An Updated Review of the Antimicrobial Potential of Selenium Nanoparticles and Selenium-Related Toxicological Issues
Abstract
:1. Introduction
2. Chemistry of Selenium
3. The Two Faces of Selenium: From Toxicity to Essentiality
3.1. Selenium Distribution on Planet Earth
3.2. Toxicity
3.3. Essentiality
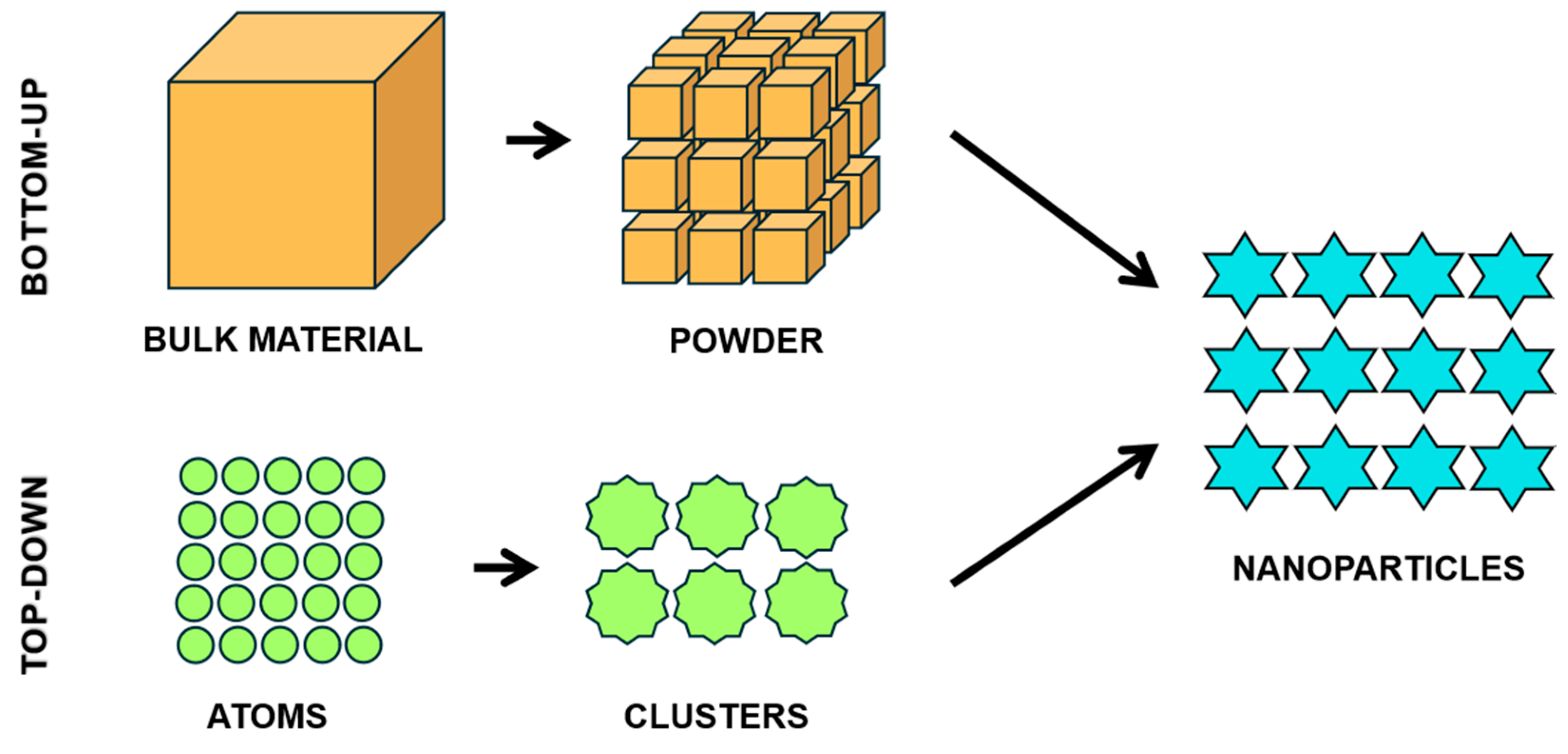
4. Nanoscience and Nanotechnology: SeNPs
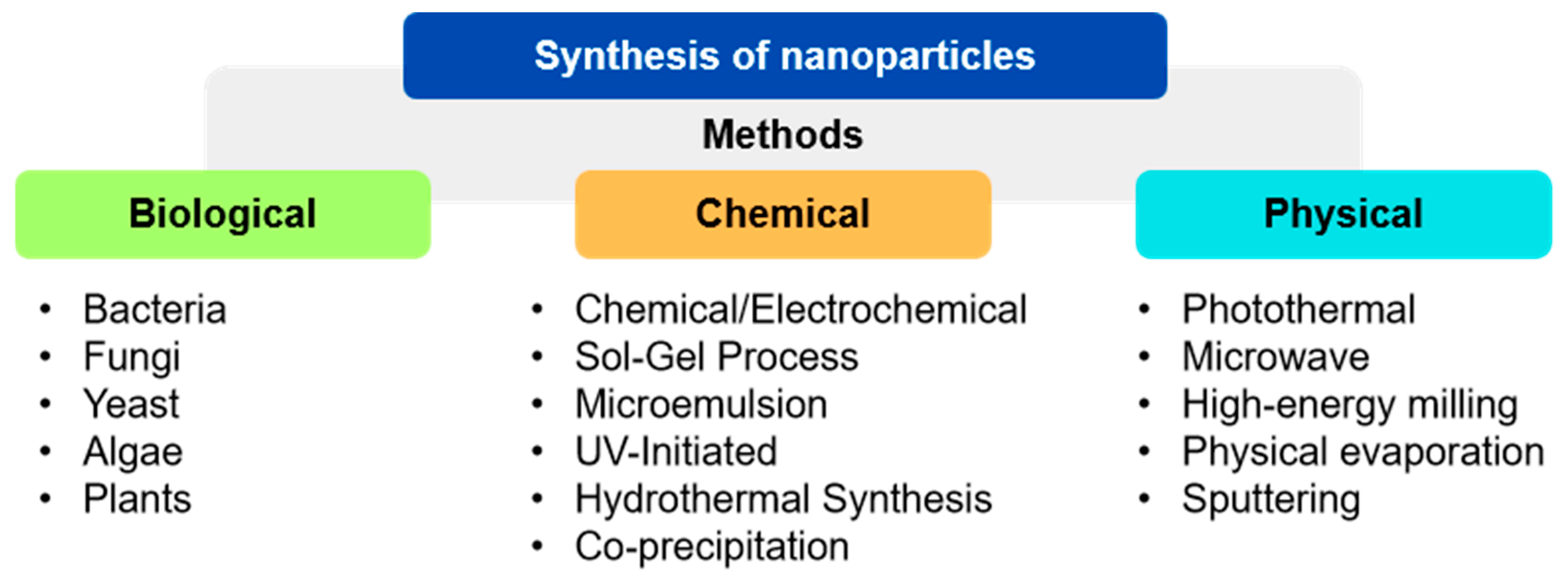
4.1. Preparation of SeNPs
4.1.1. Chemical Synthesis Methods
4.1.2. Physical Synthesis Methods
4.1.3. Biological Synthesis Methods
5. Antimicrobial Activities of SeNPs Synthesized by Different Routes
5.1. Biogenic SeNPs
5.1.1. Antifungal Properties
| NPs | Species | NP Size (nm) | DLS | Color NP Solution | Evaluated Microorganisms | MIC_SeNPs | Ref. | |
|---|---|---|---|---|---|---|---|---|
| FUNGI | Ca-SeNPs | C. albicans TIMML-1306 | (XRD) 38 | (PZ) −19 mV | Red | C. glabrata TIMML-1316 C. krusei TIMML-1321 C. glabrata TIMML-368 | 0.25–0.5 µg/mL | [85] |
| C. albicans TIMML-1306 C. albicans TIMML-183 C. albicans TIMML-491 C. albicans TIMML-1291 | <1 µg/mL 0.25 µg/mL 0.5 µg/mL 0.125–64 µg/mL | |||||||
| C. parapsilosis ATCC-2201 | 0.125–64 µg/mL | |||||||
| Af-SeNPs | A. flavus TIMML-050 | (XRD) 37 | (PZ) −21 mV | Red | A. fumigatus TIMML-025 A. flavus TIMML-050 | 1 µg/mL | ||
| PLANTS | Ne-SeNPs | Nepeta (gender) | (SEM) 37–46 | - | Red | C. albicans TIMML-1306 C. albicans TIMML-1291 C. albicans TIMML-491 | 1 µg/mL 1 µg/mL 0.5 µg/mL | [96] |
| A. flavus TIMML-050 C. tropicalis TIMML-1316 C. krusei TIMML-1321 | 0.125 µg/mL 0.125 µg/mL 0.125 µg/mL | |||||||
| PPPW-SeNPs | Prickly Pear Peel Waste (PPPW) | (TEM) 50–150 | - | Reddish | C. albicans C. neoformans | 3.9 µg/mL 7.81 µg/mL | [97] |
5.1.2. Antibacterial Properties
| NPs | Species | NP Size (nm) | DLS | Color NP Solution | Evaluated Microorganisms | MIC_SeNPs | Ref. | |
|---|---|---|---|---|---|---|---|---|
| BACTERIA | Bs-SeNP | B. subtilis BSN313 | (DLS) 530 (TEM) 280–630 | (PZ) −26.9 mV | Red | E. coli ATCC 8739 Staphylococcus aureus ATCC 9027 Pseudomonas aeruginosa ATCC 25923 | 200 µg/mL | [36] |
| La-SeNP | L. acidophilus | (DLS) 34.13 (TEM) 2–15 | (PDI) 0.28 (PZ) +37.86 mV | Reddish-brown | E. coli S. aureus B. subtilis P. aeruginosa K. pneumoniae | 9.4 µg/mL 1.2 µg/mL 3.5 µg/mL 6.5 µg/mL 4 µg/mL | [99] | |
| ALGAE | Sp-SeNP | Spirulina platensis | (TEM) 79.40 ± 44.26 >100 nm (53.33%) | (PZ) −32.9 ± 8.12 mV | Red orange | S. abony NCTC 6017 E. coli ATCC 8739 K. pneumonia ATCC700603 | 25 µg/mL | [98] |
| PLANTS | PPPW-SeNP | Prickly Pear Peel Waste (PPPW) | (TEM) 50–150 | - | Reddish | E. coli P. aeruginosa | 125 µg/mL | [97] |
| B. subtilis S. aureus | 62.5 µg/mL 15.62 µg/mL | |||||||
| PPAE-SeNPs PPAE-SeNPs@CuO | Punica granatum | (HRTEM) 1.97–10.56 (HRTEM) 92.18 | - | Red Orange | H. pylori | 8 µg/mL | [100] | |
| Va-SeNP | V. Arctostaphylos (L.) | (DLS) 246.2 ± 4.51 (FESEM) 50 ± 1.23 | (PDI) 0.267 (PZ) −11.5 mV ± 1.24 | Red | S. aureus E. coli C. diphtheriae | 500—31.25 µg/mL | [101] |
5.2. Chemical SeNPs
5.3. Physical SeNPs
6. Antimicrobial Success of SeNPs: Mechanism of Action
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trofast, J. Berzelius’ discovery of selenium. Chem. Int. 2011, 33, 16. [Google Scholar]
