Evaluation of Agro-Industrial Carbon and Energy Sources for Lactobacillus plantarum M8 Growth †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Activation of the Lactobacillus plantarum Strain on an Erlenmeyer Scale
2.2. Analysis of the Growth Kinetics of Lactobacillus plantarum M8 in Different Culture Conditions
2.3. Diluted Molasses Preparation
2.4. Whey Pretreatment
2.5. Activation of the Lactobacillus plantarum M8
2.6. Lactobacillus plantarum M8 Growth Kinetics under Different Carbon Sources
3. Results and Discussion
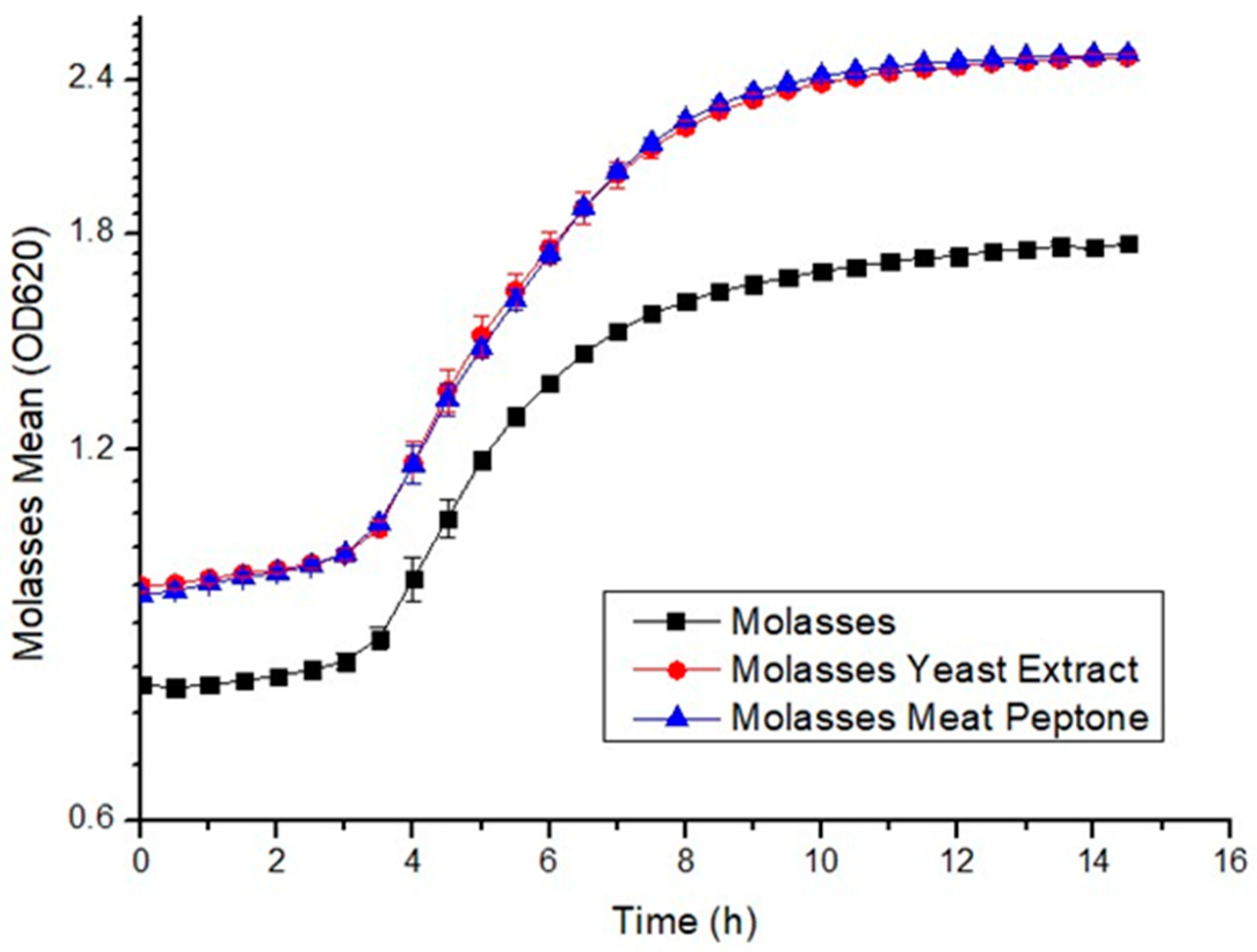
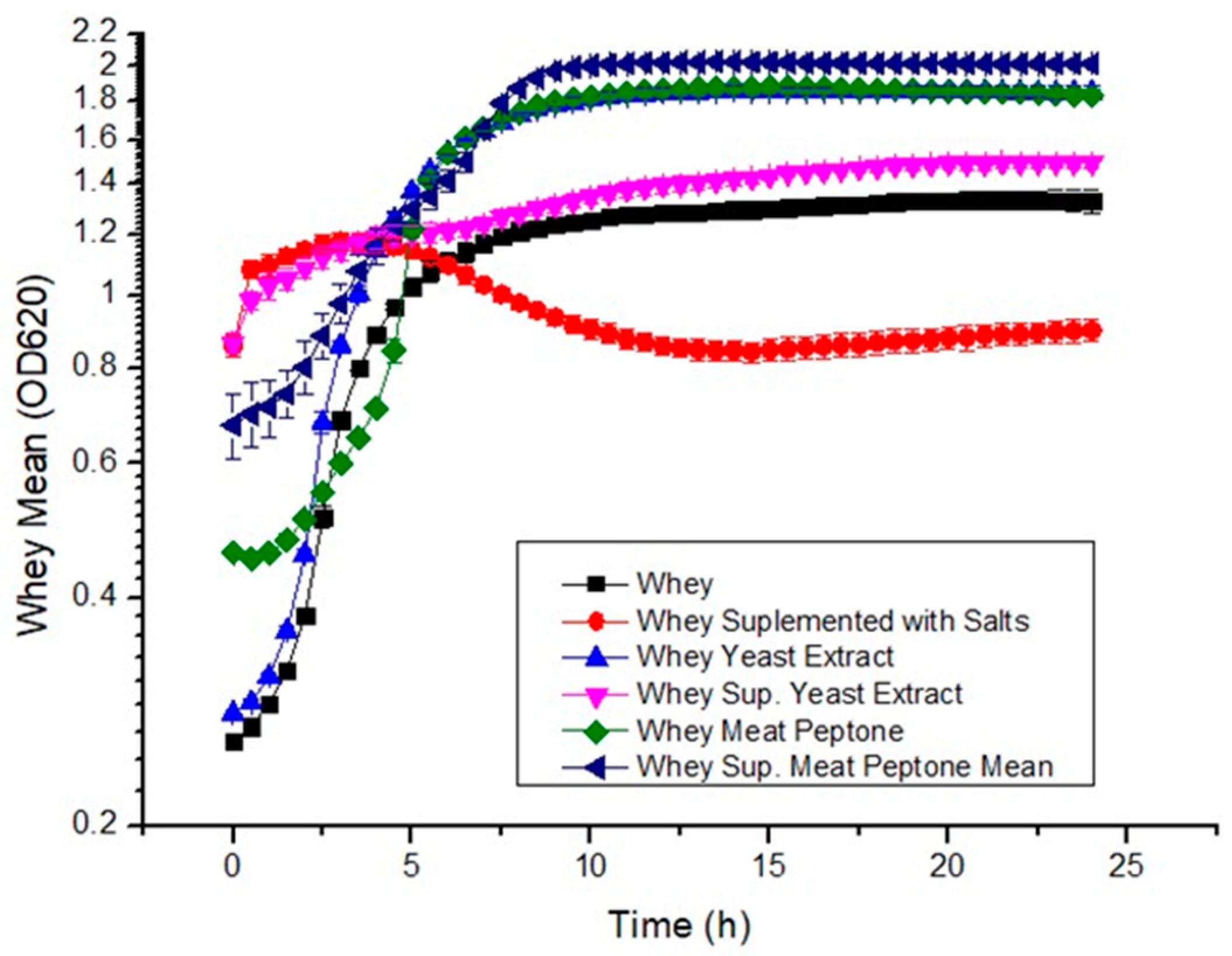
3.1. Growth Curves and Biomass Concentration
3.2. Maximum Specific Growth Grates (μMax) Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komesu, A.; de Oliveira, J.A.R.; da Silva Martins, L.H.; Maciel, M.R.W.; Maciel Filho, R. Lactic acid production to purification: A review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuels Bioprod. Biorefin. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- de Albuquerque, T.L.; Junior, J.E.M.; de Queiroz, L.P.; Ricardo, A.D.S.; Rocha, M.V.P. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Wang, Y.; Chen, C.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. The optimization of L-lactic acid production from sweet sorghum juice by mixed fermentation of Bacillus coagulans and Lactobacillus rhamnosus under unsterile conditions. Bioresour. Technol. 2016, 218, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Y.; Qian, H.; Zhang, W.G. Improvement of L-lactic acid production from Jerusalem artichoke tubers by mixed culture of Aspergillus niger and Lactobacillus sp. Bioresour. Technol. 2009, 100, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Ma, R.; Zheng, Z.; Cai, C.; Zhang, M.; Jiang, T. Open fermentative production of L-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresour. Technol. 2013, 135, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Kwon, E.Y.; Bae, S.J.; Cho, B.R.; Kim, S.Y.; Hahn, J.S. Improvement of d-lactic acid production in Saccharomyces cerevisiae under acidic conditions by evolutionary and rational metabolic engineering. Biotechnol. J. 2017, 12, 1700015. