Environmentally Friendly Water-Based Electrolyte for Dye-Sensitized Solar Cells: Future Prospective and Outlook
Abstract
1. Introduction
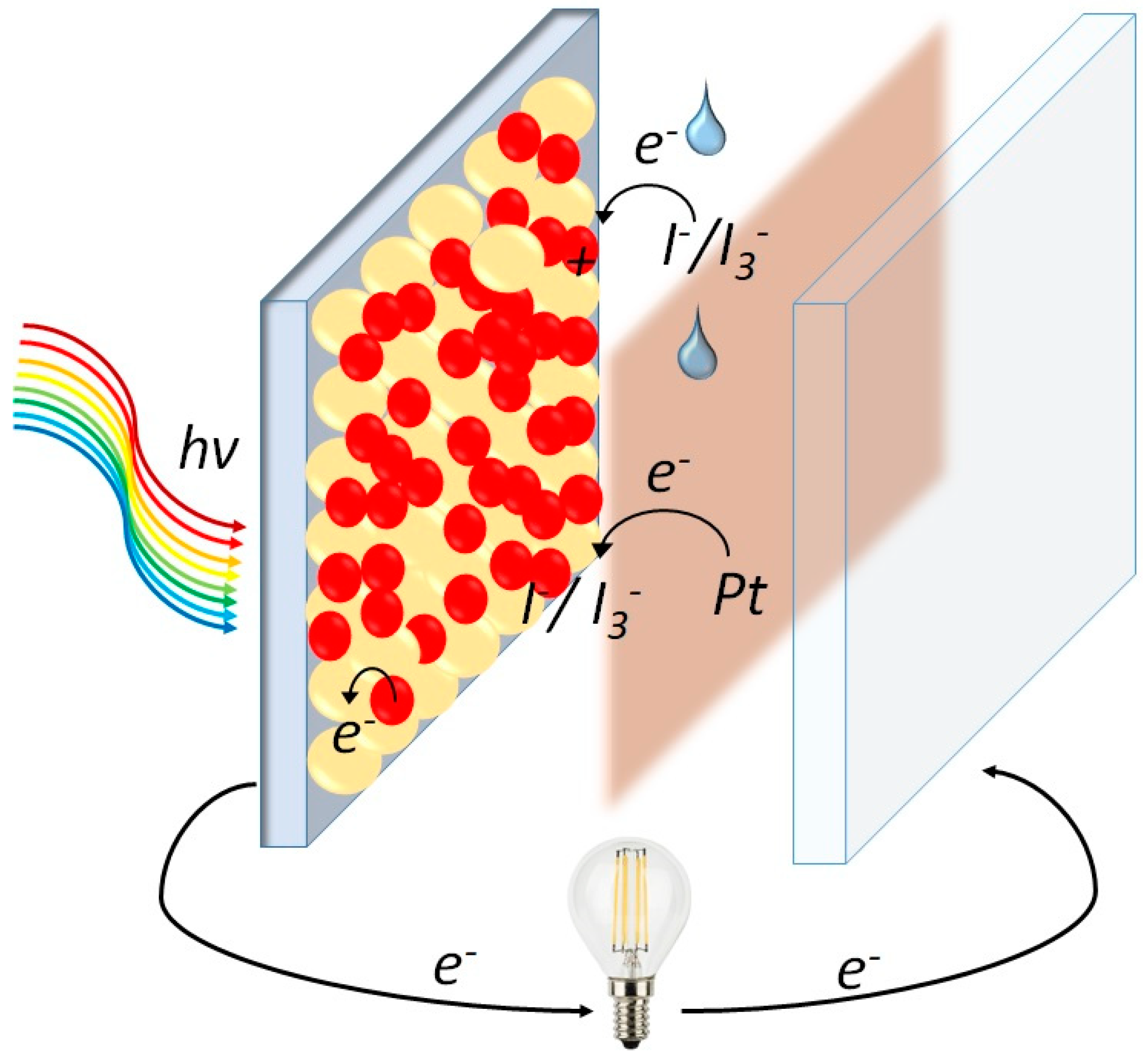
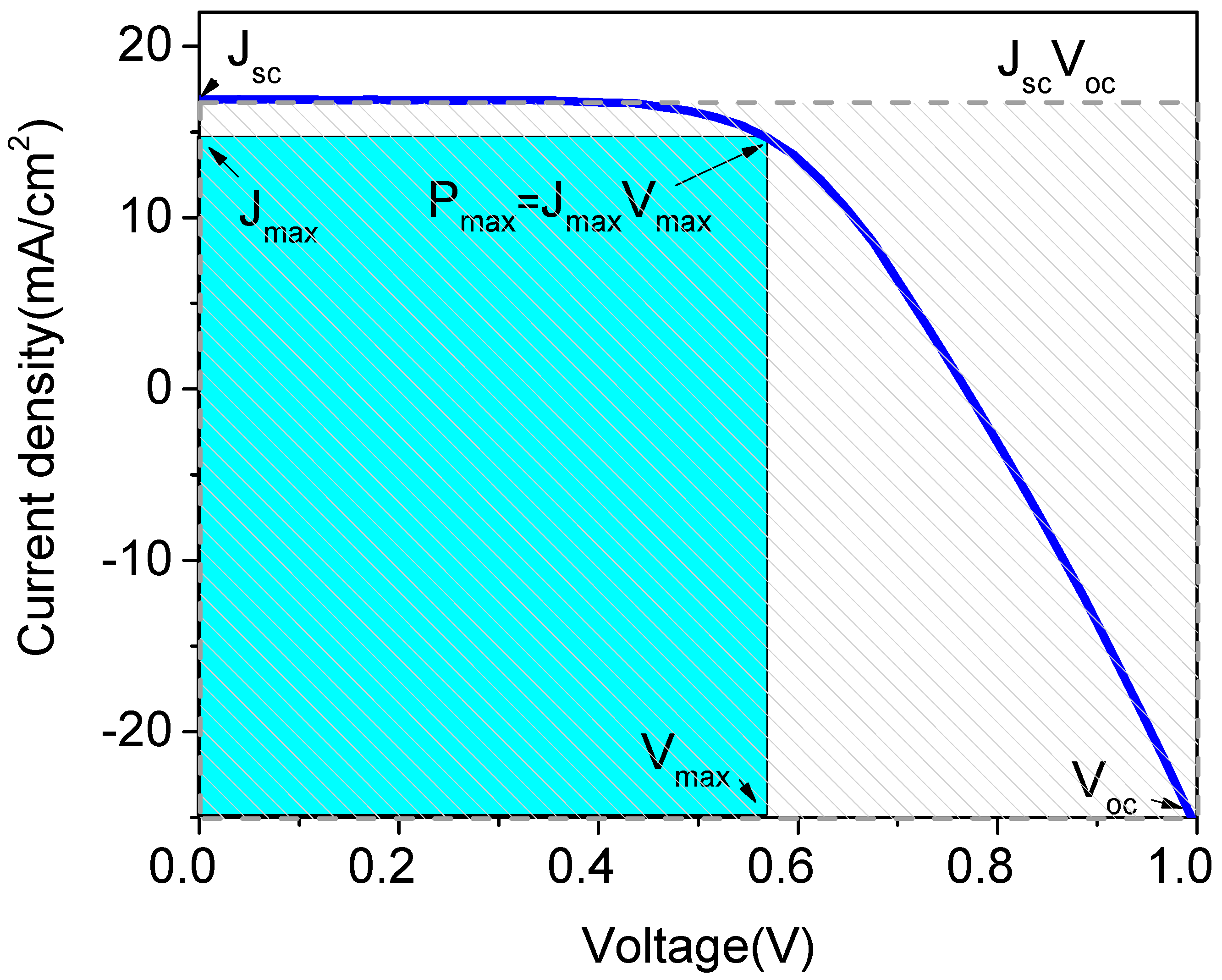
2. Concepts and Working Principles of DSSCs
 Dye* Light absorption
Dye* Light absorption
 Dye+ + eCB (TiO2) Electron injection
Dye+ + eCB (TiO2) Electron injection
 2 Dye + I3− Dye regeneration
2 Dye + I3− Dye regeneration
 3I− Electrolyte reduction
3I− Electrolyte reduction
Critical Aspects in Water-Based DSSCs
3. Water-Based DSSCs
3.1. Water DSSCs Based on Synthetic Dyes
| Dye | Electrolyte | Jsc (mA/cm2) | Voc (V) | FF | ƞ (%) | Ref |
|---|---|---|---|---|---|---|
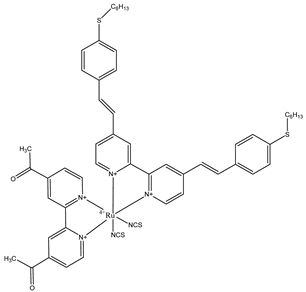 TG6 | 2 M PMMI, 0.05 M I2, 0.1 M GuSCN, 0.5 TBP, 1%TRITON X-100 | 4.7 | 0.74 | 0.69 | 2.4 | [37] |
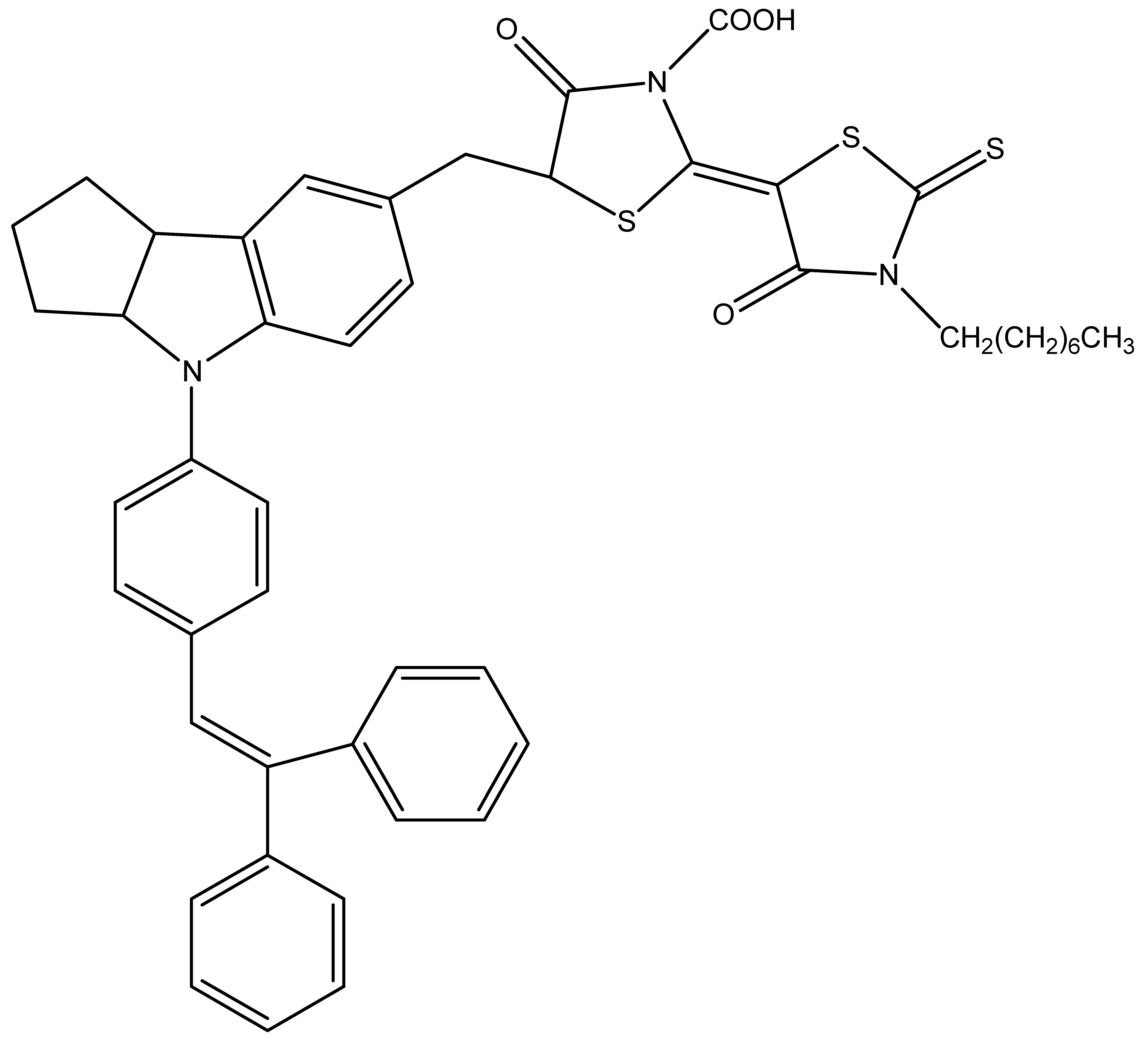 D205 | 1 M TEMPO in NaBF4 solution on Nafion | 4.5 | 0.69 | 0.64 | 2.1 | [58] |
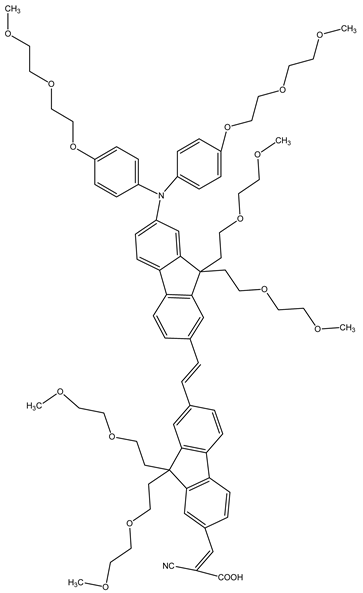 JK-259 | 2 M PMMI, 0.05 M I2, 0.1 M GuSCN, 0.5 M TBP, 1%TRITON X-100 | 2.28 | 0.66 | 0.79 | 1.16 | [59] |
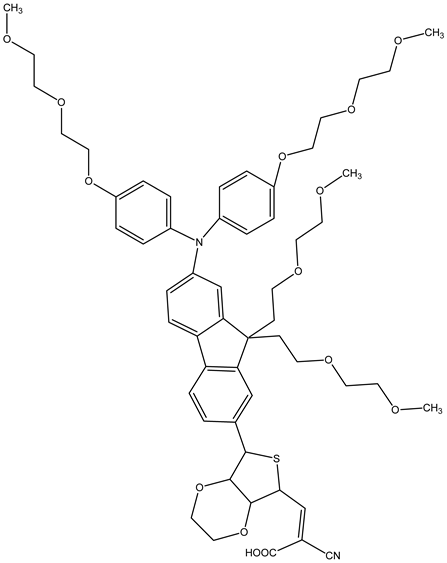 JK-262 | 3.78 | 0.64 | 0.82 | 2.1 | ||
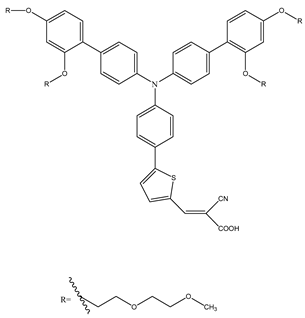 V35 | 2 M KI, 0.01 M I2 in an aqueous solution saturated CDCA on PEDOT | 6.85 | 0.65 | 0.67 | 3.01 | [60] |
 LEG4 | 0.15 M TEMPO, 0.05 M TEMPOBF4, LiClO4, 0.2 M NMBI | 5.78 | 0.95 | 0.75 | 4.14 | [61] |
 T169 | T−/DS | 13.3 | 0.54 | 0.62 | 4.50 | [63] |
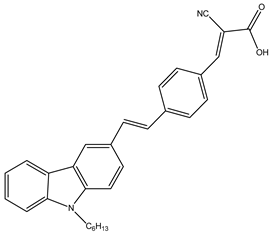 SK3 | 2 M LiI, 0.02 M I2, 1 M GuSCN | 6.2 | 0.43 | 0.44 | 1.27 | [39] |
 D131 | 5 M NaI, 0.01 M I2 in an aqueous solution saturated CDCA | 5.46 | 0.62 | 0.70 | 2.37 | [63,64] |
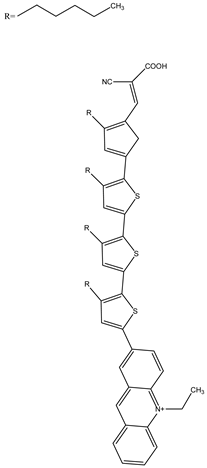 MK2 | 0.21 M [Co(bpy)3]Cl2, 0.07 M [Co(bpy)3]Cl3, 1.5 wt% of XG | 7.52 | 0.79 | 0.75 | 4.47 | [65] |
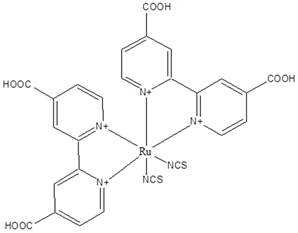 N3 | 0.5 M LiI, 0.025 M I2 on SnO2/TiO2 | 4 | 0.39 | 0.42 | 0.66 | [66] |
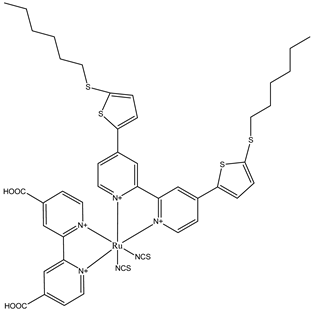 C106 | PMII (2.5 M), I2 (0.05 M), LiClO4 (0.1 M), and 0.5% Triton X-100 in water | 5.2 | 0.65 | 0.72 | 2.44 | [68] |
 N719 | [Cu (bnbip) (X)]1+/2+ redox mediator, Co-MOF additive, and aqueous geltrite polymer host | 6.51 | 0.72 | 0.73 | 3.42 | [69] |
3.2. Water DSSCs Based on Natural Dyes
- The choice of cations to be used in the electrolyte solution with iodide anions depends on their mobility, size, and local density accumulated [35];
4. Underwater-Based DSSCs
5. Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barichello, J.; Vesce, L.; Mariani, P.; Leonardi, E.; Braglia, R.; Di Carlo, A.; Canini, A.; Reale, A. Stable Semi-Transparent Dye-Sensitized Solar Modules and Panels for Greenhouse Application. Energies 2021, 14, 6393. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on electrolytes development in dye-sensitized solar cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, H.C.; Huang, F.; Cheng, Y.B. Fabrication of flexible dye sensitized solar cells on plastic substrates. Nano Energy 2013, 2, 174–189. [Google Scholar] [CrossRef]