- Murugesan, G.; Nagaraj, K.; Sunmathi, D.; Subramani, K. Methods involved in the synthesis of selenium nanoparticles and their different applications-a review. Eur. J. Biomed. 2019, 6, 189–194. [Google Scholar]
- Wisniak, J. Jöns Jacob Berzelius a guide to the perplexed chemist. Chem. Educ. 2000, 5, 343–350. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Seixas, T.G.; do AKehrig, H. O selênio no meio ambiente. Oecologia Bras. 2007, 11, 264–276. [Google Scholar] [CrossRef]
- Boyd, R. Selenium stories. Nat. Chem. 2011, 3, 570. [Google Scholar] [CrossRef]
- Garousi, F. The toxicity of different selenium forms and compounds—Review. Acta Agrar. Debreceniensis 2015, 64, 33–38. [Google Scholar] [CrossRef]
- Perrone, D.; Monteiro, M.; Nunes, J.C. The Chemistry of Selenium; Royal Society of Chemistry: London, UK, 2015. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Wang, K.; Fang, Q.; He, P.; Tu, Y.; Liu, Z.; Li, B. Unveiling the potential of selenium-enriched tea: Compositional profiles, physiological activities, and health benefits. Trends Food Sci. Technol. 2024, 145, 104356. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Handy, D.E.; Joseph, J.; Loscalzo, J. Selenium, a micronutrient that modulates cardiovascular health via redox enzymology. Nutrients 2021, 13, 3238. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim AM, E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Rosaiah, G.; Mangamuri, U.K.; Sikharam, A.S.; Devaraj, K.; Kalagatur, N.K.; Kadirvelu, K. Biosynthesis of selenium nanoparticles from Annona muricata fruit aqueous extract and investigation of their antioxidant and antimicrobial potentials. Curr. Trends Biotechnol. Pharm. 2022, 16, 101–107. [Google Scholar]
- Safaei, M.; Mozaffari, H.R.; Moradpoor, H.; Imani, M.M.; Sharifi, R.; Golshah, A. Optimization of green synthesis of selenium nanoparticles and evaluation of their antifungal activity against oral Candida albicans infection. Adv. Mater. Sci. Eng. 2022, 2022, 1376998. [Google Scholar] [CrossRef]
- Speckmann, B.; Grune, T. Epigenetic effects of selenium and their implications for health. Epigenetics 2015, 10, 179–190. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An antioxidant with a critical role in anti-aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Durán, N.; Mattoso, L.H.C.; Morais, P.C.d. Textbook of Nanotecnologia: Introdução, Preparação e Caracterização de Nanomateriais e Exemplos de Aplicação; Artliber: São Paulo, Brazil, 2006; pp. 87–92. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Madkour, L.H.; Madkour, L.H. Introduction to nanotechnology (NT) and nanomaterials (NMs). In Nanoelectronic Materials: Fundamentals and Applications; Springer: Cham, Switzerland, 2019; pp. 1–47. [Google Scholar] [CrossRef]
- Ojha, B.; Jain, V.K.; Mehra, N.K.; Jain, K. Nanotechnology: Introduction and basic concepts. In Dendrimers in Nanomedicine; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–17. [Google Scholar]
- Selmani, A.; Kovačević, D.; Bohinc, K. Nanoparticles: From synthesis to applications and beyond. Adv. Colloid Interface Sci. 2022, 303, 102640. [Google Scholar] [CrossRef]