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, Z.; Zheng, Y.; Zhou, J.; Xiu, Z. Efficient production of lactic acid from sugarcane molasses by a newly microbial consortium CEE-DL15. Process Biochem. 2019, 81, 132–138. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.D.; El-Din, M.N.; Azab, M.S.; El-Belely, E.F.; Alrefaey, H.M.A.; Elsakhawy, T. One-factor-at-a-time and response surface statistical designs for improved lactic acid production from beet molasses by Enterococcus hirae ds10. SN Appl. Sci. 2020, 2, 573. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Z.J.; Xu, Q.Q.; Qian, Z.J.; Liu, J.H.; Ouyang, J. Valorization of dairy waste for enhanced D-lactic acid production at low cost. Process Biochem. 2018, 71, 18–22. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Fernando Escobar, L.; Andres Rojas, C.; Giraldo, G.; Antonio, G.; Padilla Sanabria, L. Evaluation of lactobacillus casei growth and production of lactic acid using as substrate the whey of bovine milk. Res. J.-Univ. Quindio 2010, 20, 42–49. [Google Scholar]
- Lech, M. Optimization of protein-free waste whey supplementation used for the industrial microbiological production of lactic acid. Biochem. Eng. J. 2020, 157, 107531. [Google Scholar] [CrossRef]
- Sandoval-Espinola, W.J.; Chinn, M.; Bruno-Barcena, J.M. Inoculum optimization of Clostridium beijerinckii for reproducible growth. FEMS Microbiol. Lett. 2015, 362, fnv164. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. L-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.; Fabbricino, M. Repeated-batch fermentation of cheese whey for semi-continuous lactic acid production using mixed cultures at uncontrolled pH. Sustainability 2019, 11, 3330. [Google Scholar] [CrossRef]


| Substrates | Proportion |
|---|---|
| Glucose | 7% (m/v) |
| Glucose with Yeast Extract | Glucose 7%; 10 g/L yeast extract |
| Glucose with Meat Peptone | Glucose 7%; 20 g/L meat peptone |
| Saccharose | 7% (m/v) |
| Saccharose with Yeast Extract | Sucrose 7%; 10 g/L yeast extract |
