Effect of Acid-Extrusion Cooking on Some Properties of Quinoa Starch †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Quinoa Starch
2.2. Acid Extrusion of Starch
2.3. Starch, Resistant Starch and Glucose Content Determination
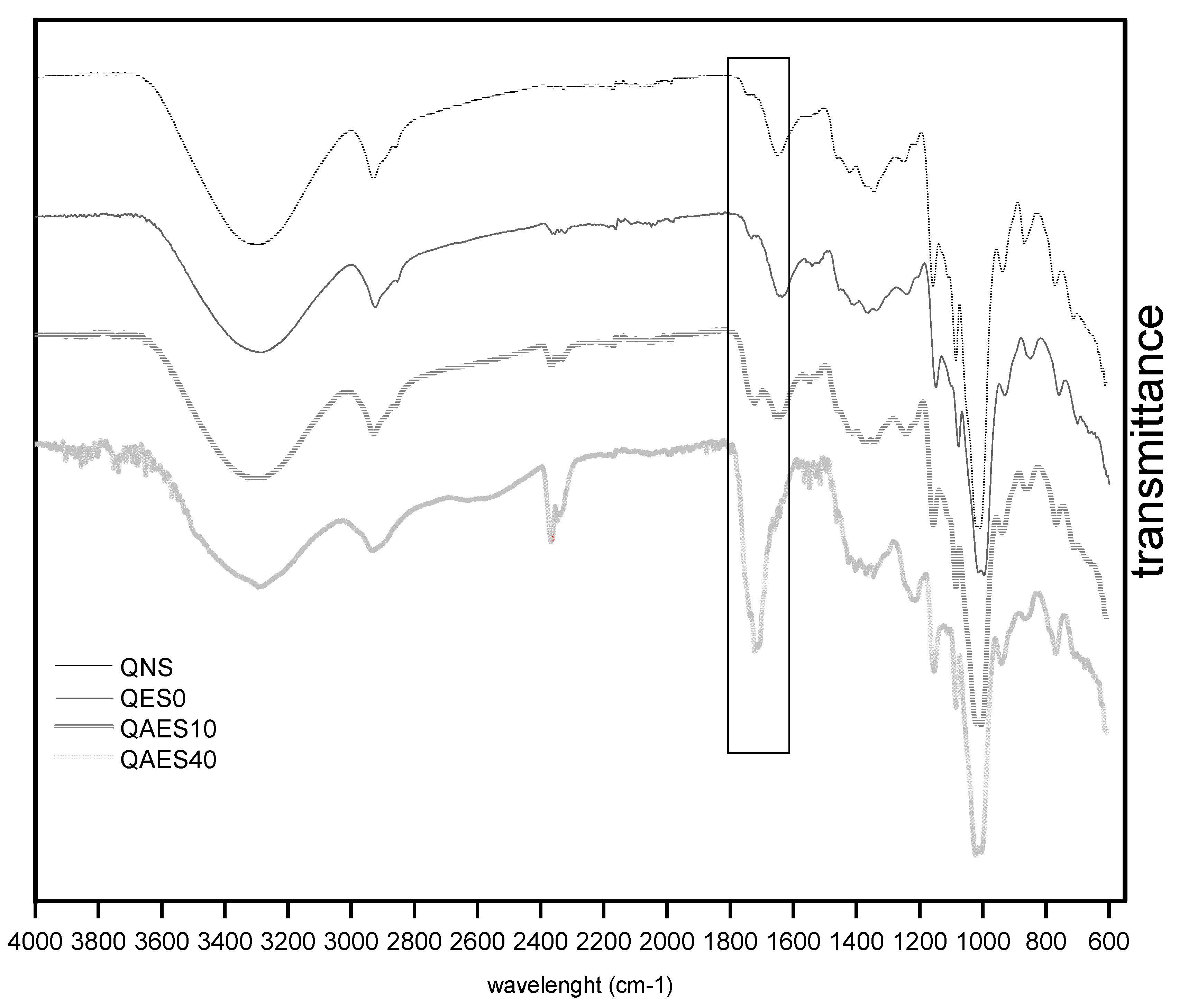
2.4. FTIR Spectrum of Starch Samples
2.5. Particle Size Distribution of Milled Starch
2.6. Optical Microphotography Characterization
3. Results and Discussion
3.1. Changes in the Resistant Starch Content and Molecular Characterization
3.2. Effect of Acid Extrusion on Particle Starch Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Contreras-jiménez, B.; Torres-vargas, O.L.; Rodríguez-garcía, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef] [PubMed]
- Butt, N.A.; Ali, T.M.; Moin, A.; Hasnain, A. Comparative study on morphological, rheological and functional characteristics of extruded rice starch citrates and lactates. Int. J. Biol. Macromol. 2021, 180, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Dunrar, A.N.; Gocmen, D. Effects of autoclaving temperature and storing time on resistant starch formation and its functional and physicochemical properties. Carbohydr. Polym. 2013, 97, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, D.J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Masatcioglu, T.M.; Sumer, Z.; Koksel, H. An innovative approach for signi fi cantly increasing enzyme resistant starch type 3 content in high amylose starches by using extrusion cooking. J. Cereal Sci. 2017, 74, 95–102. [Google Scholar] [CrossRef]
- Neder-Suárez, D.; Amaya-Guerra, C.A.; Pérez-Carrillo, E.; Quintero-Ramos, A.; Mendez-Zamora, G.; Sánchez-Madrigal, M.Á.; Lardizábal-Gutiérrez, D. Optimization of an extrusion cooking process to increase formation of resistant starch from corn starch with addition of citric acid. Starch-Stärke 2020, 72, 1900150. [Google Scholar] [CrossRef]
- Rueda, J.; Lobo, M.O.; Sammán, N. Changes in the Antioxidant Activity of Peptides Released during the Hydrolysis of Quinoa (Chenopodium quinoa willd) Protein Concentrate. Proceedings 2020, 53, 12. [Google Scholar] [CrossRef]
