Evaluation of the Relationship between Stream Habitat Quality and Taxa and Trait Richness and Diversity in Piedmont Streams in North Carolina
Abstract
:1. Introduction
2. Materials and Methods
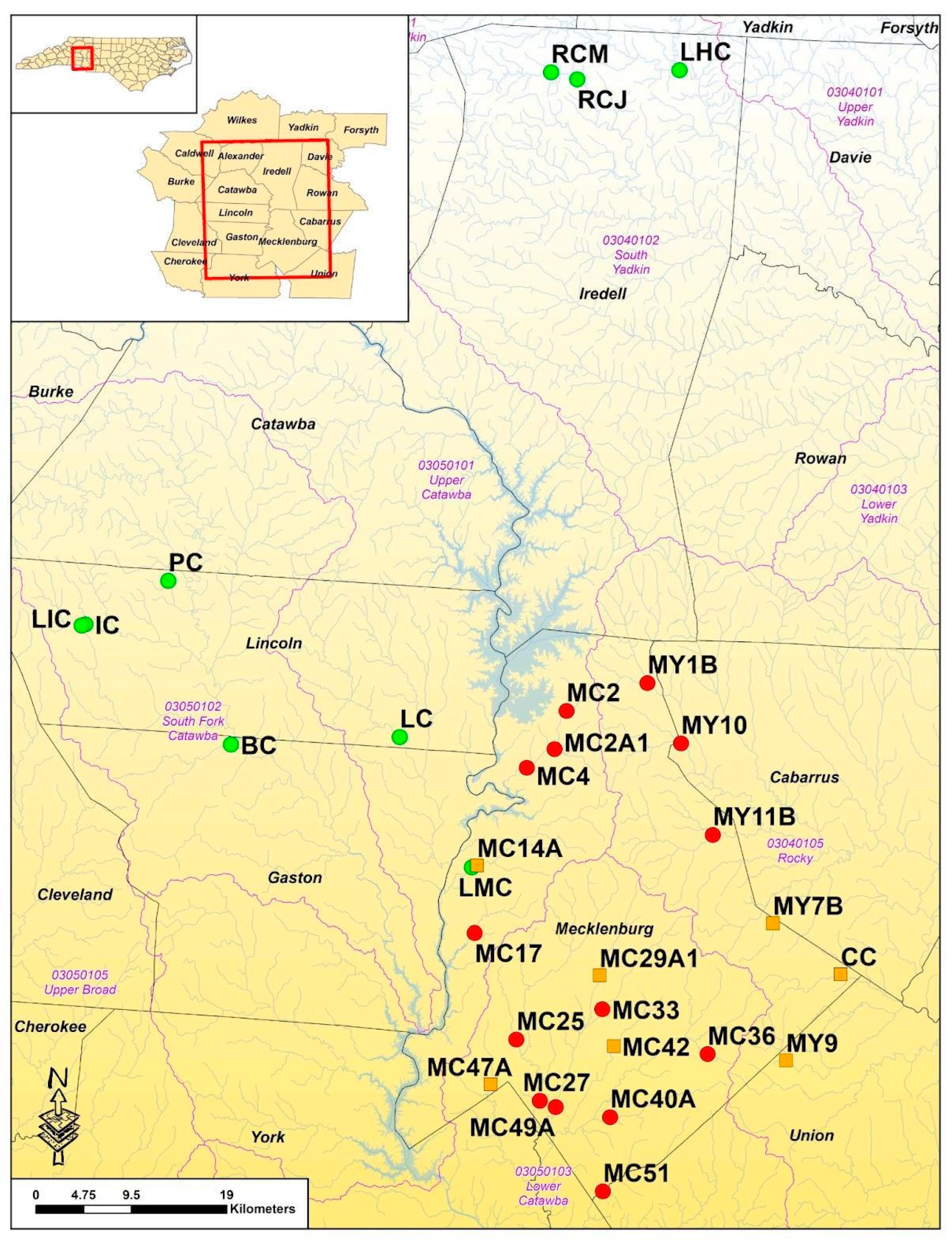
2.1. Study Sites
2.2. Aquatic Insects
2.3. Habitat Diversity
2.4. Data Analysis
3. Results
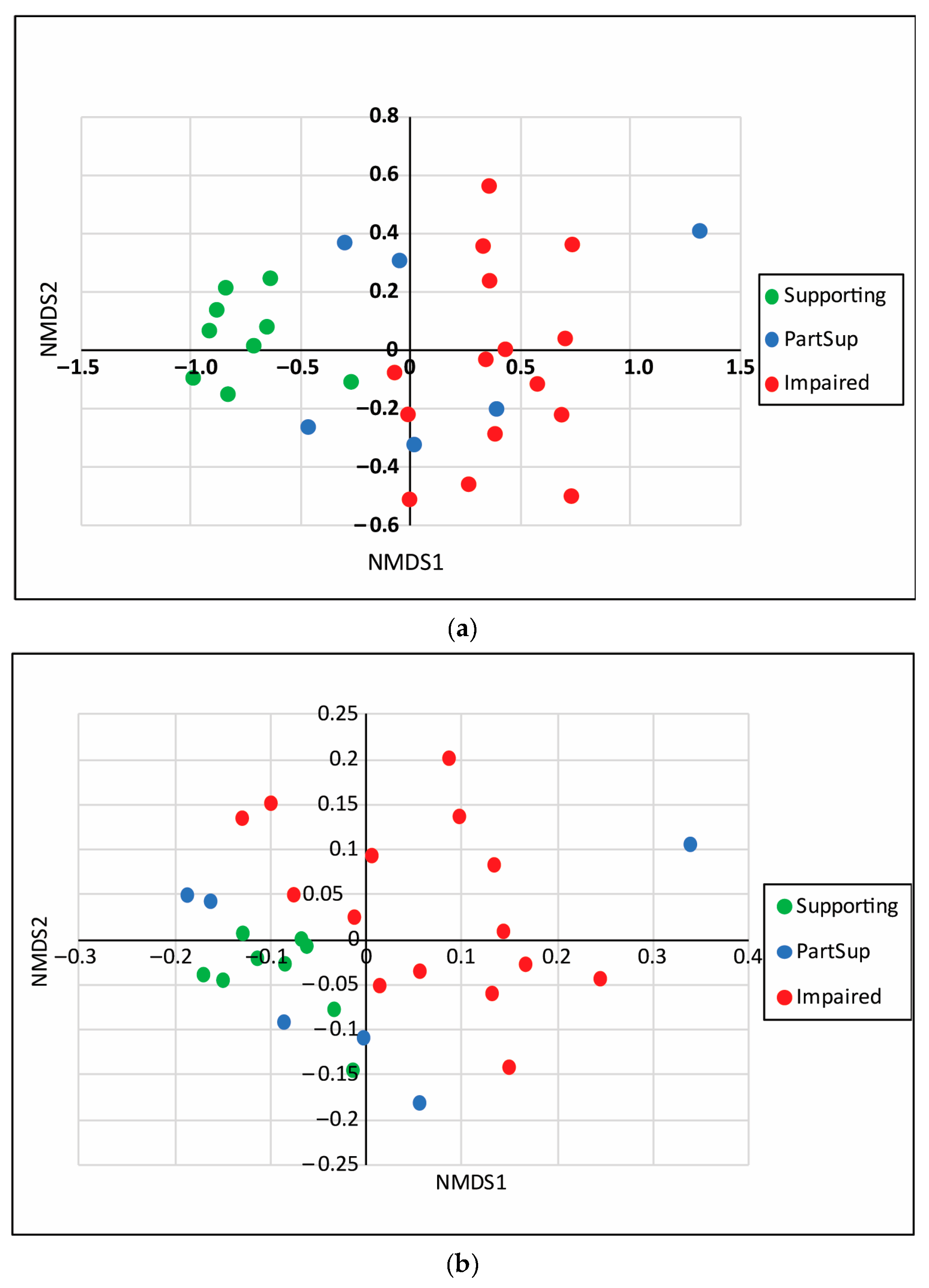
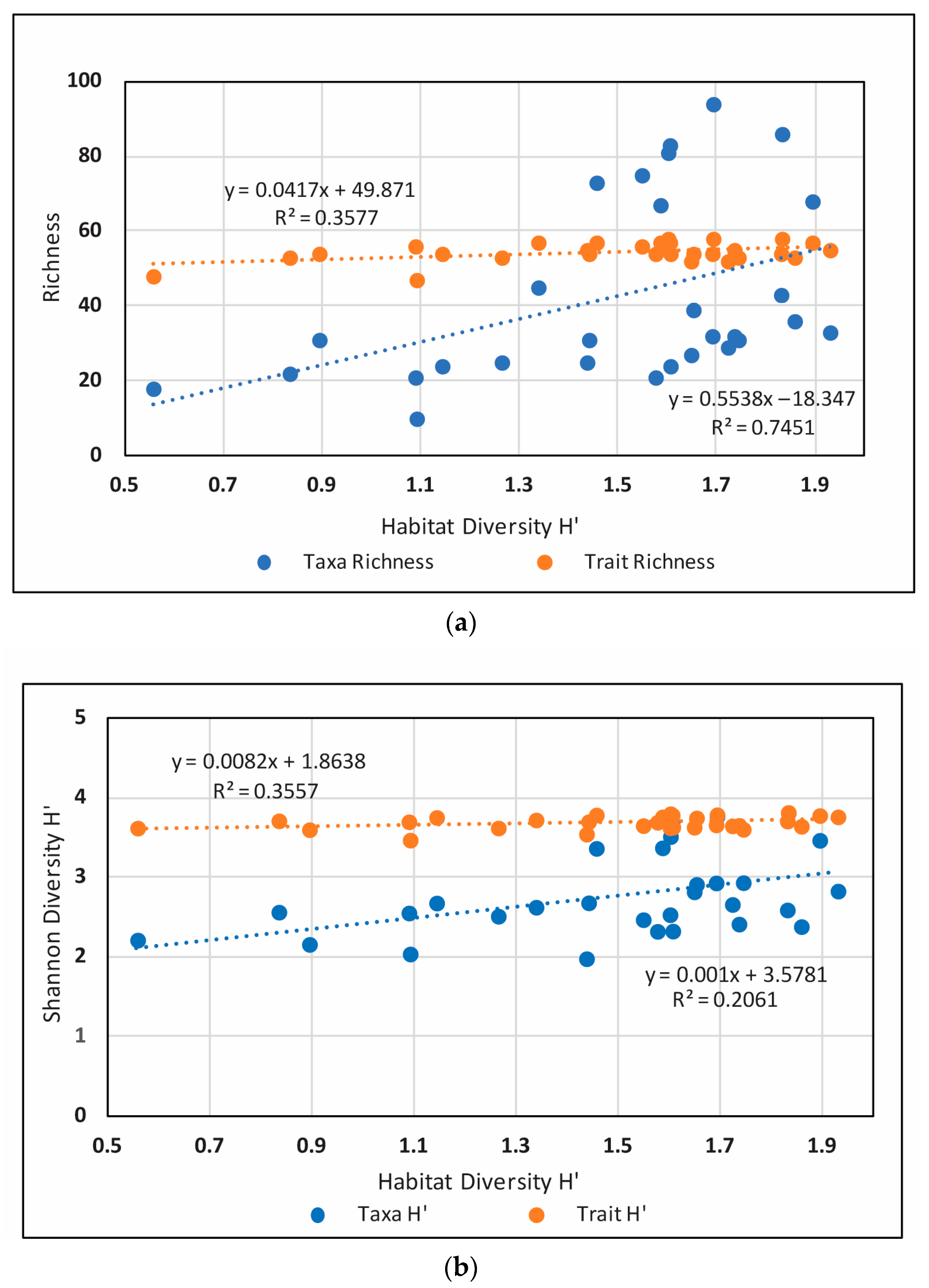
3.1. Taxa and Trait Richness and Diversity
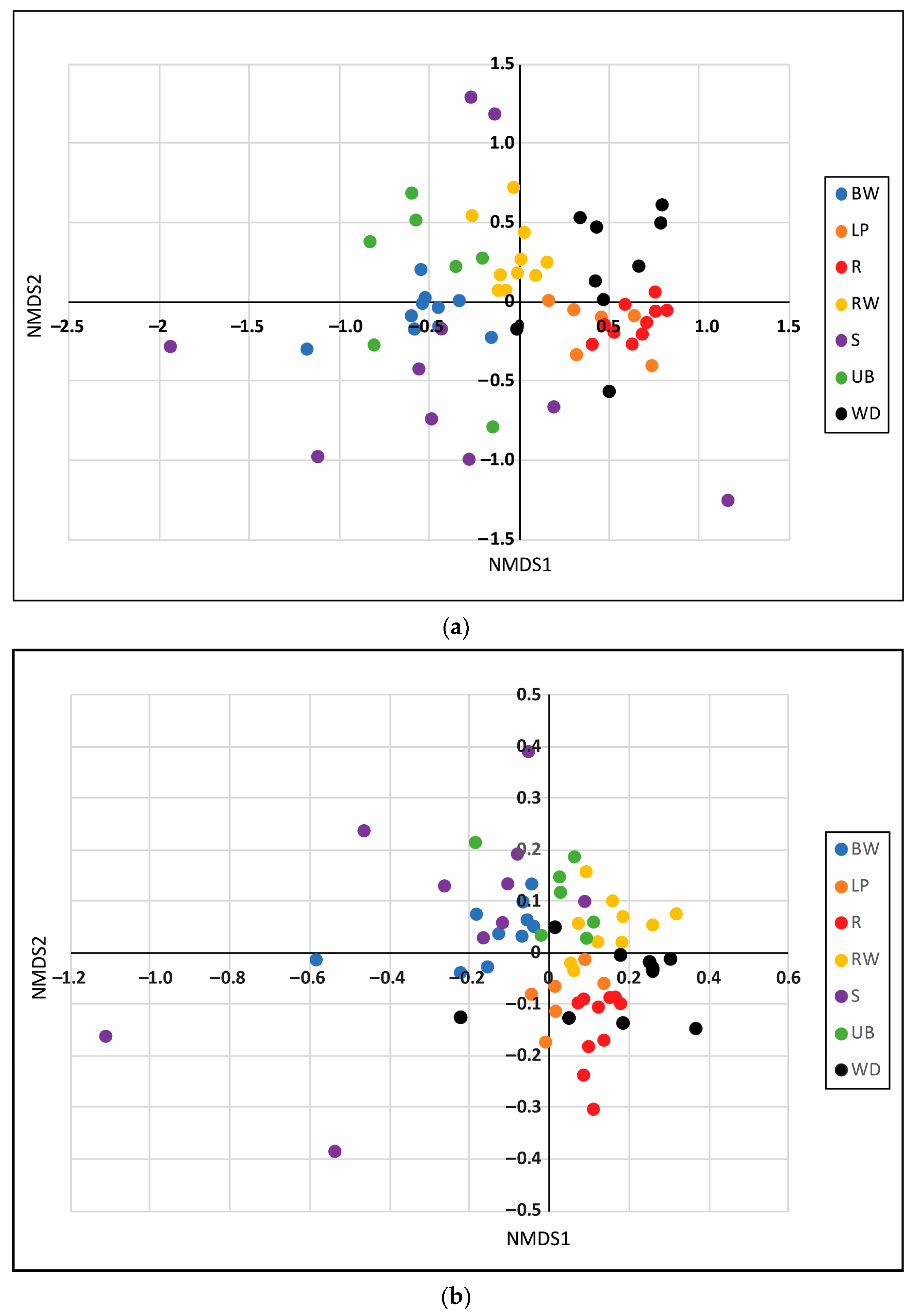
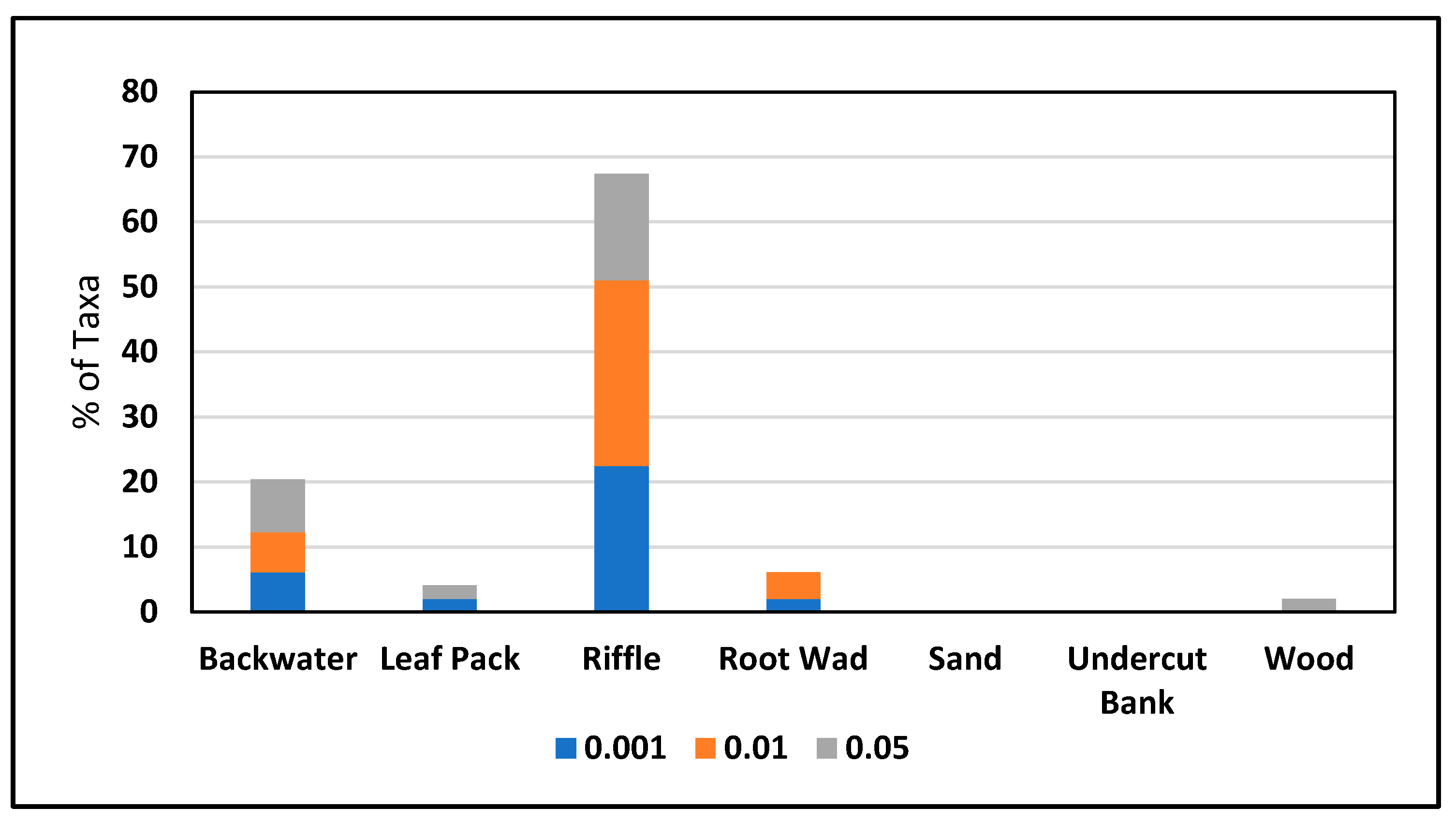
3.2. Microhabitats
4. Discussion
4.1. Taxa and Trait Patterns at the Watershed Scale
4.2. Taxa and Trait Patterns at the Reach Scale
5. Application to Stream Restorations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hynes, H.B.N. The Ecology of Running Waters; Liverpool University Press: Liverpool, UK, 1970. [Google Scholar]
- Cummins, K.W. Structure and Function of Stream Ecosystems. BioScience 1974, 24, 631–641. [Google Scholar] [CrossRef]
- Cummins, K.W.; Lauff, G.H. The Influence of Substrate Particle Size on the Microdistribution of Stream Macrobenthos. Hydrobiologia 1969, 34, 145–181. [Google Scholar] [CrossRef]
- Rabeni, C.F.; Minshall, G.W. Factors Affecting Microdistribution of Stream Benthic Insects. Oikos 1977, 29, 33–43. [Google Scholar] [CrossRef]
- Erman, D.C.; Erman, N.A. The Response of Stream Macroinvertebrates to Substrate Size and Heterogeneity. Hydrobiologia 1984, 108, 75–82. [Google Scholar] [CrossRef]
- Wohl, D.L.; Wallace, J.B.; Meyer, J.L. Benthic Macroinvertebrate Community Structure, Function and Production with Respect to Habitat Type, Reach and Drainage Basin in the Southern Appalachians (U.S.A.). Freshw. Biol. 1995, 34, 447–464. [Google Scholar] [CrossRef]
- Beisel, J.-N.; Usseglio-Polatera, P.; Moreteau, J.-C. The Spatial Heterogeneity of a River Bottom: A Key Factor Determining Macroinvertebrate Communities. Hydrobiologia 2000, 422/423, 163–171. [Google Scholar] [CrossRef]
- Lamouroux, N.; Dolédec, S.; Gayraud, S. Biological Traits of Stream Macroinvertebrate Communities: Effects of Microhabitat, Reach, and Basin Filters. J. N. Am. Benthol. Soc. 2004, 23, 449–466. [Google Scholar] [CrossRef]