- Khalil, H.S.; Maulu, S.; Verdegem, M.; Abdel-Tawwab, M. Embracing nanotechnology for selenium application in aquafeeds. Rev. Aquac. 2023, 15, 112–129. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.F.; Abd-Elraoof, W.A.; Tayel, A.A.; Alzuaibr, F.M.; Abonama, O.M. Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. J. Nanobiotechnol. 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Biointerface Res. Appl. Chem. 2022, 13, 41. [Google Scholar] [CrossRef]
- Xiao, X.; Deng, H.; Lin, X.; Ali AS, M.; Viscardi, A.; Guo, Z.; Qiao, L.; He, Y.; Han, J. Selenium nanoparticles: Properties, preparation methods, and therapeutic applications. Chem.-Biol. Interact. 2023, 378, 110483. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.; Wang, J.; Chen, T. Theranostic applications of selenium nanomedicines against lung cancer. J. Nanobiotechnol. 2023, 21, 96. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2023, 63, 12360–12371. [Google Scholar] [CrossRef]
- Niranjan, R.; Zafar, S.; Lochab, B.; Priyadarshini, R. Synthesis and characterization of sulfur and sulfur-selenium nanoparticles loaded on reduced graphene oxide and their antibacterial activity against gram-positive pathogens. Nanomaterials 2022, 12, 191. [Google Scholar] [CrossRef]
- Galan-Chilet, I.; Grau-Perez, M.; De Marco, G.; Guallar, E.; Martin-Escudero, J.C.; Dominguez-Lucas, A.; Gonzalez-Manzano, I.; Lopez-Izquierdo, R.; Briongos-Figuero, L.S.; Redon, J.; et al. A gene-environment interaction analysis of plasma selenium with prevalent and incident diabetes: The Hortega study. Redox Biol. 2017, 12, 798–805. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Kopel, J.; Fralick, J.; Reid, T.W. The potential antiviral effects of selenium nanoparticles and coated surfaces. Antibiotics 2022, 11, 1683. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Abbas, K.; Qadir, M.I. Synthesis, characterization and evaluation of biological properties of selenium nanoparticles from Solanum lycopersicum. Arab. J. Chem. 2022, 15, 103901. [Google Scholar] [CrossRef]
- Ullah, A.; Yin, X.; Wang, F.; Xu, B.; Mirani, Z.A.; Xu, B.; Chan, M.W.H.; Ali, A.; Usman, M.; Ali, N.; et al. Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities. Molecules 2021, 26, 5559. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Aziz, R.; Rafiq, M.T.; Abbasi, M.; Taneez, M.; Azhar, M.U.; El Askary, A.; Elesawy, B.H.; Eed, E.M.; Khalifa, A.S.; et al. Qayyum, A. Efficient Removal of Lead and Chromium from Aqueous Media Using Selenium Based Nanocomposite Supported by Orange Peel. Front. Environ. Sci. 2022, 10, 94. [Google Scholar] [CrossRef]
- Hardy, G.; Hardy, I.; Manzanares, W. Selenium supplementation in the critically ill. Nutr. Clin. Pract. 2012, 27, 21–33. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Staicu, L.C.; Lazuén-López, G.; Merroun, M.L. Allotropy of selenium nanoparticles: Colourful transition, synthesis, and biotechnological applications. Microb. Biotechnol. 2023, 16, 877–892. [Google Scholar] [CrossRef]
- Bouroushian, M.; Bouroushian, M. Chalcogens and metal chalcogenides. In Electrochemistry of Metal Chalcogenides; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–56. [Google Scholar] [CrossRef]
- Scopigno, T.; Steurer, W.; Yannopoulos, S.N.; Chrissanthopoulos, A.; Krisch, M.; Ruocco, G.; Wagner, T. Vibrational dynamics and surface structure of amorphous selenium. Nat. Commun. 2011, 2, 195. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Oldfield, J.E. The two faces of selenium. J. Nutr. 2015, 117, 2002–2008. [Google Scholar] [CrossRef]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef]
