malta, M.; Soares, L.G.P.; de Almeida, P.F.; Pinheiro, A.L.B. Evaluation of 1,9-Dimethyl-Methylene Blue Nanoencapsulation Using Rhamnolipid Nanoparticles to Potentiate the Photodynamic Therapy Technique in Candida Albicans: In Vitro Study. J. Photochem. Photobiol. B 2024, 256, 112943. [Google Scholar] [CrossRef] [PubMed]
- Habibah, F.F.; Sri Rizki, W.O.; Ivansyah, A.L.; Astuti, D.I.; Hertadi, R. Green Synthesis of Copper Ions Nanoparticles Functionalized with Rhamnolipid as Potential Antibacterial Agent for Pathogenic Bacteria. Heliyon 2024, 10, e24242. [Google Scholar] [CrossRef]
- Shu, X.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Composite Hydrogels Filled with Rhamnolipid-Based Nanoemulsion, Nanostructured Lipid Carrier, or Solid Lipid Nanoparticle: A Comparative Study on Gel Properties and the Delivery of Lutein. Food Hydrocoll. 2024, 146, 109264. [Google Scholar] [CrossRef]
- Kadakia, P.; Valentin, J.D.P.; Hong, L.; Watts, S.; Hameed, O.A.; Walch, M.; Salentinig, S. Biocompatible Rhamnolipid Self-Assemblies with PH-Responsive Antimicrobial Activity. Adv. Healthc. Mater. 2024, 13, e2302596. [Google Scholar] [CrossRef]
- Jiang, G.; Guo, J.; Yan, C.; He, Y.; Chen, J.; Zhang, M.; Xiang, K.; Xiang, X.; Zhang, C.; Wang, Y.; et al. Biomimetic Hybrid Nanovesicles Improve Infected Diabetic Wound via Enhanced Targeted Delivery. J. Control. Release 2024, 365, 193–207. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camilios-Neto, D.; Nascimento, R.R.d.; Ratko, J.; Caldas Pan, N.; Casagrande, R.; Verri, W.A.; Vignoli, J.A. Pseudomonas aeruginosa: A Bacterial Platform for Biopharmaceutical Production. Future Pharmacol. 2024, 4, 892-918. https://doi.org/10.3390/futurepharmacol4040047
Camilios-Neto D, Nascimento RRd, Ratko J, Caldas Pan N, Casagrande R, Verri WA, Vignoli JA. Pseudomonas aeruginosa: A Bacterial Platform for Biopharmaceutical Production. Future Pharmacology. 2024; 4(4):892-918. https://doi.org/10.3390/futurepharmacol4040047
Chicago/Turabian StyleCamilios-Neto, Doumit, Rodolfo Ricken do Nascimento, Jonathan Ratko, Nicole Caldas Pan, Rubia Casagrande, Waldiceu A. Verri, and Josiane A. Vignoli. 2024. "Pseudomonas aeruginosa: A Bacterial Platform for Biopharmaceutical Production" Future Pharmacology 4, no. 4: 892-918. https://doi.org/10.3390/futurepharmacol4040047
APA StyleCamilios-Neto, D., Nascimento, R. R. d., Ratko, J., Caldas Pan, N., Casagrande, R., Verri, W. A., & Vignoli, J. A. (2024). Pseudomonas aeruginosa: A Bacterial Platform for Biopharmaceutical Production. Future Pharmacology, 4(4), 892-918. https://doi.org/10.3390/futurepharmacol4040047