- Milesi, S.V.; Dolédec, S.; Melo, A.S. Substrate Heterogeneity Influences the Trait Composition of Stream Insect Communities: An Experimental in Situ Study. Freshw. Sci. 2016, 35, 1321–1329. [Google Scholar] [CrossRef]
- Verdonschot, R.C.M.; Kail, J.; McKie, B.G.; Verdonschot, P.F.M. The Role of Benthic Microhabitats in Determining the Effects of Hydromorphological River Restoration on Macroinvertebrates. Hydrobiologia 2016, 769, 55–66. [Google Scholar] [CrossRef]
- Poff, N.L.; Olden, J.D.; Vieira, N.K.M.; Finn, D.S.; Simmons, M.P.; Kondratieff, B.C. Functional Trait Niches of North American Lotic Insects: Traits-Based Ecological Applications in Light of Phylogenetic Relationships. J. N. Am. Benthol. Soc. 2006, 25, 730–755. [Google Scholar] [CrossRef]
- Naeem, S.; Thompson, L.J.; Lawler, S.P.; Lawton, J.H.; Woodfin, R.M. Declining Biodiversity Can Alter the Performance of Ecosystems. Nature 1994, 368, 734–737. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity and Ecosystem Functioning. In Nature’s Services Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 1997; pp. 93–112. [Google Scholar]
- Naeem, S. Species Redundancy and Ecosystem Reliability. Conserv. Biol. 1998, 12, 39–45. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Hillebrand, H.; Charles, D.F. Geographic Patterns of Diversity in Streams Are Predicted by a Multivariate Model of Disturbance and Productivity. J. Ecol. 2006, 94, 609–618. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Weis, J.J.; Forbes, A.E.; Tilmon, K.J.; Ives, A.R. Biodiversity as Both a Cause and Consequence of Resource Availability: A Study of Reciprocal Causality in a Predator-Prey System. J. Anim. Ecol. 2006, 75, 497–505. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond Species: Functional Diversity and the Maintenance of Ecological Processes and Services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Cardinale, B.J. Impacts of Biodiversity Loss. Science 2012, 336, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Giller, P.S.; Hillebrand, H.; Berninger, U.-G.; Gessner, M.O.; Hawkins, S.; Inchausti, P.; Inglis, C.; Leslie, H.; Malmqvist, B.; Monaghan, M.T.; et al. Biodiversity Effects on Ecosystem Functioning: Emerging Issues and Their Experimental Test in Aquatic Environments. Oikos 2004, 104, 423–436. [Google Scholar] [CrossRef]
