Comparison of Proximal Remote Sensing Devices of Vegetable Crops to Determine the Role of Grafting in Plant Resistance to Meloidogyne incognita †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Material and Trial Design
2.3. Measurements and Sampling Methods
2.4. Statistical Processing
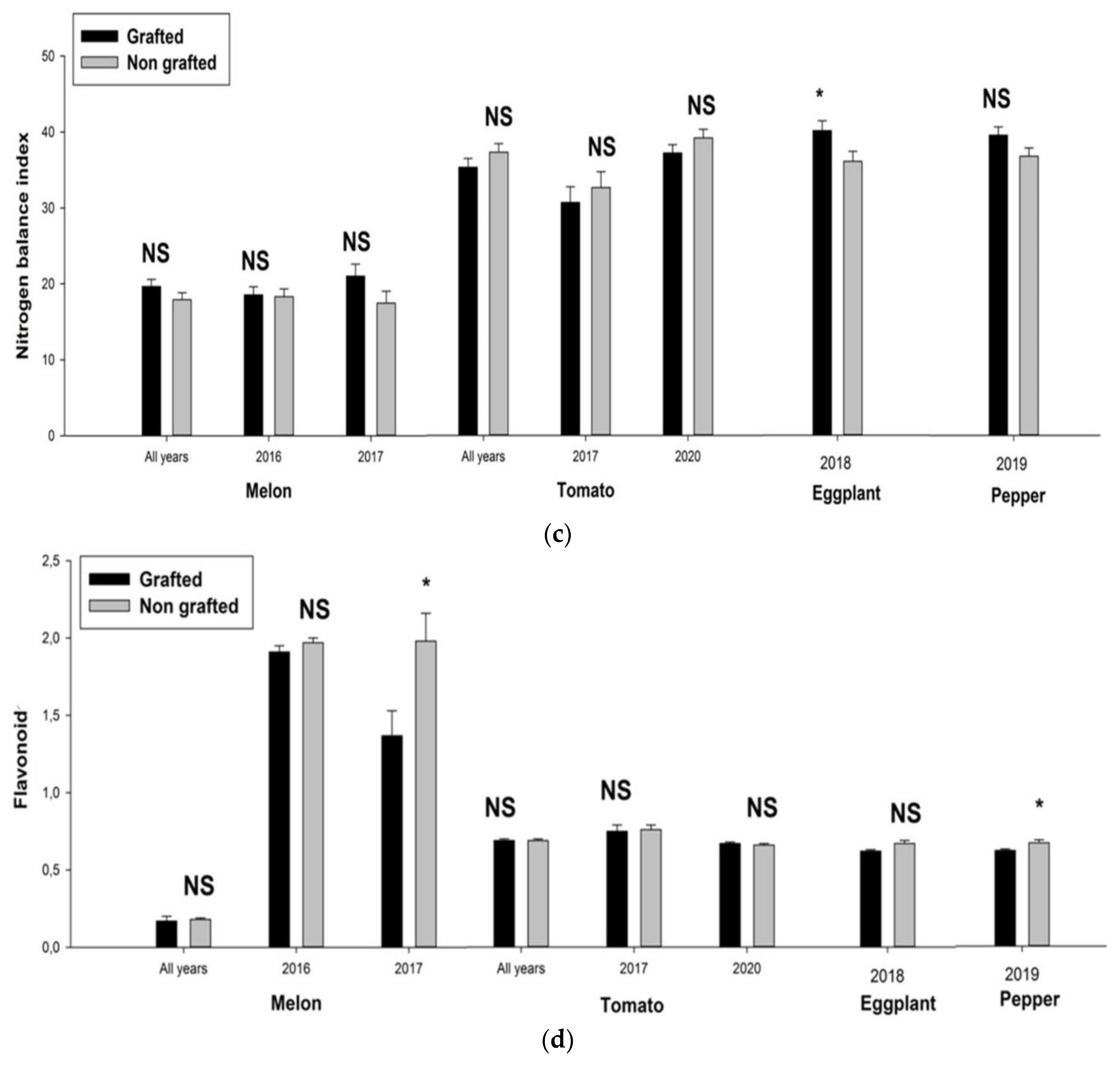
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koenning, S.R.; Overstreet, C.; Noling, J.W.; Donald, P.A.; Becker, J.O.; Fortnum, B.A. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J. Nematol. 1999, 31, 587. [Google Scholar] [PubMed]
- Gracia-Romero, A.; Kefauver, S.C.; Fernandez-Gallego, J.A.; Vergara-Díaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and ground image-based phenotyping: A proof of concept with Durum wheat. Remote Sens. 2019, 11, 1244. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C. Breeding to adapt agriculture to climate change: Affordable phenotyping solutions. Curr. Opin. Plant Biol. 2018, 45, 237–247. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Evans, R.D. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001, 6, 121–126. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive Diagnostic Test for Nitrogen Nutrition of Grapevine (Vitis vinifera L.) Based on Dualex Leaf-Clip Measurements in the Field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.R.; Daughtry, C.S.T.; Eitel, J.U.; Long, D.S. Remote sensing leaf chlorophyll content using a visible band index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef]
- Stern, A.; Doraiswamy, P.C.; Hunt Jr, E.R. Changes of crop rotation in Iowa determined from the United States Department of Agriculture, National Agricultural Statistics Service cropland data layer product. J. Appl. Remote Sens. 2012, 6, 063590. [Google Scholar] [CrossRef]
- Vergara-Díaz, O.; Zaman-Allah, M.A.; Masuka, B.; Hornero, A.; Zarco-Tejada, P.; Prasanna, B.M.; Araus, J.L. A novel remote sensing approach for prediction of maize yield under different conditions of nitrogen fertilization. Front. Plant Sci. 2016, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Montague, T.; Hellman, E.; Krawitzky, M. Comparison of greenhouse grown, containerized grapevine stomatal conductance measurements using two differing porometers. In Proceedings of the 2nd Annual National Viticulture Research Conference, Davis, CA, USA, 9–11 July 2008; pp. 58–61. [Google Scholar]
- Duncan, G.A.; Gates, R.; Montross, M.D. Measuring Relative Humidity in Agricultural Environments; Agricultural Engineering Extension Publications-Uknowledge: Lexington, KY, USA, 2005. [Google Scholar]
- Konica, M.O. Chlorophyll Meter SPAD-502 Plus-A Lightweight Handheld Meter for Measuring the Chlorophyll Content of Leaves without Causing Damage to Plants. 2012. Available online: http://www.konikcaminolta.com/instrments/download/catalog/color/pdf/spad502plus_e1.pdf (accessed on 12 March 2019).