- Contreras, A.R.; Pacheco, J.S.L.; Alvarado, J.; Pal, U.; Cab, J.V. Water-Induced Fine-Structure Disorder and Its Effect on the Performance of Photoelectrodes in Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5, 4817–4828. [Google Scholar]
- Wu, C.-G. Ruthenium-based complex dyes for dye-sensitized solar cells. J. Chin. Chem. Soc. 2022, 69, 1242–1252. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.-H.; So, J.-H.; Koo, H.-J. Toward Eco-Friendly Dye-Sensitized Solar Cells (DSSCs): Natural Dyes and Aqueous Electrolytes. Energies 2022, 15, 219. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3244–3294. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Muller, E.; Liska, P.; Vlachopoulos, N.; Gratzel, M. Conversion of Light to Electricity by Cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) Charge-Transfer Sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on Nanocrystalline Titanium Dioxide Electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Graetzel, M. Light-Induced Redox Reactions in Nanocrystalline Systems. Chem. Rev. 1995, 95, 49. [Google Scholar] [CrossRef]
- Graetzel, M. Sol-Gel Processed TiO2 Films for Photovoltaic Applications. J. SolGel Sci. Technol. 2001, 22, 7. [Google Scholar] [CrossRef]
- Argazzi, R.; Bignozzi, C.A.; Heimer, T.A.; Castellano, F.N.; Meyer, G.J. Enhanced spectral sensitivity from ruthenium (II) polypyridyl based photovoltaic devices. Inorg. Chem. 1994, 33, 5741. [Google Scholar] [CrossRef]
- Tennakone, K.; Kumara, G.R.R.A.; Kumarasinghe, A.R.; Wijayantha, K.G.U.; Sirimanne, P.M. A dye-sensitized nano-porous solid-state photovoltaic cell. Semicond. Sci. Technol. 1995, 10, 1689. [Google Scholar] [CrossRef]
- Tennakone, K.; Kumara, G.R.R.A.; Kumarasinghe, A.R.; Sirimanne, P.M.; Wijayantha, K.G.U. Efficient photosensitization of nanocrystalline TiO2 films by tannins and related phenolic substances. J. Photochem. Photobiol. A Chem. 1996, 94, 217. [Google Scholar] [CrossRef]
- Fang, J.H.; Mao, H.F.; Wu, J.W.; Zhang, X.Y.; Lu, Z.H. The photovoltaic study of co-sensitized microporous TiO2 electrode with porphyrin and phthalocyanine molecules. Appl. Surf. Sci. 1997, 119, 237. [Google Scholar] [CrossRef]
- Tennakone, K.; Jayaweera, P.V.V.; Bandaranayake, P.K.M. Dye-sensitized photoelectrochemical and solid-state solar cells: Charge separation, transport and recombination mechanisms. J. Photochem. Photobiol. A Chem. 2003, 158, 125–130. [Google Scholar] [CrossRef]
- Rosa-Clot, M.; Rosa-Clot, P.; Tina, G.M.; Scandura, P.F. Submerged photovoltaic solar panel: SP2. Renew. Energy 2010, 35, 1862–1865. [Google Scholar] [CrossRef]