- Pool, T.K.; Cucherousset, J.; Boulêtreau, S.; Villéger, S.; Strecker, A.L.; Grenouillet, G. Increased Taxonomic and Functional Similarity Does Not Increase the Trophic Similarity of Communities. Glob. Ecol. Biogeogr. 2016, 25, 46–54. [Google Scholar] [CrossRef]
- Tilman, D. Functional Diversity. Encycl. Biodivers. 2001, 3, 109–120. [Google Scholar] [CrossRef]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argillier, C. A User’s Guide to Functional Diversity Indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Habitat, the Template for Ecological Strategies? J. Anim. Ecol 1977, 46, 337–365. [Google Scholar] [CrossRef]
- Poff, N.L.; Ward, J.V. Physical Habitat Template of Lotic Systems: Recovery in the Context of Historical Pattern of Spatiotemporal Heterogeneity. Environ. Manag. 1990, 14, 629–645. [Google Scholar] [CrossRef]
- Townsend, C.R.; Hildrew, A.G. Species Traits in Relation to a Habitat Templet for River Systems. Freshw. Biol. 1994, 31, 265–275. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan II, R.P. The Urban Stream Syndrome: Current Knowledge and the Search for a Cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Schueler, T.R. The Importance of Imperviousness. Watershed Prot. Tech. 1994, 1, 100–111. [Google Scholar]
- Bledsoe, B.P.; Watson, C.C. Effects of Urbanization on Channel Instability. JAWRA J. Am. Water Resour. Assoc. 2001, 37, 255–270. [Google Scholar] [CrossRef]
- Paul, M.J.; Meyer, J.L. Streams in the Urban Landscape. Annu. Rev. Ecol. Syst. 2001, 32, 333–365. [Google Scholar] [CrossRef]
- Center for Watershed Protection. Impacts of Impervious Cover on Aquatic Systems. Watershed Protection Research Monograph; Center for Watershed Protection: Ellicott City, MD, USA, 2003; pp. 1–142. [Google Scholar]
