Precision Agriculture as Input for the Rice Grain (Oryza sativa L.) Biofortification with Selenium †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fields
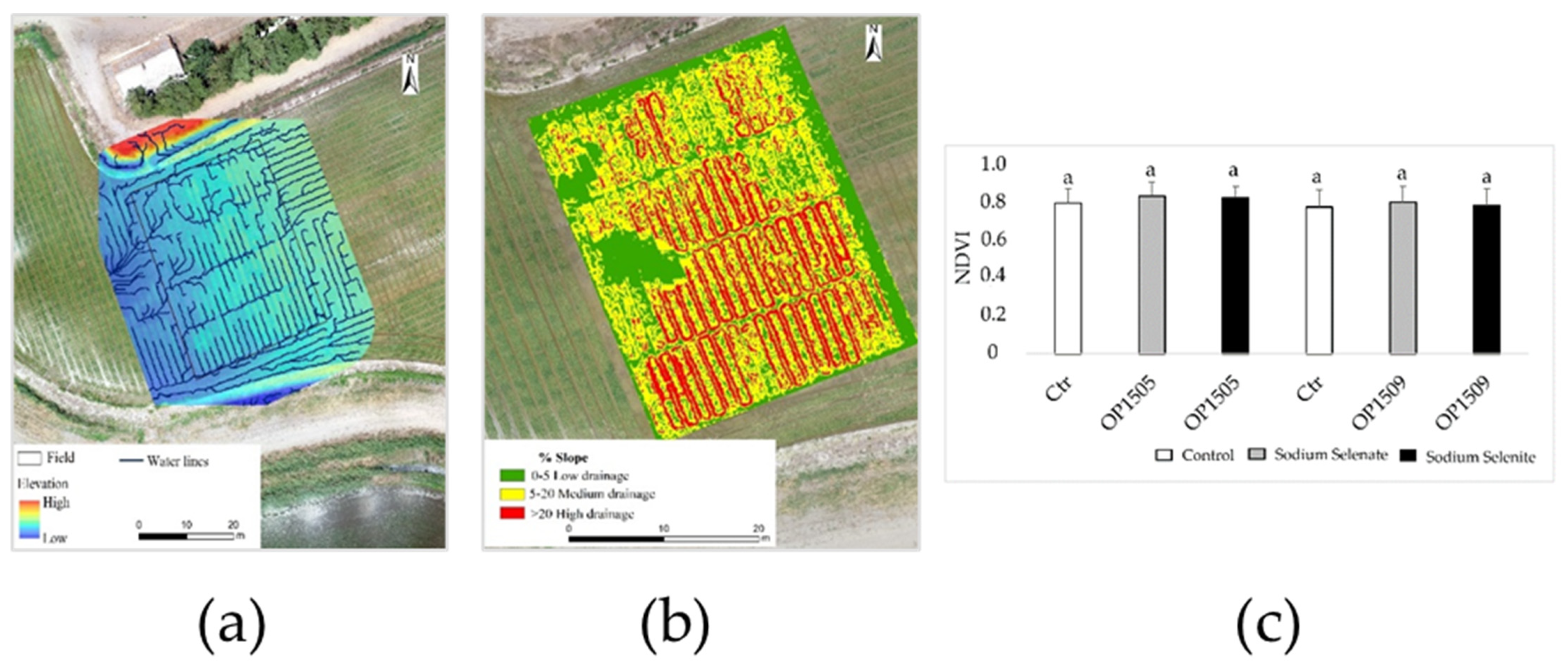
2.2. Precision Agriculture—Characterize the Experimental Fields Production and Monitor the State of the Culture
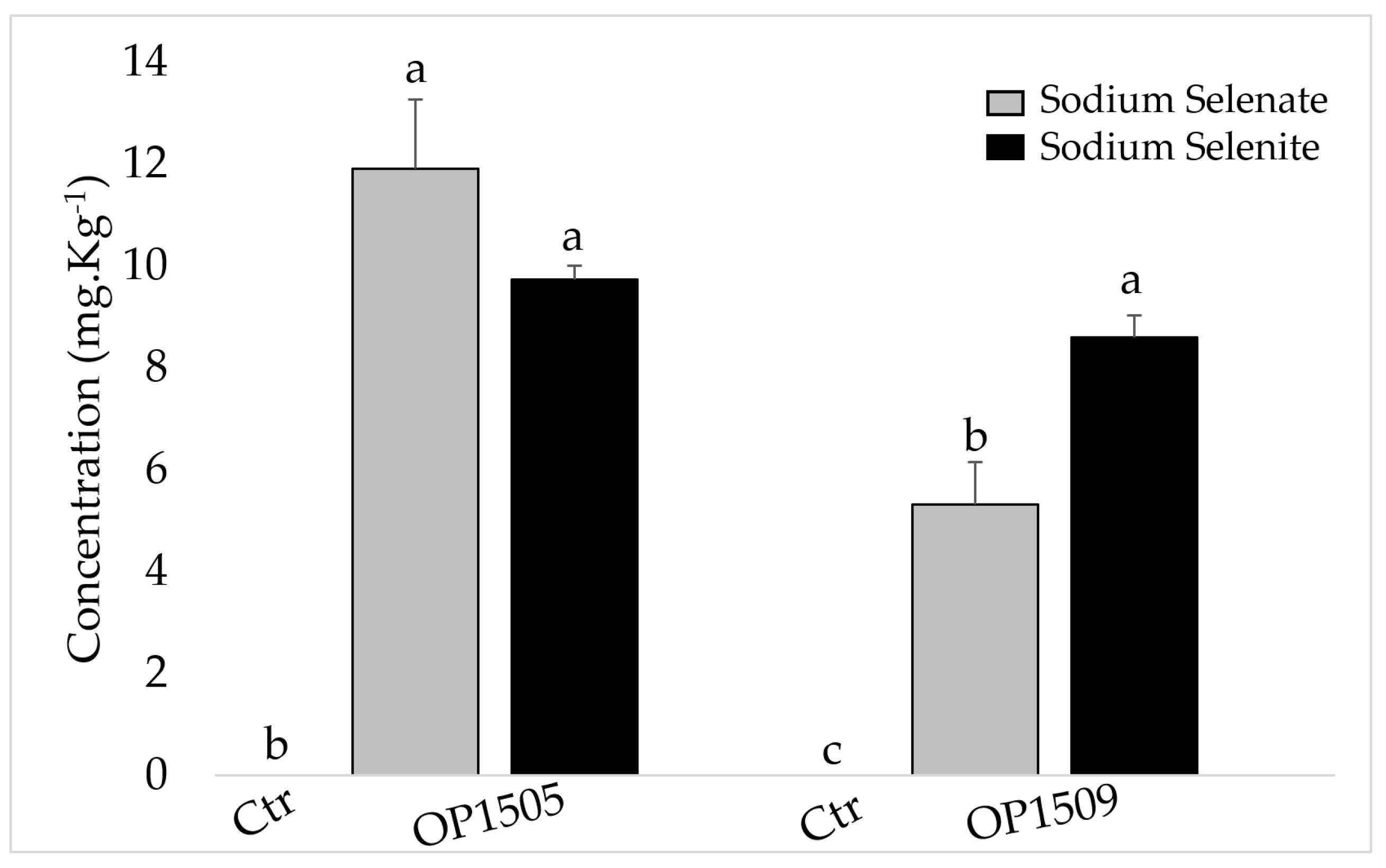
2.3. Leaf Gas Exchange Measurements and Analysis of Selenium Contents
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.; Iqbal, N. Precision agriculture techniques and practices: From considerations to applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Xu, T.; Wang, Y.; Du, W.; Shen, A. Research on vision navigation and position system of agricultural unmanned aerial vehicle. Int. J. Comput. Integr. Manuf. 2020, 33, 1185–1196. [Google Scholar] [CrossRef]
- Garcia-Bañuelos, M.L.; Hermosillo-Cereceres, M.A.; Sanchez, E. The importance of selenium biofortification in food crops. Curr. Nutr. Food Sci. 2011, 7, 181–190. [Google Scholar] [CrossRef]
- Reis, A.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of selenium deficiency and toxicity worldwide: Affected areas, selenium-related health issues, and case studies. In Plant Ecophysiology; De Kok, L.J., Hakesford, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 11, pp. 209–230. [Google Scholar] [CrossRef]
- Lidon, F.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.; Pelica, J.; Pataco, I.; Ramalho, J.; Leitão, A.; Almeida, A.; et al. Selenium biofortification of rice through foliar application with selenite and selenate. Exp. Agric. 2018, 55, 528–542. [Google Scholar] [CrossRef]
