Analysis of Polyphenol Content and Antioxidant Capacity of Hybrid Mandarin Peel †
Abstract
:1. Introduction
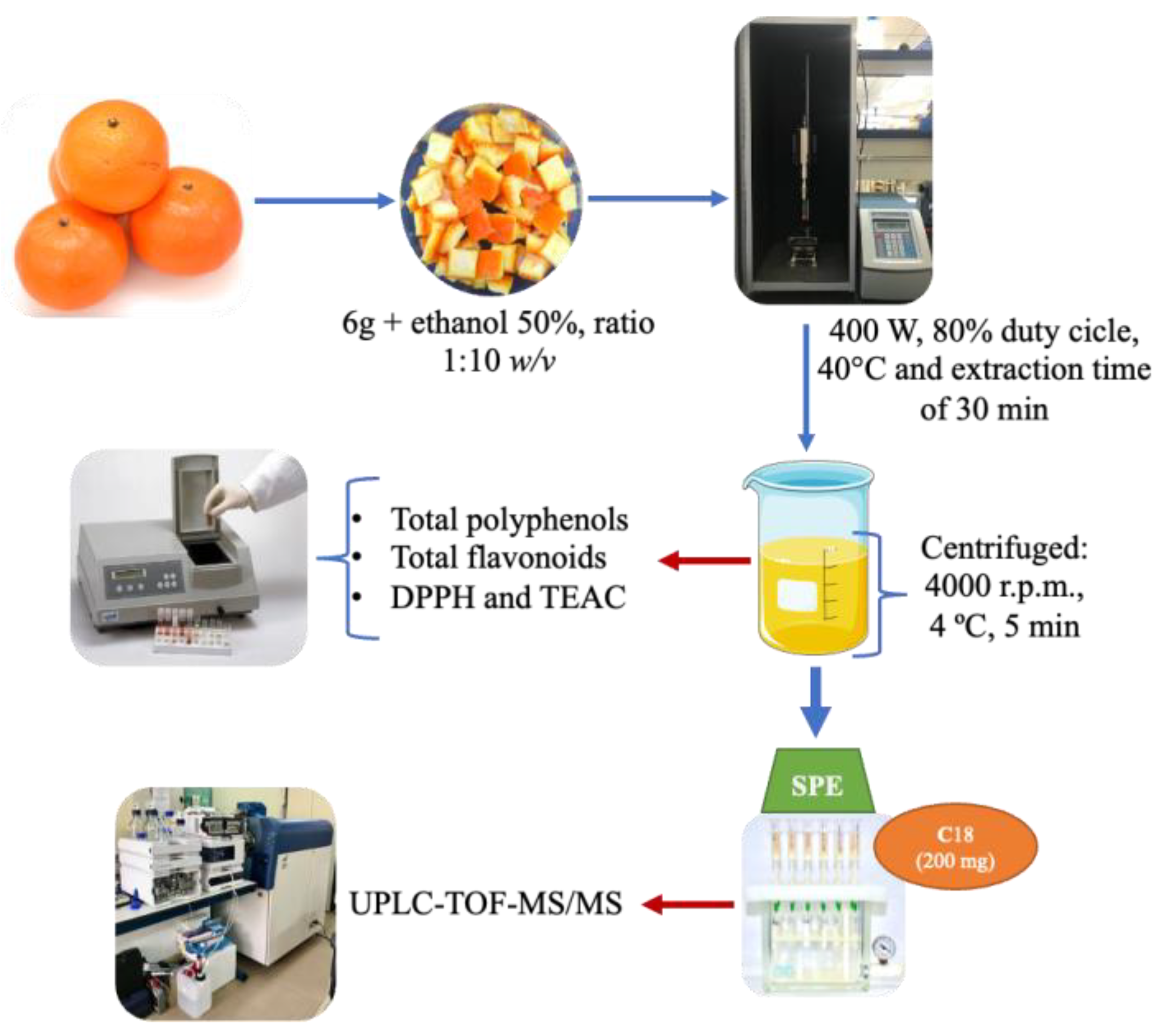
2. Materials and Methods
2.1. Plant Materials and Extraction Method
2.2. Chemical Analysis Methods
2.3. Chromatographic Analysis
3. Results and Discussion
3.1. Bioactive Compounds
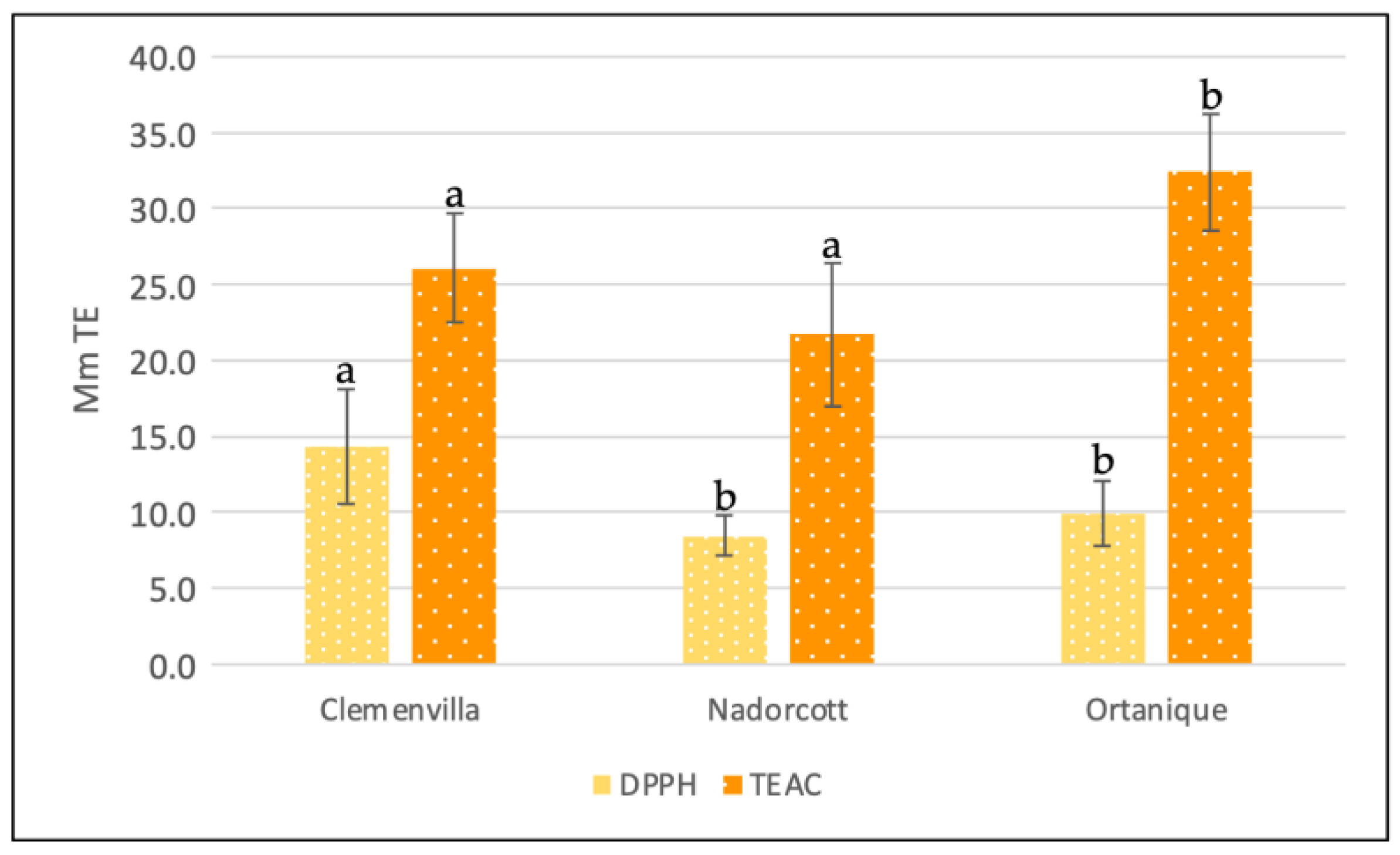
3.2. Antioxidant Capacity
3.3. Identification and Quantification of Polyphenols by Ultra-Performance Liquid Chromatography System Coupled with a Quadrupole Time-of-Flight Mass Spectrometer Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Citrus Fresh and Processed–Statistical Bulletin 2016. 2017. Available online: http://www.fao.org/3/a-i8092e.pdf (accessed on 24 January 2020).