- Coleman, J.C.; Miller, M.C.; Mink, F.L. Hydrologic Disturbance Reduces Biological Integrity in Urban Streams. Environ. Monit. Assess. 2011, 172, 663–687. [Google Scholar] [CrossRef]
- Vietz, G.J.; Walsh, C.J.; Fletcher, T.D. Urban Hydrogeomorphology and the Urban Stream Syndrome: Treating the Symptoms and Causes of Geomorphic Change. Prog. Phys. Geogr. 2016, 40, 480–492. [Google Scholar] [CrossRef]
- Rosgen, D. The Reference Reach—A Blueprint for Natural Channel Design. In Proceedings of the Wetlands Engineering and River Restoration Conference, Denver, CO, USA, 20–29 March 1998; pp. 1009–1016. [Google Scholar] [CrossRef]
- Doll, B.A.; Grabow, G.L.; Hall, K.R.; Halley, J.; Harman, W.A.; Jennings, G.D.; Wise, D.E. Stream Restoration: A Natural Channel Design Handbook; NC Stream Restoration Institute, NC State University: Raleigh, NC, USA, 2003. [Google Scholar]
- Sudduth, E.B.; Hassett, B.A.; Cada, P.; Bernhardt, E.S. Testing the Field of Dreams Hypothesis: Functional Responses to Urbanization and Restoration in Stream Ecosystems. Ecol. Appl. 2011, 21, 1972–1988. [Google Scholar] [CrossRef]
- Suren, A.M.; McMurtrie, S. Assessing the Effectiveness of Enhancement Activities in Urban Streams: II. Responses of Invertebrate Communities. River Res. Appl. 2005, 21, 439–453. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Palmer, M.A. Restoring Streams in an Urbanizing World. Freshw. Biol. 2007, 52, 738–751. [Google Scholar] [CrossRef]
- Palmer, M.A.; Menninger, H.L.; Bernhardt, E. River Restoration, Habitat Heterogeneity and Biodiversity: A Failure of Theory or Practice? Freshw. Biol. 2010, 55, 205–222. [Google Scholar] [CrossRef]
- Violin, C.R.; Cada, P.; Sudduth, E.B.; Hassett, B.A.; Penrose, D.L.; Bernhardt, E.S. Effects of Urbanization and Urban Stream Restoration on the Physical and Biological Structure of Stream Ecosystems. Ecol. Appl. 2011, 21, 1932–1949. [Google Scholar] [CrossRef]
- Stranko, S.A.; Hilderbrand, R.H.; Palmer, M.A. Comparing the Fish and Benthic Macroinvertebrate Diversity of Restored Urban Streams to Reference Streams. Restor. Ecol. 2012, 20, 747–755. [Google Scholar] [CrossRef]