- Adoram-Kershner, L.; Bruce, T.; Morris, C. Modeling and testing solar power for globally migrating submarine systems. In Proceedings of the OCEANS 2017—Anchorage, Anchorage, AK, USA, 18–21 September 2017; IEEE: New York, NY, USA, 2017; pp. 1–9. [Google Scholar]
- Mayville, P.; Patil, N.V.; Pearce, J.M. Distributed manufacturing of after market flexible floating photovoltaic modules. Sustain. Energy Technol. Assess. 2020, 42, 100830. [Google Scholar] [CrossRef]
- Mittal, D.; Saxena, B.K.; Rao, K.V. Potential of floating photovoltaic system for energy generation and reduction of water evaporation at four different lakes in Rajasthan. In Proceedings of the 2017 International Conference On Smart Technologies For Smart Nation (SmartTechCon), Bengaluru, India, 17–19 August 2017; IEEE: New York, NY, USA, 2018; pp. 238–243. [Google Scholar]
- Spencer, R.S.; Macknick, J.; Aznar, A.; Warren, A.; Reese, M.O. Floating photovoltaic systems: Assessing the technical potential of photovoltaic systems on man-made water bodies in the continental United States. Env. Sci. Technol. 2019, 53, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Calnan, S.; Bagacki, R.; Bao, F. Development of various photovoltaic-driven water electrolysis technologies for green solar hydrogen generation. Sol. RRL 2021, 6, 2100479. [Google Scholar] [CrossRef]
- Hayibo, K.S.; Mayville, P.; Kailey, R.K.; Pearce, J.M. Water conservation potential of self-funded foam-based flexible surface-mounted floatovoltaics. Energies 2020, 13, 6285. [Google Scholar] [CrossRef]
- Stachiw, J.D. Performance of photovoltaic cells in an undersea environment. J. Eng. Ind. 1980, 102, 51–59. [Google Scholar] [CrossRef]
- Bella, F.; Porcarelli, L.; Mantione, D.; Gerbaldi, C.; Barolo, C.; Grätzel, M.; Mecerreyes, D. Water-based and metal-free dye solar cell exceeding 7% efficiency using a cationic poly(3,4-ethylenedioxythiophene) derivative. Chem. Sci. 2020, 11, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic acid preadsorption raises efficiency of cosensitized solar cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Khushboo, S.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar]
- Delices, A.; Zhang, J.; Santoni, M.P.; Dong, C.Z.; Maurel, F.; Bellynck, S.; Chevillot, A.; Vlachopoulos, N.; Hagfeldt, A.; Jouini, M. Experimental and theoretical study of organic sensitizers for solid-state dye sensitized solar cells. J. Photochem. Photobiol. A Chem. 2022, 428, 113890. [Google Scholar] [CrossRef]