- Tan, J.A.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Goswami, L.; Lee, J.; Sonne, C.; Brown, R.J.; Kim, K.H. Selenium in soil-microbe-plant systems: Sources, distribution, toxicity, tolerance, and detoxification. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2383–2420. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the health-promoting effect of adequate selenium status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, R.J.; Diamond, A.M. Selenium deficiency and human disease. In Selenium: Its Molecular Biology and Role in Human Health; Springer US: Boston, MA, USA, 2001; pp. 219–233. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review: Selenium interactions and toxicity. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, C.; Zhang, T. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Burk, R.F. (Ed.) Selenium in Biology and Human Health; Springer: New York, NY, USA, 1994; pp. 14–221. [Google Scholar]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Imran, M.; Chen, Z.; Mehmood, A.; Rukh, S.; Weixie, W.; Asghar, W.; Iftikhar, F. Distribution of selenium in soils and human health. In Selenium and Human Health; IntechOpen: London, UK, 2023. [Google Scholar]
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. Nutrition 1999, 15, 255. [Google Scholar] [CrossRef]
- Pinsent, J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem. J. 1954, 57, 10. [Google Scholar] [CrossRef]
- Patterson, E.L.; Milstrey, R.; Stokstad EL, R. Effect of selenium in preventing exudative diathesis in chicks. Proc. Soc. Exp. Biol. Med. 1957, 95, 617–620. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Senthamarai, M.D.; Hillary, V.E.; Rajan, M.R.; Ceasar, S.A. Biosynthesis of selenium nanoparticles and its biological applications: A systematic review. Nano Struct. Nano Objects 2024, 39, 101261. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- Karthik, K.K.; Cheriyan, B.V.; Rajeshkumar, S.; Gopalakrishnan, M. A review on selenium nanoparticles and their biomedical applications. Biomed. Technol. 2024, 6, 61–74. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef]
- Wong, C.W.; Chan, Y.S.; Jeevanandam, J.; Pal, K.; Bechelany, M.; Abd Elkodous, M.; El-Sayyad, G.S. Response surface methodology optimization of mono-dispersed MgO nanoparticles fabricated by ultrasonic-assisted sol–gel method for outstanding antimicrobial and antibiofilm activities. J. Clust. Sci. 2020, 31, 367–389. [Google Scholar] [CrossRef]
- Agilan, S.; Velauthapillai, D.; Muthukumarasamy, N.; Thambidurai, M.; Senthil, T.S.; Balasundaraprabhu, R. Synthesis and characterization of selenium nanowires. Int. Sch. Res. Not. 2011, 2011, 589073. [Google Scholar] [CrossRef]
- Xi, G.; Xiong, K.; Zhao, Q.; Zhang, R.; Zhang, H.; Qian, Y. Nucleation− dissolution− recrystallization: A new growth mechanism for t-selenium nanotubes. Cryst. Growth Des. 2006, 6, 577–582. [Google Scholar] [CrossRef]
- Nagar, D.N.; Ghosh, N.N.; Braganca, J.M. Green synthesis of selenium nanospheres and nanoneedles by halophilic archaea. Appl. Nanosci. 2022, 12, 3983–3994. [Google Scholar] [CrossRef]