- Longchamp, M.; Angeli, N.; Castrec-Rouelle, M. Effects on the accumulation of calcium, magnesium, iron, manganese, copper and zinc of adding the two inorganic forms of selenium to solution cultures of Zea mays. Plant Physiol. Biochem. 2016, 98, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.R.F.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Luís, I.C.; Caleiro, J.C.; Simões, M.; Kullberg, J.; Legoinha, P.; Brito, G.; et al. Can foliar pulverization with CaCl2 and Ca(NO3)2 trigger Ca enrichment in Solanum Tuberosum L. tubers? Plants 2021, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulao, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and Coffea canephora species. Glob. Chang. Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Luís, I.; Lidon, F.; Pessoa, C.; Marques, A.; Coelho, A.; Simões, M.; Patanita, M.; Dôres, J.; Ramalho, J.; Silva, M.; et al. Zinc enrichment in two contrasting genotypes of Triticum aestivum L. grains: Interactions between edaphic conditions and foliar fertilizers. Plants 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Skakun, S.; Justice, C.O.; Vermote, E.; Roger, J.C. Transitioning from MODIS to VIIRS: An analysis of inter-consistency of NDVI data sets for agricultural monitoring. Int. J. Remote Sens. 2018, 39, 971–992. [Google Scholar] [CrossRef] [PubMed]
- Daroya, R.; Ramos, M. NDVI image extraction of an agricultural land using an autonomous quadcopter with a filter-modified camera. In Proceedings of the 2017 7th IEEE International Conference on Control System, Computing and Engineering (ICCSCE), Penang, Malaysia, 24–26 November 2017; pp. 110–114. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.; Shi, W. Selenium Biofortification and Interaction With Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Lyons, G.H.; Genc, Y.; Stangoulis, J.C.; Palmer, L.T.; Graham, R.D. Selenium distribution in wheat grain, and the effect of postharvest processing on wheat selenium content. Biol. Trace Elem. Res. 2005, 103, 155–168. [Google Scholar] [CrossRef]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at different stages. Field Crops Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