- Hasjim, J.; Jane, J.L. Production of resistant starch by extrusion cooking of acid-modified normal-maize starch. J. Food Sci. 2009, 74, C556–C562. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.N.; Panesar, P.S.; Singh, S. Process standardization for isolation of quinoa starch and its characterization in comparison with other starches. J. Food Meas. Charact. 2017, 11, 1919–1927. [Google Scholar] [CrossRef]

| Parameter | QNS | QES0 | QAES10 | QAES40 |
|---|---|---|---|---|
| Resistant starch (g/100 g starch) | 0.63 b ± 0.08 | 0.56 b ± 0.07 | 0.20 c ± 0.04 | 1.10 a ± 0.09 |
| Free glucose (mg/100 g sample) | 801.36 ab ± 176.73 | 1064.37 a ± 50.15 | 904.62 a ± 88.31 | 368.56 c ± 6.76 |
| Parameter | Unit | Treatments | |||
|---|---|---|---|---|---|
| QNS | QES0 | QAES10 | QAES40 | ||
| D10 | µm | 26.81 d ± 19.12 | 90.67 bc ± 2.72 | 71.09 c ± 2.73 | 71.83 c ± 1.67 |
| D50 | µm | 319.45 bc ± 53.48 | 305.75 bcd ± 4.87 | 284.99 bcd ± 7.94 | 262.80 d ± 5.18 |
| D90 | µm | 560.72 c ± 4.04 | 567.63 c ± 5.47 | 558.56 c ± 6.10 | 517.84 d ± 6.05 |
| D[3.2] | µm | 45.99 d ± 18.79 | 145.53 c ± 10.91 | 132.78 c ± 5.17 | 136.071 c ± 2.54 |
| D[4.3] | µm | 295.8643 c ± 7.25 | 321.4907 c ± 4.09 | 303.55 c ± 6.39 | 282.3167 cd ± 4.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda, J.; Lobo, M.O.; Sammán, N.; Haros, C.M. Effect of Acid-Extrusion Cooking on Some Properties of Quinoa Starch. Biol. Life Sci. Forum 2022, 17, 8. https://doi.org/10.3390/blsf2022017008
Rueda J, Lobo MO, Sammán N, Haros CM. Effect of Acid-Extrusion Cooking on Some Properties of Quinoa Starch. Biology and Life Sciences Forum. 2022; 17(1):8. https://doi.org/10.3390/blsf2022017008
Chicago/Turabian StyleRueda, Julio, Manuel Oscar Lobo, Norma Sammán, and Claudia Monika Haros. 2022. "Effect of Acid-Extrusion Cooking on Some Properties of Quinoa Starch" Biology and Life Sciences Forum 17, no. 1: 8. https://doi.org/10.3390/blsf2022017008
APA StyleRueda, J., Lobo, M. O., Sammán, N., & Haros, C. M. (2022). Effect of Acid-Extrusion Cooking on Some Properties of Quinoa Starch. Biology and Life Sciences Forum, 17(1), 8. https://doi.org/10.3390/blsf2022017008