- Sawant, V.J.; Sawant, V.J. Biogenic capped selenium nano rods as naked eye and selective hydrogen peroxide spectrometric sensor. Sens. Bio Sens. Res. 2020, 27, 100314. [Google Scholar] [CrossRef]
- Sowmiya, P.; Dhas, T.S.; Inbakandan, D.; Mani, R.; Natarajan, A.; Dharani, G.; Govindaraju, K.; Kannan, M.; Velu, K.; Kumar, C.M.V. Antagonistic activity of a novel chitosan-selenium nanoflower against common aquaculture pathogen Aeromonas caviae. Aquac. Int. 2023, 31, 3109–3123. [Google Scholar] [CrossRef]
- Shanthappa, R.; Ankinapalli, O.R.; Kakarla, A.K.; Narsimulu, D.; Bandi, H.; Syed, W.A.; Yu, J.S. Selenium incorporated sodium vanadate nanobelts as high-performance electrode material for long-lasting aqueous zinc-ion batteries and supercapacitors. Chem. Eng. J. 2023, 476, 146777. [Google Scholar] [CrossRef]
- Shah, V.; Medina-Cruz, D.; Vernet-Crua, A.; Truong, L.B.; Sotelo, E.; Mostafavi, E.; González, M.U.; García-Martín, J.M.; Cholula-Díaz, J.L.; Webster, T.J. Pepper-Mediated Green Synthesis of Selenium and Tellurium Nanoparticles with Antibacterial and Anticancer Potential. J. Funct. Biomater. 2022, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zuo, Y.; Dai, L.; Zhang, L.; Yu, Y.; Zhou, L. Effect of ultrasonic-induced selenium crystallization behavior during selenium reduction. Ultrason. Sonochem. 2023, 95, 106392. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium nanoparticles for biomedical applications: From development and characterization to therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef]
- Ashraf, H.; Cossu, D.; Ruberto, S.; Noli, M.; Jasemi, S.; Simula, E.R.; Sechi, L.A. Latent Potential of Multifunctional Selenium Nanoparticles in Neurological Diseases and Altered Gut Microbiota. Materials 2023, 16, 699. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying AN, J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, characterization, and applications of metal nanoparticles. In Biomaterials and Bionanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Davoodbasha, M.A.; Banerjee, J.; Chanda, S.; Ray, K.; Roychowdhury, T.; Acharya, K.; Sarkar, J. A state-of-the-art systemic review on selenium nanoparticles: Mechanisms and factors influencing biogenesis and its potential applications. Biol. Trace Elem. Res. 2023, 201, 5000–5036. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.U.R. Biomedical Potential of Plant-Based Selenium Nanoparticles: A Comprehensive Review on Therapeutic and Mechanistic Aspects. Int. J. Nanomed. 2021, 16, 249–268. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sun, H.; Liu, X.; Leng, X. Physicochemical stability and functional properties of selenium nanoparticles stabilized by chitosan, carrageenan, and gum Arabic. Carbohydr. Polym. 2021, 255, 117379. [Google Scholar] [CrossRef]
- Indhira, D.; Aruna, A.; Manikandan, K.; Albeshr, M.F.; Alrefaei, A.F.; Vinayagam, R.; Kathirvel, A.; Priyan, S.R.; Kumar, G.S.; Srinivasan, R. Antimicrobial and Photocatalytic Activities of Selenium Nanoparticles Synthesized from Elaeagnus indica Leaf Extract. Processes 2023, 11, 1107. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Dertli, E. A green nano-biosynthesis of selenium nanoparticles with Tarragon extract: Structural, thermal, and antimicrobial characterization. LWT 2021, 141, 110969. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2021, 12, 467–480. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Selenium Nanoparticles: Green Synthesis and Biomedical Application. Molecules 2023, 28, 8125. [Google Scholar] [CrossRef] [PubMed]