| Saccharose with Meat Peptone | 7% sucrose; 20 g/L meat peptone |
| Molasses | 7% (v/v) |
| Molasses with Yeast Extract | Molasses 7%; 10 g/L yeast extract |
| Molasses with Beef Peptone | Molasses 7%; 20 g/L meat peptone |
| Whey | Clarified whey |
| Supplemented Whey | MgSO4 0.05 g/L; (NH4)2HPO4 2.5 g/L; MnSO4 0.005 g/L |
| Whey with Meat Peptone | 20 g/L meat peptone |
| Whey with Yeast Extract | 10 g/L yeast extract |
| Whey Supplemented with Meat Peptone | MgSO4 0.05 g/L; (NH4)2HPO4 2.5 g/L; MnSO4 0.005 g/L; 20 g/L meat peptone |
| Whey Supplemented with Yeast Extract | MgSO4 0.05 g/L; (NH4)2HPO4 2.5 g/L; MnSO4 0.005 g/L; 10 g/L yeast extract |
| Condition | μmax (h−1) |
|---|---|
| MRS | 0.67 |
| Molasses | 0.2246 |
| Molasses with Beef Peptone | 0.2326 |
| Molasses with Yeast Extract | 0.26 |
| Saccharose | 0 |
| Sucrose with Meat Peptone | 0.2519 |
| Sucrose with Yeast Extract | 0.7258 |
| Glucose | 0 |
| Glucose with Meat Peptone | 0.7714 |
| Glucose with Yeast Extract | 0.216 |
| Whey | 0.59 |
| Whey Supplemented with Salts | 0.027 |
| Whey with Yeast Extract | 0.63 |
| Whey with Meat Peptone | 0.167 |
| Supplemented Whey + Yeast Extract | 0.046 |
| Supplemented Whey + Meat Peptone | 0.193 |
| MRS Broth | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escurra, J.; Ferreira, F.P.; López, T.R.; Sandoval-Espinola, W.J. Evaluation of Agro-Industrial Carbon and Energy Sources for Lactobacillus plantarum M8 Growth. Biol. Life Sci. Forum 2023, 28, 1. https://doi.org/10.3390/blsf2023028001
Escurra J, Ferreira FP, López TR, Sandoval-Espinola WJ. Evaluation of Agro-Industrial Carbon and Energy Sources for Lactobacillus plantarum M8 Growth. Biology and Life Sciences Forum. 2023; 28(1):1. https://doi.org/10.3390/blsf2023028001
Chicago/Turabian StyleEscurra, José, Francisco P. Ferreira, Tomás R. López, and Walter J. Sandoval-Espinola. 2023. "Evaluation of Agro-Industrial Carbon and Energy Sources for Lactobacillus plantarum M8 Growth" Biology and Life Sciences Forum 28, no. 1: 1. https://doi.org/10.3390/blsf2023028001