- Sudduth, E.B.; Meyer, J.L. Effects of Bioengineered Streambank Stabilization on Bank Habitat and Macroinvertebrates in Urban Streams. Environ. Manag. 2006, 38, 218–226. [Google Scholar] [CrossRef]
- Hering, D.; Aroviita, J.; Baattrup-Pedersen, A.; Brabec, K.; Buijse, T.; Ecke, F.; Friberg, N.; Gielczewski, M.; Januschke, K.; Köhler, J.; et al. Contrasting the Roles of Section Length and Instream Habitat Enhancement for River Restoration Success: A Field Study of 20 European Restoration Projects. J. Appl. Ecol. 2015, 52, 1518–1527. [Google Scholar] [CrossRef]
- Poff, N.L.; Bledsoe, B.P.; Cuhaciyan, C.O. Hydrologic Variation with Land Use across the Contiguous United States: Geomorphic and Ecological Consequences for Stream Ecosystems. Geomorphology 2006, 79, 264–285. [Google Scholar] [CrossRef]
- Richardson, C.J.; Flanagan, N.E.; Ho, M.; Pahl, J.W. Integrated Stream and Wetland Restoration: A Watershed Approach to Improved Water Quality on the Landscape. Ecol. Eng. 2011, 37, 25–39. [Google Scholar] [CrossRef]
- Walsh, C.J.; Fletcher, T.D.; Burns, M.J. Urban Stormwater Runoff: A New Class of Environmental Flow Problem. PLoS ONE 2012, 7, e45814. [Google Scholar] [CrossRef]
- Walsh, C.J.; Booth, D.B.; Burns, M.J.; Fletcher, T.D.; Hale, R.L.; Hoang, L.N.; Livingston, G.; Rippy, M.A.; Roy, A.H.; Scoggins, M. Principles for Urban Stormwater Management to Protect Stream Ecosystems. J. Freshw. Sci. 2016, 35, 1–13. [Google Scholar] [CrossRef]
- Shields, F.D.; Cooper, C.M.; Knight, S.S.; Moore, M.T. Stream Corridor Restoration Research: A Long and Winding Road. Ecol. Eng. 2003, 20, 441–454. [Google Scholar] [CrossRef]
- Palmer, M.A.; Bernhardt, E.S.; Allan, J.D.; Lake, P.S.; Alexander, G.; Brooks, S.; Carr, J.; Clayton, S.; Dahm, C.N.; Follstad Shah, J.; et al. Standards for Ecologically Successful River Restoration. J. Appl. Ecol. 2005, 42, 208–217. [Google Scholar] [CrossRef]
- NCDEQ. Standard Operating Procedures for Collection and Analysis of Benthic Macroinvertebrates; North Carolina Department of Environment and Natural Resources: Raleigh, NC, USA, 2016; p. 71.