- Bafghi, M.H.; Darroudi, M.; Zargar, M.; Zarrinfar, H.; Nazari, R. Biosynthesis of selenium nanoparticles by Aspergillus flavus and Candida albicans for antifungal applications. Micro Nano Lett. 2021, 16, 656–669. [Google Scholar] [CrossRef]
- Chaudhary, S.; Jain, V.P.; Sharma, D.; Jaiswar, G. Implementation of agriculture waste for the synthesis of metal oxide nanoparticles: Its management, future opportunities and challenges. J. Mater. Cycles Waste Manag. 2023, 25, 3144. [Google Scholar] [CrossRef]
- Kobayashi, R.K.T.; Nakazato, G. Editorial: Nanotechnology for Antimicrobials. Front. Microbiol. 2020, 11, 1421. [Google Scholar] [CrossRef]
- Weldick, P.J.; Wang, A.; Halbus, A.F.; Paunov, V.N. Emerging nanotechnologies for targeting antimicrobial resistance. Nanoscale 2022, 14, 4018–4041. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Asante, J. Emerging Mechanisms of Antimicrobial Resistance in Bacteria and Fungi: Advances in the Era of Genomics. Future Microbiol. 2018, 13, 241–262. [Google Scholar] [CrossRef]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed. 2014, 53, 8840–8869. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Adefisoye, M.A.; Okoh, A.I. Nanotechnology as a viable alternative for the removal of antimicrobial resistance determinants from discharged municipal effluents and associated watersheds: A review. J. Environ. Manag. 2020, 275, 111234. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, T.; Pasic, P.; Easton, C.D.; Voelcker, N.H.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J.; Thissen, H. One step antimicrobial coatings for medical device applications based on low fouling polymers containing selenium nanoparticles. Chem. Eng. J. 2023, 467, 143546. [Google Scholar] [CrossRef]
- Al-Awsi, G.R.L.; Alameri, A.A.; Al-Dhalimy, A.M.B.; Gabr, G.A.; Kianfar, E. Application of nano-antibiotics in the diagnosis and treatment of infectious diseases. Braz. J. Biol. 2023, 84, e264946. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Taha, T.F.; Najjar, A.A.; Zabermawi, N.M.; Nader, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Salama, A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021, 28, 6782–6794. [Google Scholar] [CrossRef]
- Nile, S.H.; Thombre, D.; Shelar, A.; Gosavi, K.; Sangshetti, J.; Zhang, W.; Sieniawska, E.; Patil, R.; Kai, G. Antifungal properties of biogenic selenium nanoparticles functionalized with nystatin for the inhibition of Candida albicans biofilm formation. Molecules 2023, 28, 1836. [Google Scholar] [CrossRef]
- Hosseini Bafghi, M.; Safdari, H.; Nazari, R.; Darroudi, M.; Sabouri, Z.; Zargar, M.; Zarrinfar, H. Evaluation and comparison of the effects of biosynthesized selenium and silver nanoparticles using plant extracts with antifungal drugs on the growth of Aspergillus and Candida species. Rendiconti Lincei. Sci. Fis. Nat. 2021, 32, 791–803. [Google Scholar] [CrossRef]
- Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah DH, M.; Al Jaouni, S.K.; Salem, S.S. Unveiling antimicrobial and insecticidal activities of biosynthesized selenium nanoparticles using prickly pear peel waste. J. Funct. Biomater. 2022, 13, 112. [Google Scholar] [CrossRef]