- Yu, X.; Zhang, X.; Jiang, D.; Zhu, S.; Cao, L.; Liu, X.; Shen, W.; Zhao, W.; Zhao, X. Genetic diversity of the ease of peeling in mandarins. Sci. Hortic. 2021, 278, 109852. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Kou, G.; Chen, Q.; Li, Y.; Zhou, Z. Protection and delivery of mandarin (Citrus reticulata Blanco) peel extracts by encapsulation of whey protein concentrate nanoparticles. LWT 2019, 99, 24–33. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Effects of ultrasound-assisted extraction on physicochemical properties, bioactive compounds, and antioxidant capacity for the valorization of hybrid Mandarin peels. Food Biosci. 2021, 42, 101185. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Raes, K.; Vanhoutte, H.; Coelus, S.; Smagghe, G.; Van Camp, J. Liquid chromatography–mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams. J. Chromatogr. A 2015, 1402, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Londoño, J.; de Lima, V.R.; Lara, O.; Gil, A.; Pasa, T.B.C.; Arango, G.J.; Pineda, J.R. Clean recovery of antioxidant flavonoids from citrus peel: Optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010, 119, 81–87. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Ho, S.C.; Lin, C.C. Investigation of Heat Treating Conditions for Enhancing the Anti-Inflammatory Activity of Citrus Fruit (Citrus reticulata) Peels. J. Agric. Food Chem. 2008, 56, 7976–7982. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, M.S.; Wannes, W.A.; Ouerghemmi, I.; Jegham, S.; Njima, Y.B.; Hamdaoui, G.; Zemni, H.; Marzouk, B. Juice components and antioxidant capacity of four Tunisian Citrus varieties. J. Sci. Food Agric. 2011, 91, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.A.; Moreno, D.; García-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.T.; Ballester, A.R.; Calejero, J.; González-Candelas, L. Effect of high-temperature-conditioning treatments on quality, flavonoid composition and vitamin C of cold stored ‘Fortune’ mandarins. Food Chem. 2011, 128, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and Ultrasound-Assisted Extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef] [PubMed]
- M’hiri, N.; Ioannou, I.; Mihoubi Boudhrioua, N.; Ghoul, M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Process. 2015, 96, 161–170. [Google Scholar] [CrossRef]
- Fayek, N.M.; Farag, M.A.; Abdel Monem, A.R.; Moussa, M.Y.; Abd-Elwahab, S.M.; El-Tanbouly, N.D. Comparative Metabolite Profiling of Four Citrus Peel Cultivars via Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-of-Flight-Mass Spectrometry and Multivariate Data Analyses. J. Chromatogr. Sci. 2019, 57, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Zhang, X.; Chen, H.; Xia, S.; Jia, C.; Zhong, F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 2017, 3, 371–376. [Google Scholar] [CrossRef]
- Zhao, X.J.; Chen, D.; Kilmartin, P.A.; Jiao, B.N. Simultaneous Determination of Phenolics and Polymethoxylated Flavones in Citrus Fruits by Ultra-High Performance Liquid Chromatography Coupled with Triple-Quadrupole Mass Spectrometry (UHPLC-QqQ-MS). Anal. Lett. 2019, 52, 1926–1938. [Google Scholar] [CrossRef]
| Bioactive Compound | Clemenvilla | Nadorcott | Ortanique |
|---|---|---|---|
| TP (mg GAE/100 g FW ± SD) | 828.4 ± 95.8 a | 724.2 ± 43.0 a | 1155.2 ± 171.3 b |
| TF (mg CE/100 g FW ± SD) | 89.6 ± 15.5 a | 70.9 ± 12.5 a | 71.7 ± 17.8 a |
| Compound | Molecular Formula | [M-H]− m/z (−) | Clemenvilla | Nadorcott | Ortanique |
|---|---|---|---|---|---|
| 4-hidroxibenzoic acid | C7H6O3 | 137.02442 | 4.1 ± 10.7 a | 1.9 ± 0.8 b | 2.1 ± 1.2 b |
| Rutin | C27H30O16 | 609.14611 | 7.3 ± 3.8 a | 5.6 ± 3.3 ab | 6.9 ± 2.2 ab |
| Ferulic acid | C10H10O4 | 193.05063 | 1.5 ± 0.7 a | 6.8 ± 0.9 b | 2.2 ± 0.5 c |
| Narirutin | C27H32O14 | 579.17193 | 4.3 ± 2.6 a | 31.8 ± 6.8 b | 33.1 ± 6.3 b |
| Hesperidin | C28H34O15 | 609.18249 | 63.7 ± 10.7 a | 72.3 ± 7.0 b | 63.7 ± 6.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anticona, M.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J.; Blesa, J. Analysis of Polyphenol Content and Antioxidant Capacity of Hybrid Mandarin Peel. Biol. Life Sci. Forum 2021, 6, 25. https://doi.org/10.3390/Foods2021-11100
Anticona M, Lopez-Malo D, Frigola A, Esteve MJ, Blesa J. Analysis of Polyphenol Content and Antioxidant Capacity of Hybrid Mandarin Peel. Biology and Life Sciences Forum. 2021; 6(1):25. https://doi.org/10.3390/Foods2021-11100
Chicago/Turabian StyleAnticona, Mayra, Daniel Lopez-Malo, Ana Frigola, Maria Jose Esteve, and Jesus Blesa. 2021. "Analysis of Polyphenol Content and Antioxidant Capacity of Hybrid Mandarin Peel" Biology and Life Sciences Forum 6, no. 1: 25. https://doi.org/10.3390/Foods2021-11100
APA StyleAnticona, M., Lopez-Malo, D., Frigola, A., Esteve, M. J., & Blesa, J. (2021). Analysis of Polyphenol Content and Antioxidant Capacity of Hybrid Mandarin Peel. Biology and Life Sciences Forum, 6(1), 25. https://doi.org/10.3390/Foods2021-11100