- Barichello, J.; Spadaro, D.; Gullace, S.; Sinopoli, A.; Calandra, P.; Irrera, A.; Matteocci, F.; Calogero, G.; Caramori, S.; Bignozzi, C.A. Optically Transparent Gold Nanoparticles for DSSC Counter-Electrode: An Electrochemical Characterization. Molecules 2022, 27, 4178. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Guerin, V.M.; Tiginyanu, I.M. Well-aligned arrays of vertically oriented ZnOnanowires electrodeposited on ITO-coated glass and their integration in dye sensitized solar cells. J. Photochem. Photobiol. A Chem. 2010, 211, 65–73. [Google Scholar] [CrossRef]
- Han, D.-W.; Heo, J.-H.; Kwak, D.-J.; Han, C.-H.; Sung, Y.-M. Texture, morphology and photovoltaic characteristics of nanoporous F:SnO2 films. J. Electr. Eng. Technol. 2009, 4, 93–97. [Google Scholar] [CrossRef]
- Wang, Z.S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G.; Cazzanti, S. Efficient dyesensitized solar cells using red turnip and purple wild Sicilian prickly pear fruits. Int. J. Mol. Sci. 2010, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Gerrit, B.; Anders, H. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar]
- Cassone, G.; Calogero, G.; Sponer, J.; Saija, F. Mobilities of iodide anions in aqueous solutions for applications in natural dye-sensitized solar cell. Phys. Chem. Chem. Phys. 2018, 20, 13038. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Galliano, S.; Falco, M.; Viscardi, G.; Barolo, C.; Graetzel, M.; Gerbaldi, C. Unveiling iodine-based electrolytes chemistry in aqueous dye-sensitized solar cells. Chem. Sci. 2016, 7, 4880. [Google Scholar] [CrossRef]
- Law, C.H.; Pathirana, S.C.; Li, X.; Anderson, A.Y.; Barnes, P.R.F.; Listorti, A.; Ghaddar, T.H.; O’Regan, B.C. Water-Based Electrolytes for Dye-Sensitized Solar Cells. Adv. Mater. 2010, 22, 4505–4509. [Google Scholar] [CrossRef]
- Zhu, K.; Jang, S.-R.; Frank, A.J. Effects of water intrusion on the charge-carrier dynamics, performance, and stability of dye sensitized solar cells. Energy Environ. Sci. 2012, 5, 9492. [Google Scholar] [CrossRef]
- Keval, K.; Sonigara, J.V.; Machhi, V.; Hiren, K.; Prasad, J.; Gibaud, A.; Soni, S.S. Anisotropic One-Dimensional Aqueous Polymer Gel Electrolyte for Photoelectrochemical Devices: Improvement in Hydrophobic TiO2−Dye/Electrolyte Interface. ACS Appl. Energy Mater. 2018, 1, 3665–3673. [Google Scholar]
- Park, S.J.; Yoo, K.; Kim, J.-Y.; Kim, J.Y.; Lee, D.-K.; Kim, B.S.; Kim, H.; Kim, J.H.; Cho, J.; Ko, M.J. Water-Based Thixotropic Polymer Gel Electrolyte for Dye-Sensitized Solar Cells. ACS Nano 2013, 7, 4050–4056. [Google Scholar] [CrossRef]
- Dai, Q.; Rabani, J. Photosensitization of nanocrystalline TiO2 films by pomegranate pigments with unusually high efficiency in aqueous medium. Chem. Commun. 2001, 20, 2142–2143. [Google Scholar] [CrossRef]
- Dai, Q.; Rabani, J. Photosensitization of nanocrystalline TiO2 films by anthocyanin dyes. J. Photochem. Photobiol. A Chem. 2002, 148, 17–24. [Google Scholar] [CrossRef]