- Abbas, H.S.; Abou Baker, D.H.; Ahmed, E.A. Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol. 2021, 203, 523–532. [Google Scholar] [CrossRef]
- Alam, H.; Khatoon, N.; Khan, M.A.; Husain, S.A.; Saravanan, M.; Sardar, M. Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria. J. Clust. Sci. 2020, 31, 1003–1011. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Abbas, H.S. Antimicrobial activity of Biosynthesized Cuo/Se nanocomposite against Helicobacter pylori. Arab. J. Chem. 2023, 16, 105095. [Google Scholar] [CrossRef]
- Khudier, M.A.; Hammadi, H.A.; Atyia, H.T.; Al-Karagoly, H.; Albukhaty, S.; Sulaiman, G.M.; Dewir, Y.H.; Mahood, H.B. Antibacterial activity of green synthesized selenium nanoparticles using Vaccinium arctostaphylos (L.) fruit extract. Cogent Food Agric. 2023, 9, 2245612. [Google Scholar] [CrossRef]
- Dorazilová, J.; Muchová, J.; Šmerková, K.; Kočiová, S.; Diviš, P.; Kopel, P.; Veselý, R.; Pavliňáková, V.; Adam, V.; Vojtová, L. Synergistic effect of chitosan and selenium nanoparticles on biodegradation and antibacterial properties of collagenous scaffolds designed for infected burn wounds. Nanomaterials 2020, 10, 1971. [Google Scholar] [CrossRef]
- Golmohammadi, R.; Najar-Peerayeh, S.; Tohidi Moghadam, T.; Hosseini, S.M.J. Synergistic antibacterial activity and wound healing properties of selenium-chitosan-mupirocin nanohybrid system: An in vivo study on rat diabetic staphylococcus aureus wound infection model. Sci. Rep. 2020, 10, 2854. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Holden, J.A.; Reynolds, E.C.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Multifunctional antimicrobial polypeptide-selenium nanoparticles combat drug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 55696–55709. [Google Scholar] [CrossRef] [PubMed]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked selenium nanoparticles for antibacterial and anticancer treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef]
- Menazea, A.A.; Ismail, A.M.; Awwad, N.S.; Ibrahium, H.A. Physical characterization and antibacterial activity of PVA/Chitosan matrix doped by selenium nanoparticles prepared via one-pot laser ablation route. J. Mater. Res. Technol. 2020, 9, 9598–9606. [Google Scholar] [CrossRef]
- Escobar-Ramírez, M.C.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Rodríguez-Serrano, G.M.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Añorve-Morga, J.; Jaimez-Ordaz, J.; González-Olivares, L.G. Antimicrobial activity of Se-nanoparticles from bacterial biotransformation. Fermentation 2021, 7, 130. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Martínez, B.; Rodríguez, A.; Kulakauskas, S.; Chapot-Chartier, M.P. Cell wall homeostasis in lactic acid bacteria: Threats and defences. FEMS Microbiol. Rev. 2020, 44, 538–564. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, Y.; Liu, Y.; An, H.; Deng, K.; Ashraf, M.A.; Zou, L.; Wang, J. Breaking down the cell wall: Still an attractive antibacterial strategy. Front. Microbiol. 2022, 13, 952633. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Chen, Q.; Yu, Q.; Sun, D.; Liu, J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Dai, C.; Wang, P.; Fan, S.; Yu, B.; Qu, Y. Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ. Res. 2021, 194, 110630. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, S.; Sundar, K.; Muthukumaran, A. Reducing agents influence the shapes of selenium nanoparticles (SeNPs) and subsequently their antibacterial and antioxidant activity. Mater. Res. Express 2019, 6, 0850i2. [Google Scholar] [CrossRef]