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Gutiérrez, N.A.; Nieto-Taladriz, M.T.; Araus, J.L. Wheat ear counting in-field conditions: High throughput and low-cost approach using RGB images. Plant Methods 2018, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Miguel, A.; Maroto, J.V.; San Bautista, A.; Baixauli, C.; Cebolla, V.; Pascual, B.; Guardiola, J.L. The grafting of triploid watermelon is an advantageous alternative to soil fumigation by methyl bromide for control of Fusarium wilt. Sci. Hortic. 2004, 103, 9–17. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Vergara, O.; Araus, J.L.; Tarekegne, A.; Magorokosho, C.; Zarco-Tejada, P.J.; Hornero, A.; Albà, A.H.; Das, B.; Craufurd, P.; et al. Unmanned aerial platform-based multi-spectral imaging for field phenotyping of maize. Plant Methods 2015, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- De Guiran, G. Protection des Cultures Maraîchères et Fruitières Face Aux Capacités D’adaptation des Nématodes Meloidogyne; Comptes Rendus de l’Académie d’Agriculture: Paris, France, 1983. [Google Scholar]
- Sinclair, T.R.; Rufty, T.W. Nitrogen and water resources commonly limit crop yield increases, not necessarily plant genetics. Glob. Food Secur. 2012, 1, 94–98. [Google Scholar] [CrossRef]
- Silva-Sánchez, A.; Buil-Salafranca, J.; Cabral, A.C.; Uriz-Ezcaray, N.; García-Mendívil, H.A.; Sorribas, F.J.; Gracia-Romero, A. Comparison of proximal remote sensing devices for estimating physiological responses of eggplants to root-knot nematodes. Proceedings 2019, 18, 9. [Google Scholar]
- Goverse, A.; Smant, G. The activation and suppression of plant innate immunity by parasitic nematodes. Ann Rev Phytopath. 2014, 52, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Rivard, C.L.; Louws, F.J. Grafting to manage soilborne diseases in heirloom tomato production. HortScience 2008, 43, 2104–2111. [Google Scholar] [CrossRef]
- Expósito, A.; García, S.; Giné, A.; Escudero, N.; Sorribas, F.J. Cucumis metuliferus reduces Meloidogyne incognita virulence against the Mi1.2 resistance gene in a tomato–melon rotation sequence. Pest Manag. Sci. 2019, 75, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Bosquet, L.; Albrizio, R.; Nogues, S.; Araus, J.L. Dual Delta 13C/delta 18O response to water and nitrogen availability and its relationship with yield in field-grown durum wheat. Plant Cell Environ. 2011, 34, 418–433. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdane, Y.; Gracia-Romero, A.; Buchaillot, M.L.; Sanchez-Bragado, R.; Fullana, A.M.; Sorribas, F.J.; Araus, J.L.; Kefauver, S.C. Comparison of Proximal Remote Sensing Devices of Vegetable Crops to Determine the Role of Grafting in Plant Resistance to Meloidogyne incognita. Biol. Life Sci. Forum 2021, 3, 61. https://doi.org/10.3390/IECAG2021-09718
Hamdane Y, Gracia-Romero A, Buchaillot ML, Sanchez-Bragado R, Fullana AM, Sorribas FJ, Araus JL, Kefauver SC. Comparison of Proximal Remote Sensing Devices of Vegetable Crops to Determine the Role of Grafting in Plant Resistance to Meloidogyne incognita. Biology and Life Sciences Forum. 2021; 3(1):61. https://doi.org/10.3390/IECAG2021-09718
Chicago/Turabian StyleHamdane, Yassine, Adrian Gracia-Romero, Ma. Luisa Buchaillot, Rut Sanchez-Bragado, Aida Magdalena Fullana, Francisco Javier Sorribas, José Luis Araus, and Shawn C. Kefauver. 2021. "Comparison of Proximal Remote Sensing Devices of Vegetable Crops to Determine the Role of Grafting in Plant Resistance to Meloidogyne incognita" Biology and Life Sciences Forum 3, no. 1: 61. https://doi.org/10.3390/IECAG2021-09718
APA StyleHamdane, Y., Gracia-Romero, A., Buchaillot, M. L., Sanchez-Bragado, R., Fullana, A. M., Sorribas, F. J., Araus, J. L., & Kefauver, S. C. (2021). Comparison of Proximal Remote Sensing Devices of Vegetable Crops to Determine the Role of Grafting in Plant Resistance to Meloidogyne incognita. Biology and Life Sciences Forum, 3(1), 61. https://doi.org/10.3390/IECAG2021-09718