Gastrointestinal Digestion and Absorption of Antioxidant Phenolic Compounds and Caffeine from the Coffee Pulp under Simulated Conditions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Simulated Digestion
2.3. Colorimetric Analysis of Total Phenolic Compounds and Antioxidant Capacity
2.3.1. Total Phenolic Content
2.3.2. In Vitro Antioxidant Capacity
2.4. HPLC–DAD–ESI/MSn Qualitative and Quantitative Analyses of Bioactive Compounds
2.5. Bioaccessibility of the Bioactive Compounds in the Coffee Pulp Flour
2.6. In Silico Potential Bioavailability Estimation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Main Bioactive Compounds in the Coffee Pulp Flour Were Caffeine and Chlorogenic Acids
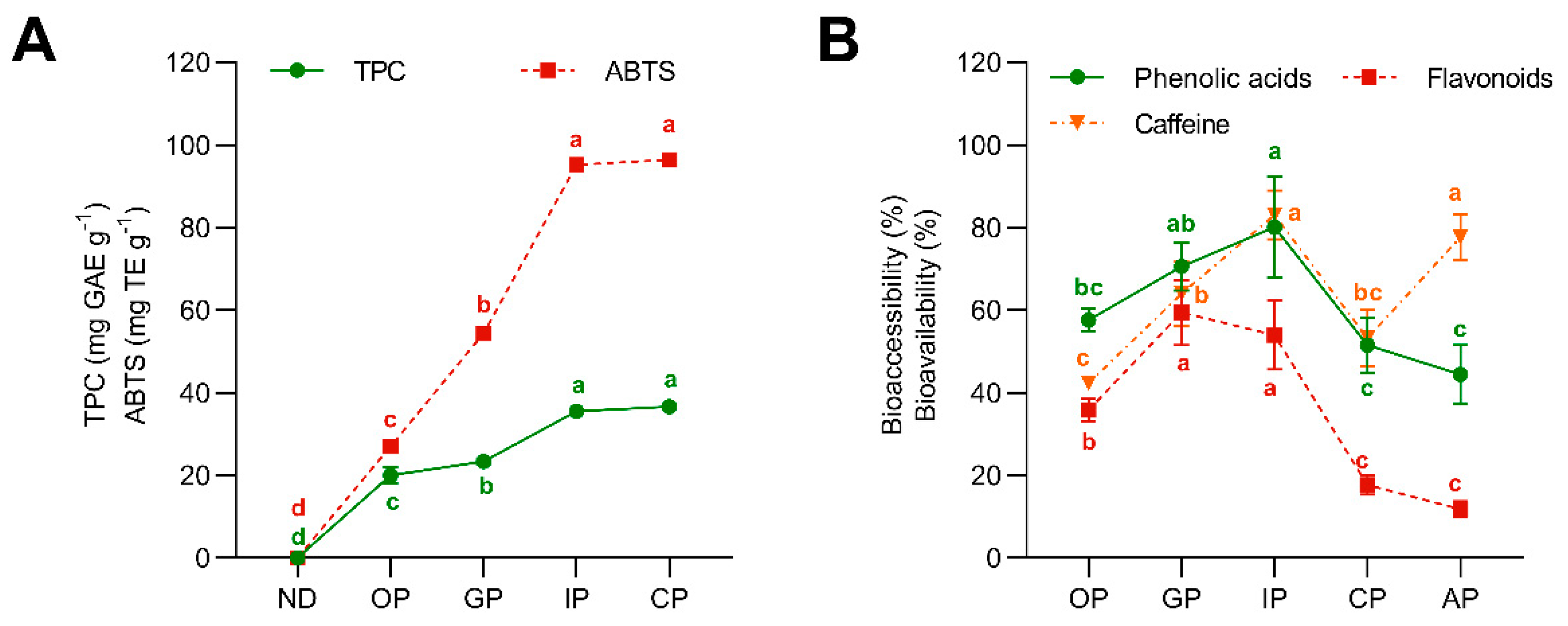
3.2. Gastrointestinal Digestion Reduced Phenolic Compounds and Caffeine Bioaccessibility
3.3. Phenolic Compounds Exhibited a Lower Potential Bioavailability than Caffeine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.A.; Vinha, A.F.; Oliveira, M.B.P.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing By-Products: Sustainable Applications; Galanakis, C., Ed.; Academic Press-Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–26. ISBN 978-0-12-811290-8. [Google Scholar]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Dos Santos, É.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Moreno, J.; Cozzano, S.; Mercedes Pérez, A.; Arcia, P.; Curutchet, A. Coffee Pulp Waste as a Functional Ingredient: Effect on Salty Cookies Quality. J. Food Nutr. Res. 2019, 7, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Delgado, S.R.; Arbelaez, A.F.A.; Rojano, B. Antioxidant capacity, bioactive compounds in coffee pulp and implementation in the production of infusions. Acta Sci. Pol. Technol. Aliment. 2019, 18, 235–248. [Google Scholar]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Benítez, V.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Revalorization of coffee husk: Modeling and optimizing the green sustainable extraction of phenolic compounds. Foods 2021, 10, 653. [Google Scholar] [CrossRef]
- Benítez, V.; Rebollo-Hernanz, M.; Aguilera, Y.; Bejerano, S.; Cañas, S.; Martín-Cabrejas, M.A. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food Funct. 2021, 12, 1097. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Activating Effects of the Bioactive Compounds From Coffee By-Products on FGF21 Signaling Modulate Hepatic Mitochondrial Bioenergetics and Energy Metabolism in vitro. Front. Nutr. 2022, 9, 866233. [Google Scholar] [CrossRef]
- Wu, H.; Gu, J.; Bk, A.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of processing on bioavailability and bioaccessibility of bioactive compounds in coffee beans. Food Biosci. 2021, 46, 101373. [Google Scholar] [CrossRef]