| NPs | Species | NP Size (nm) | DLS | Color NP Solution | Evaluated Microorganisms | Antifungal Parameters SeNPs | Ref. | |
|---|---|---|---|---|---|---|---|---|
| BACTERIA | He-SeNPs | Halomonas elongata | (SEM) 11–50 (TEM) 5–25 | - | - | C. albicans | 24 h: 55.14–66.17% 48 h: 60.96–70.86% 72 h: 58.31–61.52% | [15] |
| Lab-SeNPs | Lactobacillus paracasei (HMI) (MW390875) | (DLS) 56.91 ± 1.8 (TEM) 3–50 | (ZP) 20.1 ± 0.6 mV | Red | C. albicans ATCC 4862 C. parapsilosis ATCC 22019 F. oxysporum ATCC 62506 F. solani ATCC 38341 C. krusei ATCC 14243 C. glabrata ATCC 64677 C. tropicalis ATCC 66029 | MIC 55–70 μg/mL MFC 80–130 μg/mL | [94] | |
| Pt-SeNPs | Paenibacillus terreus | (DLS) 200–220 (FESEM) 220–240 | (PDI) 0.265 (ZP) −37.77 mV | - | C. albicans | MIC 3.90–500 μg/mL | [95] |
| NPs | Precursor Agent | Reducing Agent | Stabilizing Agent | NP Size (nm) | Tested Bacteria | Antibacterial Parameters | Ref. | |
|---|---|---|---|---|---|---|---|---|
| CHEMICAL | SeNPs | (Na2SeO3) Sodium selenite | Mercaptopropionic acid | Biopolymer | 50–300 | S. aureus MRSA S. epidermidis | 5 ppm | [102] |
| SeNPs | (Na2SeO3) Sodium selenite pentahydrate | C6H8O6 Ascorbic acid | - | (DLS) 66 ± 8 (TEM) 61 ± 7 | MRSA | MIC 32 μg/mL | [103] | |
| SeNP-ε-PL | (SeO2) Selenium dioxide | Na2S2O3 Sodium thiosulfate | PVA/ε-PL | (TEM) 80 | S. aureus MRSA E. faecalis E. coli A. baumannii P. aeruginosa K. pneumoniae K. pneumoniae (MDR) | MIC 6.0–26.2 μg/mL MBC 12.6–63 μg/mL | [104] | |
| PHYSICAL | SeNPs | Bulk Se pellets (target) | - | - | (DLS) 144 ± 46 | MDR-EC P. aeruginosa S. Aureus MRSA | MIC: 2.35 ppm MIC: 4.45 ppm MIC: 12.77 ppm MIC: 14.26 ppm | [105] |
| PVA/CS/SeNPs | Selenium plate | - | - | (XRD) 17 | E. coli P. aeruginosa S. aureus B. subtilis | 10 min 33–56% * 30 min 44–69% * | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.P.d.S.; Lima, A.K.O.; Muehlmann, L.A. An Updated Review of the Antimicrobial Potential of Selenium Nanoparticles and Selenium-Related Toxicological Issues. Future Pharmacol. 2025, 5, 3. https://doi.org/10.3390/futurepharmacol5010003
Oliveira TPdS, Lima AKO, Muehlmann LA. An Updated Review of the Antimicrobial Potential of Selenium Nanoparticles and Selenium-Related Toxicological Issues. Future Pharmacology. 2025; 5(1):3. https://doi.org/10.3390/futurepharmacol5010003
Chicago/Turabian StyleOliveira, Tainá Pereira da Silva, Alan Kelbis Oliveira Lima, and Luís Alexandre Muehlmann. 2025. "An Updated Review of the Antimicrobial Potential of Selenium Nanoparticles and Selenium-Related Toxicological Issues" Future Pharmacology 5, no. 1: 3. https://doi.org/10.3390/futurepharmacol5010003
APA StyleOliveira, T. P. d. S., Lima, A. K. O., & Muehlmann, L. A. (2025). An Updated Review of the Antimicrobial Potential of Selenium Nanoparticles and Selenium-Related Toxicological Issues. Future Pharmacology, 5(1), 3. https://doi.org/10.3390/futurepharmacol5010003