- Hui, Z.; Xiong, Y.; Heng, L.; Yuan, L.; Yu-Xiang, W. Explanation of Effect of Added Water on Dye-Sensitized Nanocrystalline TiO2 Solar Cell: Correlation between Performance and Carrier Relaxation Kinetics. Chin. Phys. Lett. 2007, 24, 3272–3275. [Google Scholar] [CrossRef]
- Galliano, S.; Bella, F.; Gerbaldi, C.; Falco, M.; Viscardi, G.; Graetzel, M.; Barolo, C. Photoanode/Electrolyte Interface Stability in Aqueous Dye-Sensitized Solar Cells. Energy Technol. 2017, 5, 300–311. [Google Scholar] [CrossRef]
- Liu, Y.; Hagfeldt, A.; Xiao, X.-R.; Lindquist, S.E. Investigation of influence of redox species on the interfacial energetics of a Dye-Sensitized nanoporous TiO2 solar cell. Sol. Energy Mater. Sol. Cells 1998, 55, 267–281. [Google Scholar] [CrossRef]
- Glinka, A.; Gierszewski, M.; Ziółek, M. Effects of Aqueous Electrolyte, Active Layer Thickness and Bias Irradiation on Charge Transfer Rates in Solar Cells Sensitized with Top Efficient Carbazole Dyes. J. Phys. Chem. C 2018, 122, 8147–8158. [Google Scholar] [CrossRef]
- Jung, Y.S.; Yoo, B.; Lim, M.K.; Lee, S.Y.; Kim, K.J. Effect of Triton X-100 in water-added electrolytes on the performance of dye-sensitized solar cells. Electrochim. Acta 2009, 54, 6286–6291. [Google Scholar] [CrossRef]
- Bella, F.; Gerbaldi, C.; Barolo, C.; Graetzel, M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3431. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, L.; Xu, D.; Zhang, W.; Yan, F. Performance enhancement for water-based dye-sensitized solar cells via addition of ionic surfactants. J. Mater. Chem. A 2014, 2, 2221–2226. [Google Scholar] [CrossRef]
- Law, C.H.; Moudam, O.; Villarroya-Lidon, S.; O’Regan, B. Managing wetting behavior and collection efficiency in photoelectrochemical devices based on water electrolytes; improvement in efficiency of water/iodide dye sensitised cells to 4%. J. Mater. Chem. 2012, 22, 23387. [Google Scholar] [CrossRef]
- Fagiolari, L.; Bonomo, M.; Cognetti, A.; Meligrana, G.; Gerbaldi, C.; Barolo, C.; Bella, F. Photoanodes for Aqueous Solar Cells: Exploring Additives and Formulations Starting from a Commercial TiO2 Paste. ChemSusChem 2020, 13, 6562–6573. [Google Scholar] [CrossRef]
- Dong, C.; Xiang, W.; Huang, F.; Fu, D.; Huang, W.; Bach, U.; Cheng, Y.B.; Li, X.; Spiccia, L. Controlling interfacial recombination in aqueous dye-sensitized solar cells by octadecyltrichlorosilane surface treatment, Angew. Chem. Int. Ed. 2014, 53, 6933–6937. [Google Scholar] [CrossRef]
- Su, Y.H.; Lai, W.H.; Teoh, L.G.; Hon, M.H.; Huang, J.L. Layer-by-layer Au nanoparticles as a Schottky barrier in a water-based dye-sensitized solar cell. Appl. Phys. A Mater. Sci. 2007, 88, 173–178. [Google Scholar] [CrossRef]
- Lai, W.H.; Su, L.H.; Teoh, L.G.; Hon, M.H. Commercial and natural dyes as photosensitizers for a water-based dye-sensitized solar cell loaded with gold nanoparticles. J. Photochem. Photobiol. A 2008, 195, 307–313. [Google Scholar] [CrossRef]