- Charlotte-Mecklenburg Storm Water Services. Mecklenburg County Benthic Macroinvertebrate Collection and Analysis Standard Operating Procedures; Mecklenburg County Land Use and Environmental Services Agency: Charlotte, NC, USA, 2017.
- National Weather Service. Available online: www.weather.gov/gsp/cltcli (accessed on 14 November 2022).
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. USEPA Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; EPA 841-b-99-002; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999; ISBN 1-111-11111-1.
- Charlotte-Mecklenburg Storm Water Services. Enhanced Mecklenburg Stream Habitat Assessment Protocol: Field Guide; Mecklenburg County Land Use and Environmental Services Agency: Charlotte, NC, USA, 2020.
- Charlotte-Mecklenburg Storm Water Services. Enhanced Mecklenburg Stream Habitat Assessment Protocol: Data Analysis; Mecklenburg County Land Use and Environmental Services Agency: Charlotte, NC, USA, 2020.
- Roux, A.J. An Examination of the Impact of Urbanization on Stream Biodiversity and Ecosystem Function. Ph.D. Thesis, The University of North Carolina at Charlotte, Charlotte, NC, USA, 2022. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America; Kendall/Hunt Publishing Co.: Dubuque, IA, USA, 2008; ISBN 978-0757563218. [Google Scholar]
- Kondratieff, B.C. Larvae of the Southeastern USA Mayfly, Stonefly, and Caddisfly Species:(Ephemeroptera, Plecoptera, and Trichoptera); Morse, J.C., McCafferty, W.P., Stark, B.P., Jacobus, L.M., Eds.; Biota of South Carolina; Clemson University Public Service Publishing, Clemson University: Clemson, SC, USA, 2017; Volume 9. [Google Scholar]
- Epler, J.H. Identification Manual for the Larval Chironomidae (Diptera) of North and South Carolina; North Carolina Department of Environment and Natural Resources: Raleigh, NC, USA, 2001; Volume 1.
- Allan, J.D.; Castillo, M.M. Stream Ecology Structure and Function of Running Waters; Springer Netherlands: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-5582-9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 24 April 2023).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Community Ecology Package ‘Vegan’. 2020, p. 298. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 24 April 2023).
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. 2020. Available online: https://cran.r-project.org/web/packages/FSA/index.html (accessed on 24 April 2023).
- De Cáceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.R.; Cuffney, T.F.; Coles, J.F.; Fitzpatrick, F.; McMahon, G.; Steuer, J.; Bell, A.H.; May, J.T. Urban Streams across the USA: Lessons Learned from Studies in 9 Metropolitan Areas. J. N. Am. Benthol. Soc. 2009, 28, 1051–1069. [Google Scholar] [CrossRef]
- Cuffney, T.F.; Brightbill, R.A.; May, J.T.; Waite, I.R. Responses of Benthic Macroinvertebrates to Environmental Changes Associated with Urbanization in Nine Metropolitan Areas. Ecol. Appl. 2010, 20, 1384–1401. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.H.; Coles, J.F.; McMahon, G.; Woodside, M.D. Urban Development Results in Stressors That Degrade Stream Ecosystems; U.S. Geological Survey: Reston, VA, USA, 2012; p. 138.
- Carlisle, D.M.; Meador, M.R.; Short, T.M.; Tate, C.M.; Gurtz, M.E.; Bryant, W.L.; Falcone, J.A.; Woodside, M.D. The Quality of Our Nation’s Waters—Ecological Health in the Nation’s Streams, 1993–2005; U.S. Geological Survey Circular 1391; U.S. Geological Survey: Reston, VA, USA, 2013.
- Coles, J.F.; Cuffney, T.F.; McMahon, G.; Beaulieu, K.M. The Effects of Urbanization on the Biological, Physical, and Chemical Characteristics of Coastal New England Streams; U.S. Geological Survey Professional Paper 1695; U.S. Geological Survey: Reston, VA, USA, 2004; 47p.
- Alberti, M. The Effects of Urban Patterns on Ecosystem Function. Int. Reg. Sci. Rev. 2005, 28, 168–192. [Google Scholar] [CrossRef]
- Coles, J.F.; McMahon, G.; Bell, A.H.; Brown, L.R.; Fitzpatrick, F.A.; Scudder Eikenberry, B.C.; Woodside, M.D.; Cuffney, T.F.; Bryant, W.L.; Cappiella, K.; et al. Effects of Urban Development on Stream Ecosystems in Nine Metropolitan Study Areas across the United States; U.S. Geological Survey: Reston, VA, USA, 2012; ISBN 978-1-4113-3447-2.