| Fertilization | OP1505 | OP1509 | ||||
|---|---|---|---|---|---|---|
| 1st Analysis | 2nd Analysis | 3rd Analysis | 1st Analysis | 2nd Analysis | 3rd Analysis | |
| Pn (μmol CO2 m−2 s−1) | ||||||
| Control | 15.53 ± 0.75 ar 1 | 12.43 ± 0.64 br | 5.57 ± 0.46 cr | 13.57 ± 0.33 as | 9.61 ± 0.44 bs | 3.65 ± 0.17 cs |
| Selenate | 18.36 ± 0.18 ar | 11.04 ± 0.76 br | 6.17 ± 0.44 cr | 13.71 ± 0.69 as | 7.81 ± 1.06 bs | 3.11 ± 0.30 cs |
| Selenite | 19.20 ± 1.60 ar | 11.84 ± 0.68 br | 6.48 ± 0.28 cr | 18.19 ± 2.08 ar | 12.80 ± 0.97 br | 8.03 ± 0.81 cr |
| gs (mmol H2O m−2 s−1) | ||||||
| Control | 190.0 ± 22.5 as | 306.4 ± 41.3 ar | 150.5 ± 22.3 ar | 173.1 ± 24.5 as | 170.4 ± 18.5 as | 87.9 ± 12.9 ar |
| Selenate | 332.8 ± 60.8 ars | 226.1 ± 33.8 abr | 120.7 ± 15.5 br | 339.0 ± 32.9 ar | 104.8 ± 17.0 bs | 84.7 ± 4.6 br |
| Selenite | 418.8 ± 27.2 ar | 278.7 ± 78.6 abr | 168.0 ± 48.2 br | 399.1 ± 22.9 ar | 276.0 ± 44.4 br | 136.4 ± 15.2 cr |
| E (mmol H2O m−2 s−1) | ||||||
| Control | 2.31± 0.23 as | 3.62 ± 0.44as | 1.65 ± 0.19ar | 2.19 ± 0.30at | 2.49 ± 0.25 as | 1.15 ± 0.55 ar |
| Selenate | 3.02 ± 0.30 as | 2.85 ± 0.31as | 2.07 ± 0.38br | 3.22 ± 0.20as | 1.69 ± 0.22 bs | 1.70 ± 0.22 br |
| Selenite | 5.12 ± 0.82 ar | 4.03 ± 0.52as | 1.81 ± 0.17ar | 5.80 ± 0.57ar | 4.06 ± 0.42 ar | 1.97 ± 0.16 br |
| iWUE (mmol CO2 m−2 s−1 H2O) | ||||||
| Control | 7.71 ± 0.75 ar | 4.12 ± 0.46br | 4.25 ± 0.63 br | 8.89 ± 1.39 ar | 4.60 ± 0.54 br | 3.87 ± 0.45 br |