- Daeneke, T.; Uemura, Y.; Duffy, N.W.; Mozer, A.J.; Koumura, N.; Bach, U.; Spiccia, L. Aqueous dye-sensitized solar cell electrolytes based on the ferricyanide-ferrocyanide redox couple. Adv. Mater. 2012, 24, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Huang, F.; Cheng, Y.B.; Bach, U.; Spiccia, L. Aqueous dye-sensitized solar cell electrolytes based on the cobalt(II)/(III) tris(bipyridine) redox couple. Energy Environ. Sci. 2013, 6, 121–127. [Google Scholar] [CrossRef]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent advances in eco-friendly and cost-effective materials towards sustainable dye-sensitized solar cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Kato, R.; Kato, F.; Oyaizu, K.; Nishide, H. Redox-active hydroxy-TEMPO radical immobilized in Nafion layer for an aqueous electrolyte-based and dye-sensitized solar cell. Chem. Lett. 2014, 43, 480–482. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, B.-S.; Do, K.; Ju, M.J.; Song, K.; Ko, J. Aqueous electrolyte based dye-sensitized solar cells using organic sensitizers. New J. Chem. 2013, 37, 329–336. [Google Scholar] [CrossRef]
- Leandri, V.; Ellis, H.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. An organic hydrophilic dye for water-based dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2014, 16, 19964–19971. [Google Scholar] [CrossRef]
- Yang, W.; Söderberg, M.; Eriksson, A.I.K.; Boschloo, G. Efficient aqueous dye-sensitized solar cell electrolytes based on a TEMPO/TEMPO+ redox couple. RSC Adv. 2015, 5, 26706. [Google Scholar] [CrossRef]
- Fayad, R.; Shoker, T.A.; Ghaddar, T.H. High photo-currents with a zwitterionic thiocyanate-free dye in aqueous-based dye sensitized solar cells. Dalton Trans. 2016, 45, 5622–5628. [Google Scholar] [CrossRef]
- Bella, F.; Galliano, S.; Falco, M.; Viscardi, G.; Barolo, C.; Grätzel, M.; Gerbaldi, C. Approaching truly sustainable solar cells by the use of water and cellulose derivatives. Green. Chem. 2017, 19, 1043. [Google Scholar] [CrossRef]
- Bella, F.; Galliano, S.; Piana, G.; Giacona, G.; Viscardi, G.; Grätzel, M.; Barolo, C.; Gerbaldi, C. Boosting the efficiency of aqueous solar cells: A photoelectrochemical estimation on the effectiveness of TiCl4 treatment. Electrochim. Acta 2019, 302, 31–37. [Google Scholar] [CrossRef]
- Galliano, S.; Bella, F.; Bonomo, M.; Giordano, F.; Grätzel, M.; Viscardi, G.; Hagfeldt, A.; Gerbaldi, C.; Barolo, C. Xanthan-Based Hydrogel for Stable and Efficient Quasi-Solid Truly Aqueous Dye-Sensitized Solar Cell with Cobalt Mediator. Sol. RRL 2021, 5, 2000823. [Google Scholar] [CrossRef]
- Pham, B.; Willinger, D.; McMillan, N.K.; Roye, J.; Burnett, W.; D’Achille, A.; Coffer, J.L.; Sherman, B.D. Tin (IV) oxide nanoparticulate films for aqueous dye-sensitized solar cells. Sol. Energy 2021, 224, 984–991. [Google Scholar] [CrossRef]