- Baumgartner, S.D.; Robinson, C.T. Changes in Macroinvertebrate Trophic Structure along a Land-Use Gradient within a Lowland Stream Network. Aquat. Sci. 2017, 79, 407–418. [Google Scholar] [CrossRef]
- Wang, L.; Lyons, J.; Kanehl, P.; Gatti, R. Influence of Watershed Land Use on Habitat Quality and Biotic Integrity in Wisconsin Streams. Fisheries 1997, 22, 7–12. [Google Scholar] [CrossRef]
- Gage, M.S.; Spivak, A.; Paradise, C.J. Effects of Land Use and Disturbance on Benthic Insects in Headwater Streams Draining Small Watersheds North of Charlotte, NC. Southeast. Nat. 2004, 3, 345–358. [Google Scholar] [CrossRef]
- Péru, N.; Dolédec, S. From Compositional to Functional Biodiversity Metrics in Bioassessment: A Case Study Using Stream Macroinvertebrate Communities. Ecol. Indic. 2010, 10, 1025–1036. [Google Scholar] [CrossRef]
- Bêche, L.A.; McElravy, E.P.; Resh, V.H. Long-Term Seasonal Variation in the Biological Traits of Benthic-Macroinvertebrates in Two Mediterranean-Climate Streams in California, U.S.A. Freshw. Biol. 2006, 51, 56–75. [Google Scholar] [CrossRef]
- Lamothe, K.A.; Alofs, K.M.; Jackson, D.A.; Somers, K.M. Functional Diversity and Redundancy of Freshwater Fish Communities across Biogeographic and Environmental Gradients. Divers. Distrib. 2018, 24, 1612–1626. [Google Scholar] [CrossRef]
- Rosenfeld, J. Functional Redundancy in Ecology and Conservation. Oikos 2002, 98, 156–162. [Google Scholar] [CrossRef]
- Bêche, L.A.; Statzner, B. Richness Gradients of Stream Invertebrates across the USA: Taxonomy- and Trait-Based Approaches. Biodivers. Conserv. 2009, 18, 3909–3930. [Google Scholar] [CrossRef]
- Walker, B.H. Biodiversity and Ecological Redundancy. Conserv. Biol. 1992, 6, 18–23. [Google Scholar] [CrossRef]
- Heatherly, T.; Whiles, M.R.; Royer, T.V.; David, M.B. Relationships between Water Quality, Habitat Quality, and Macroinvertebrate Assemblages in Illinois Streams. J. Environ. Qual. 2007, 36, 1653–1660. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of Functional Diversity under Land Use Intensification across Multiple Taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef]
- Richards, C.; Haro, R.J.; Johnson, L.B.; Host, G.E. Catchment and Reach-Scale Properties as Indicators of Macroinvertebrate Species Traits. Freshw. Biol. 1997, 37, 219–230. [Google Scholar] [CrossRef]
- Dovciak, A.L.; Perry, J.A. In Search of Effective Scales for Stream Management: Does Agroecoregion, Watershed, or Their Intersection Best Explain the Variance in Stream Macroinvertebrate Communities? Environ. Manag. 2002, 30, 365–377. [Google Scholar] [CrossRef]
- Waite, I.R. Agricultural Disturbance Response Models for Invertebrate and Algal Metrics from Streams at Two Spatial Scales within the U.S. Hydrobiologia 2014, 726, 285–303. [Google Scholar] [CrossRef]
- Krynak, E.M.; Yates, A.G. Benthic Invertebrate Taxonomic and Trait Associations with Land Use in an Intensively Managed Watershed: Implications for Indicator Identification. Ecol. Indic. 2018, 93, 1050–1059. [Google Scholar] [CrossRef]
- Bisson, P.A.; Montgomery, D.R.; Buffington, J.M. Chapter 2—Valley Segments, Stream Reaches, and Channel Units. In Methods in Stream Ecology; Academic Press: Burlington, MA, USA, 2006; pp. 23–49. ISBN 978-0-12-332908-0. [Google Scholar]
- Jowett, I.G.; Richardson, J. Microhabitat Preferences of Benthic Invertebrates in a New Zealand River and the Development of In-stream Flow-habitat Models for Deleatidium spp. N. Z. J. Mar. Freshw. Res. 1990, 24, 19–30. [Google Scholar] [CrossRef]
- White, J.C.; Krajenbrink, H.J.; Hill, M.J.; Hannah, D.M.; House, A.; Wood, P.J. Habitat-Specific Invertebrate Responses to Hydrological Variability, Anthropogenic Flow Alterations, and Hydraulic Conditions. Freshw. Biol. 2019, 64, 555–576. [Google Scholar] [CrossRef]
- Forcellini, M.; Plichard, L.; Dolédec, S.; Mérigoux, S.; Olivier, J.-M.; Cauvy-Fraunié, S.; Lamouroux, N. Microhabitat Selection by Macroinvertebrates: Generality among Rivers and Functional Interpretation. J. Ecohydraul. 2022, 7, 28–41. [Google Scholar] [CrossRef]
- Scotti, A.; Füreder, L.; Marsoner, T.; Tappeiner, U.; Stawinoga, A.E.; Bottarin, R. Effects of Land Cover Type on Community Structure and Functional Traits of Alpine Stream Benthic Macroinvertebrates. Freshw. Biol. 2020, 65, 524–539. [Google Scholar] [CrossRef]
- Gregory, M.B. Microhabitat Preferences by Aquatic Invertebrates Influence Bioassessment Metrics in Piedmont Streams of Georgia and Alabama. In Proceedings of the 2005 Georgia Water Resources Conference, Athens, GA, USA, 25–27 April 2005. [Google Scholar]
- Wang, L.; Weigel, B.W.; Kanehl, P.; Lohman, K. Influence of Riffle and Snag Habitat Specific Sampling on Stream Macroinvertebrate Assemblage Measures in Bioassessment. Environ. Monit. Assess. 2006, 119, 245–273. [Google Scholar] [CrossRef]
- Coe, H.J.; Kiffney, P.M.; Pess, G.R. A Comparison of Methods to Evaluate the Response of Periphyton and Invertebrates to Wood Placement in Large Pacific Coastal Rivers. Northwest Sci. 2006, 80, 298–307. [Google Scholar]
- Cordova, J.M.; Rosi-Marshall, E.J.; Yamamuro, A.M.; Lamberti, G.A. Quantity, Controls and Functions of Large Woody Debris in Midwestern USA Streams. River Res. Appl. 2007, 24, 21–33. [Google Scholar] [CrossRef]
- Coe, H.J.; Kiffney, P.M.; Pess, G.R.; Kloehn, K.K.; McHenry, M.L. Periphyton and Invertebrate Response to Wood Placement in Large Pacific Coastal Rivers. River Res. Appl. 2009, 25, 1025–1035. [Google Scholar] [CrossRef]
- Pilotto, F.; Harvey, G.L.; Wharton, G.; Pusch, M.T. Simple Large Wood Structures Promote Hydromorphological Heterogeneity and Benthic Macroinvertebrate Diversity in Low-Gradient Rivers. Aquat. Sci. 2016, 78, 755–766. [Google Scholar] [CrossRef]