| Selenate | 6.54 ± 0.68 as | 4.06 ± 0.29abr | 3.50 ± 0.17 br | 4.34 ± 0.18 as | 4.75 ± 0.58 ar | 1.91 ± 0.14 ar |
| Selenite | 4.18 ± 0.42 at | 3.16 ± 0.26ar | 3.91 ± 0.67 ar | 3.14 ± 0.14 as | 3.23 ± 0.14 ar | 4.16 ± 0.32 ar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.C.; Pessoa, C.C.; Daccak, D.; Luís, I.C.; Coelho, A.R.F.; Caleiro, J.; Campos, P.S.; Almeida, A.S.; Simões, M.; Brito, M.G.; et al. Precision Agriculture as Input for the Rice Grain (Oryza sativa L.) Biofortification with Selenium. Biol. Life Sci. Forum 2021, 3, 37. https://doi.org/10.3390/IECAG2021-10019
Marques AC, Pessoa CC, Daccak D, Luís IC, Coelho ARF, Caleiro J, Campos PS, Almeida AS, Simões M, Brito MG, et al. Precision Agriculture as Input for the Rice Grain (Oryza sativa L.) Biofortification with Selenium. Biology and Life Sciences Forum. 2021; 3(1):37. https://doi.org/10.3390/IECAG2021-10019
Chicago/Turabian StyleMarques, Ana Coelho, Cláudia Campos Pessoa, Diana Daccak, Inês Carmo Luís, Ana Rita F. Coelho, João Caleiro, Paula Scotti Campos, Ana Sofia Almeida, Manuela Simões, Maria Graça Brito, and et al. 2021. "Precision Agriculture as Input for the Rice Grain (Oryza sativa L.) Biofortification with Selenium" Biology and Life Sciences Forum 3, no. 1: 37. https://doi.org/10.3390/IECAG2021-10019
APA StyleMarques, A. C., Pessoa, C. C., Daccak, D., Luís, I. C., Coelho, A. R. F., Caleiro, J., Campos, P. S., Almeida, A. S., Simões, M., Brito, M. G., Kullberg, J. C., Pessoa, M. F., Reboredo, F., Ramalho, J. C., Semedo, J. M. N., Marques, P., Silva, M. M., Legoinha, P., Pais, I., & Lidon, F. C. (2021). Precision Agriculture as Input for the Rice Grain (Oryza sativa L.) Biofortification with Selenium. Biology and Life Sciences Forum, 3(1), 37. https://doi.org/10.3390/IECAG2021-10019

