- Ellis, H.; Jiang, R.; Ye, S.; Hagfeldt, A.; Boschloo, G. Development of high efficiency 100% aqueous cobalt electrolyte dye-sensitised solar cells. Phys. Chem. Chem. Phys. 2016, 18, 8419. [Google Scholar] [CrossRef]
- Jilakian, M.; Ghaddar, T.H. Eco-Friendly Aqueous Dye-Sensitized Solar Cell with a Copper(I/II) Electrolyte System: Efficient Performance under Ambient Light Conditions, ACS Appl. Energy Mater. 2022, 5, 257–265. [Google Scholar] [CrossRef]
- Selvaraj, B.; Shanmugam, G.; Kamaraj, S.; Thirugnanasambandam, E.; Gunasekeran, A.; Sambandam, A. Effect of an aqueous copper gel electrolyte with cobalt metal organic framework based additive on performance of aqueous-dye-sensitized solar cells. Sol. Energy 2022, 236, 586–598. [Google Scholar] [CrossRef]
- Olea, A.; Ponce, G.; Sebastian, P.J. Electron transfer via organic dyes for solar conversion. Sol. Energy Mater. Sol. Cells 1999, 59, 137–143. [Google Scholar] [CrossRef]
- Calogero, G.; Barichello, J.; Citro, I.; Mariani, P.; Vesce, L.; Bartolotta, A.; Di Carlo, A.; Di Marco, G. Photoelectrochemical and spectrophotometric studies on dye-sensitized solar cells (DSCs) and stable modules (DSCMs) based on natural apocarotenoids pigments. Dye. Pigment. 2018, 155, 75–83. [Google Scholar] [CrossRef]
- Dai, Q.; Rabani, J. Unusually efficient photosensitization of nanocrystalline TiO2 films by pomegranate pigments in aqueous medium. New J. Chem. 2002, 26, 421–426. [Google Scholar] [CrossRef]
- Chang, H.; Kao, M.-J.; Chen, T.-L.; Chen, C.-H.; Cho, K.-C.; Lai, X.-R. Characterization of natural dye extracted from wormwood and purple cabbage for dye-sensitized solar cells. Int. J. Photoenergy 2013, 2013, 159502. [Google Scholar] [CrossRef]
- Röhr, J.A.; Lipton, J.; Kong, J.; Maclean, S.A.; Taylor, A.D. Efficiency Limits of Underwater Solar Cells. Joule 2020, 4, 840–849. [Google Scholar] [CrossRef]
- Jenkins, P.P.; Messenger, S.; Trautz, K.M.; Maximenko, S.I.; Goldstein, D.; Scheiman, D.; Hoheisel, R.; Walters, R.J. High-Bandgap Solar Cells for Underwater Photovoltaic Applications. IEEE J. Photovolt. 2014, 4, 202–207. [Google Scholar] [CrossRef]
- Liu, C.; Dong, H.; Zhang, Z.; Chai, W.; Li, L.; Chen, D.; Zhu, W.; Xi, H.; Zhang, J.; Zhang, C.; et al. Promising applications of wide bandgap inorganic perovskites in underwater photovoltaic cells. Sol. Energy 2022, 233, 489–493. [Google Scholar] [CrossRef]
- Enaganti, P.K.; Soman, S.; Devan, S.S.; Pradhan, S.C.; Srivastava, A.K.; Pearce, J.M.; Goel, S. Dye-sensitized solar cells as promising candidates for underwater photovoltaic applications. Prog. Photovolt. Res. Appl. 2022, 30, 632–639. [Google Scholar] [CrossRef]