- de Brouwer, J.H.F.; Verdonschot, P.F.M.; Eekhout, J.P.C.; Verdonschot, R.C.M. Macroinvertebrate Taxonomic and Trait-Based Responses to Large-Wood Reintroduction in Lowland Streams. Freshw. Sci. 2020, 39, 693–703. [Google Scholar] [CrossRef]
- Entrekin, S.A.; Rosi, E.J.; Tank, J.L.; Hoellein, T.J.; Lamberti, G.A. Quantitative Food Webs Indicate Modest Increases in the Transfer of Allochthonous and Autochthonous C to Macroinvertebrates Following a Large Wood Addition to a Temperate Headwater Stream. Front. Ecol. Evol. 2020, 8, 114. [Google Scholar] [CrossRef]
- Huryn, A.D.; Wallace, J.B.; Anderson, N.H. Habitat, Life History, Secondary Production, and Behavioral Adaptations of Aquatic Insects. In An Introduction to the Aquatic Insects of North America; Merritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 2008; pp. 55–104. ISBN 978-0-7575-5049-2. [Google Scholar]
- Schröder, M.; Kiesel, J.; Schattmann, A.; Jähnig, S.C.; Lorenz, A.W.; Kramm, S.; Keizer-Vlek, H.; Rolauffs, P.; Graf, W.; Leitner, P.; et al. Substratum Associations of Benthic Invertebrates in Lowland and Mountain Streams. Ecol. Indic. 2013, 30, 178–189. [Google Scholar] [CrossRef]
- Orth, D.J.; Maughan, O.E. Microhabitat Preference of Benthic Fauna in a Woodland Stream. Hydrobiologia 1983, 106, 157–168. [Google Scholar] [CrossRef]
- Brooks, A.J.; Haeusler, T.; Reinfelds, I.; Williams, S. Hydraulic Microhabitats and the Distribution of Macroinvertebrate Assemblages in Riffles. Freshw. Biol. 2005, 50, 331–344. [Google Scholar] [CrossRef]
- Smith, R.F.; Venugopal, P.D.; Baker, M.E.; Lamp, W.O. Habitat Filtering and Adult Dispersal Determine the Taxonomic Composition of Stream Insects in an Urbanizing Landscape. Freshw. Biol. 2015, 60, 1740–1754. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Arimoro, F.O.; Odume, O.N. Exploring the Distribution Patterns of Macroinvertebrate Signature Traits and Ecological Preferences and Their Responses to Urban and Agricultural Pollution in Selected Rivers in the Niger Delta Ecoregion, Nigeria. Aquat. Ecol. 2020, 54, 553–573. [Google Scholar] [CrossRef]
- Jordt, S.; Taylor, B.W. A Rolling Stone Gathers No Eggs: The Importance of Stream Insect Egg Laying Natural History for Stream Restoration. Ecology 2021, 102, e03331. [Google Scholar] [CrossRef]
- Louhi, P.; Mykrä, H.; Paavola, R.; Huusko, A.; Vehanen, T.; Mäki-Petäys, A.; Muotka, T. Twenty Years of Stream Restoration in Finland: Little Response by Benthic Macroinvertebrate Communities. Ecol. Appl. 2011, 21, 1950–1961. [Google Scholar] [CrossRef]
- Ernst, A.G.; Warren, D.R.; Baldigo, B.P. Natural-Channel-Design Restorations That Changed Geomorphology Have Little Effect on Macroinvertebrate Communities in Headwater Streams. Restor. Ecol. 2012, 20, 532–540. [Google Scholar] [CrossRef]
- Frainer, A.; Polvi, L.E.; Jansson, R.; Mckie, B.G. Enhanced Ecosystem Functioning Following Stream Restoration: The Roles of Habitat Heterogeneity and Invertebrate Species Traits. J. Appl. Ecol. 2018, 55, 377–385. [Google Scholar] [CrossRef]
- Hasselquist, E.; Polvi, L.; Kahlert, M.; Nilsson, C.; Sandberg, L.; McKie, B. Contrasting Responses among Aquatic Organism Groups to Changes in Geomorphic Complexity Along a Gradient of Stream Habitat Restoration: Implications for Restoration Planning and Assessment. Water 2018, 10, 1465. [Google Scholar] [CrossRef]
- Merten, E.C.; Snobl, Z.R.; Wellnitz, T.A. Microhabitat Influences on Stream Insect Emergence. Aquat. Sci. 2014, 76, 165–172. [Google Scholar] [CrossRef]
| Taxa Metric | Kendall’s Rank Correlation Tau | |||
|---|---|---|---|---|
| Microhabitats | Taxa Richness (S) | Taxa Shannon Diversity (H′) | Trait Richness (S) | Trait Shannon Diversity (H′) |
| Pool | 0.363 ** | 0.277 * | 0.285 | 0.270 |
| Run | 0.423 ** | 0.250 | 0.446 ** | 0.312 * |
| Backwater | 0.359 ** | 0.117 | 0.172 | 0.032 |
| Root Wad | 0.186 | 0.118 | 0.089 | 0.069 |
| Undercut Bank | 0.071 | 0.241 | 0.028 | 0.136 |
| Leaf Pack | 0.345 ** | 0.357 ** | 0.229 | 0.29 * |
| Small Wood | 0.117 | 0.188 | 0.290 * | 0.274 * |
| Large Wood | 0.228 | 0.210 | 0.185 | 0.171 |
| Riffle Index | 0.489 ** | 0.261 | 0.558 *** | 0.341 ** |
| Habitat Diversity | 0.383 ** | 0.331 * | 0.155 | 0.210 |
| Goodness of Fit | Regression Model | ||
|---|---|---|---|
| Adj. R2 | p | ||
| Taxa Richness | 0.674 | 1.3 × 10−6 | 22.443 + 2.942 (RiffleInd) + 0.486 (LgWood) − 6.728 (Run) + 0.697 (LeafPack) |
| Taxa Diversity (H′) | 0.4144 | 6.9 × 10−4 | 2.248 + 0.026 (LeafPack) + 0.024 (RiffleInd) + 0.010 (UndercutBank) |
| Trait Richness | 0.410 | 7.6 × 10−4 | 48.736 + 0.137 (RiffleInd) + 2.568 (HabitatHʹ) + 0.014 (SmWood) |
| Trait Diversity (H′) | 0.261 | 6.4 × 10−3 | 3.636 + 0.003 (LeafPack) + 0.003 (RiffleInd) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roux, A.J.; Clinton, S.M. Evaluation of the Relationship between Stream Habitat Quality and Taxa and Trait Richness and Diversity in Piedmont Streams in North Carolina. Hydrobiology 2023, 2, 363-381. https://doi.org/10.3390/hydrobiology2020024
Roux AJ, Clinton SM. Evaluation of the Relationship between Stream Habitat Quality and Taxa and Trait Richness and Diversity in Piedmont Streams in North Carolina. Hydrobiology. 2023; 2(2):363-381. https://doi.org/10.3390/hydrobiology2020024
Chicago/Turabian StyleRoux, Anthony J., and Sandra M. Clinton. 2023. "Evaluation of the Relationship between Stream Habitat Quality and Taxa and Trait Richness and Diversity in Piedmont Streams in North Carolina" Hydrobiology 2, no. 2: 363-381. https://doi.org/10.3390/hydrobiology2020024