- Bohn, T.; Mcdougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites-a position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of melatonin from lentil sprouts and its role in the plasmatic antioxidant status in rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebollo-Hernanz, M.; Fernández-Gómez, B.; Herrero, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Uribarri, J.; Del Castillo, M.D. Inhibition of the Maillard reaction by phytochemicals composing an aqueous coffee silverskin extract via a mixed mechanism of action. Foods 2019, 8, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Olmo-García, L.; Figueiredo-González, M.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B. Application of the INFOGEST Standardized Method to Assess the Digestive Stability and Bioaccessibility of Phenolic Compounds from Galician Extra-Virgin Olive Oil. J. Agric. Food Chem. 2021, 69, 11592–11605. [Google Scholar] [CrossRef]
- Zapata, F.J.; Rebollo-Hernanz, M.; Novakofski, J.E.; Nakamura, M.T.; Gonzalez de Mejia, E. Caffeine, but not other phytochemicals, in mate tea (Ilex paraguariensis St. Hilaire) attenuates high-fat-high-sucrose-diet-driven lipogenesis and body fat accumulation. J. Funct. Foods 2020, 64, 103646. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Valverde, A.; Madrid, Y. A combined analytical-chemometric approach for the in vitro determination of polyphenol bioaccessibility by simulated gastrointestinal digestion. Anal. Bioanal. Chem. 2022, 414, 2739–2755. [Google Scholar] [CrossRef] [PubMed]
- Cañas, S.; Rebollo-Hernanz, M.; Cano-Muñoz, P.; Aguilera, Y.; Benítez, V.; Braojos, C.; Gila-Díaz, A.; Rodríguez-Rodríguez, P.; Monedero-Cobeta, I.; de Pablo, A.L.L.; et al. Critical Evaluation of Coffee Pulp as an Innovative Antioxidant Dietary Fiber Ingredient: Nutritional Value, Functional Properties and Acute and Sub-Chronic Toxicity. Proceedings 2021, 70, 65. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañas, S.; Rebollo-Hernanz, M.; Aguilera, Y.; Braojos, C.; Benítez, V.; Gil-Ramírez, A.; Dueñas, M.; Arribas, S.M.; Martín-Cabrejas, M.A. Gastrointestinal Digestion and Absorption of Antioxidant Phenolic Compounds and Caffeine from the Coffee Pulp under Simulated Conditions. Biol. Life Sci. Forum 2022, 12, 1. https://doi.org/10.3390/IECN2022-12395
Cañas S, Rebollo-Hernanz M, Aguilera Y, Braojos C, Benítez V, Gil-Ramírez A, Dueñas M, Arribas SM, Martín-Cabrejas MA. Gastrointestinal Digestion and Absorption of Antioxidant Phenolic Compounds and Caffeine from the Coffee Pulp under Simulated Conditions. Biology and Life Sciences Forum. 2022; 12(1):1. https://doi.org/10.3390/IECN2022-12395
Chicago/Turabian StyleCañas, Silvia, Miguel Rebollo-Hernanz, Yolanda Aguilera, Cheyenne Braojos, Vanesa Benítez, Alicia Gil-Ramírez, Montserrat Dueñas, Silvia M. Arribas, and María A. Martín-Cabrejas. 2022. "Gastrointestinal Digestion and Absorption of Antioxidant Phenolic Compounds and Caffeine from the Coffee Pulp under Simulated Conditions" Biology and Life Sciences Forum 12, no. 1: 1. https://doi.org/10.3390/IECN2022-12395
APA StyleCañas, S., Rebollo-Hernanz, M., Aguilera, Y., Braojos, C., Benítez, V., Gil-Ramírez, A., Dueñas, M., Arribas, S. M., & Martín-Cabrejas, M. A. (2022). Gastrointestinal Digestion and Absorption of Antioxidant Phenolic Compounds and Caffeine from the Coffee Pulp under Simulated Conditions. Biology and Life Sciences Forum, 12(1), 1. https://doi.org/10.3390/IECN2022-12395