- Enaganti, P.K.; Dwivedi, P.K.; Sudha, R.; Srivastava, A.K.; Goel, S. Underwater Characterization and Monitoring of Amorphous and Monocrystalline Solar Cells in Diverse Water Settings. IEEE Sens. J. 2020, 20, 2730–2737. [Google Scholar] [CrossRef]
- Enaganti, P.K.; Dwivedi, P.K.; Srivastava, A.K.; Goel, S. Study of solar irradiance and performance analysis of submerged monocrystalline and polycrystalline solar cells. Prog. Photovolt. Res. Appl. 2020, 28, 725–735. [Google Scholar] [CrossRef]
- Li, W.; Pu, Y.; Baosheng, G.; Wang, Y.; Yu, D.; Song, Q. Dye-sensitized solar cells based on natural and artificial phycobiliproteins to capture low light underwater. Int. J. Hydrogen Energy 2019, 44, 1182–1191. [Google Scholar] [CrossRef]
| Dye | Electrolyte | Jsc (mA/cm2) | Voc (V) | FF | ƞ (%) |
|---|---|---|---|---|---|
Z907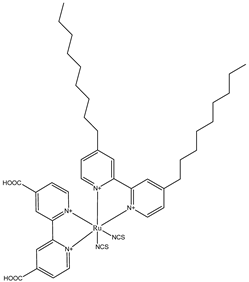 | 0.6 M MBII and 0.03 M I2 in acetonitrile (ACN)/ Valeronitrile (VN) (85:15 v/v) with deionized water 0 vol.% | 10.30 | 0.760 | 0.524 | 4.09 |
| with deionized water 2 vol.% | 11.80 | 0.751 | 0.506 | 4.46 | |
| with deionized water 5 vol.% | 12.59 | 0.717 | 0.514 | 4.76 | |
| with deionized water 10 vol.% | 9.74 | 0.720 | 0.494 | 3.65 | |
| with deionized water 20 vol.% | 4.17 | 0.678 | 0.467 | 1.32 | |
| with deionized water 40 vol.% | 1.49 | 0.571 | 0.499 | 0.45 |
| Dye | Electrolyte | Jsc (mA/cm2) | Voc (V) | FF | ƞ (%) | Ref |
|---|---|---|---|---|---|---|
| cyanidin-3-glucoside | NaI 2.5 M/I2 0.1 M | 2.2 | 0.44 | [41] | ||
| Delphinidin chloride | CsI 2.5 M/I2 0.05 M | 3.9 | 0.38 | [42] | ||
| Bongainvillea brasiliensis Raeusch. | 0.1 MCe(NO3)3/0.05 M Ce(NO3)4 | 5 | 0.249 | 0.364 | 0.45 | [54] |
| Garcinia suubelliptica. | 6.48 | 0.322 | 0.331 | 0.69 | [54] | |
| Ficus Reusa Linn. | 7.85 | 0.520 | 0.289 | 1.18 | [54] | |
| Rhoeo spathacea (Sw.) Stearn | 10.9 | 0.496 | 0.274 | 1.49 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadaro, D.; Barichello, J.; Citro, I.; Calogero, G. Environmentally Friendly Water-Based Electrolyte for Dye-Sensitized Solar Cells: Future Prospective and Outlook. Solar 2023, 3, 229-252. https://doi.org/10.3390/solar3020015
Spadaro D, Barichello J, Citro I, Calogero G. Environmentally Friendly Water-Based Electrolyte for Dye-Sensitized Solar Cells: Future Prospective and Outlook. Solar. 2023; 3(2):229-252. https://doi.org/10.3390/solar3020015
Chicago/Turabian StyleSpadaro, Donatella, Jessica Barichello, Ilaria Citro, and Giuseppe Calogero. 2023. "Environmentally Friendly Water-Based Electrolyte for Dye-Sensitized Solar Cells: Future Prospective and Outlook" Solar 3, no. 2: 229-252. https://doi.org/10.3390/solar3020015
APA StyleSpadaro, D., Barichello, J., Citro, I., & Calogero, G. (2023). Environmentally Friendly Water-Based Electrolyte for Dye-Sensitized Solar Cells: Future Prospective and Outlook. Solar, 3(2), 229-252. https://doi.org/10.3390/solar3020015








