Abstract
Exhausted olive pomace (EOP) is a waste generated in large quantities each year in the olive oil industry. This biomass contains phenolic compounds with antioxidant, antiatherogenic, anti-inflammatory, and antimicrobial properties. For the extraction of these compounds, the use of a novel and environmentally friendly technique, microwave-assisted extraction using water as extraction solvent, was proposed. A Box–Behnken design of experiments based on the response surface methodology was used to optimise the effect of the factors temperature (40–100 °C), extraction time (4–40 min), and solid loading (2–15%). The response variables were the total phenolic content analysed by Folin–Ciocalteau assay, hydroxytyrosol content by HPLC, and antioxidant activity through FRAP and ABTS assays. The optimal conditions for each response variable were determined. Overall, microwave-assisted extraction is considered a suitable technique for the extraction of bioactive compounds from EOP at short extraction times. In particular, the maximum content of hydroxytyrosol (6 mg/g of EOP) could be obtained at 99.7 °C, 3.9% (w/v) solids, and 34.3 min. Therefore, this extract has the potential to be used as a functional and antioxidant additive.
1. Introduction
Exhausted olive pomace (EOP) is the final solid residue generated after subjecting olive pomace to a drying process and a solid-liquid extraction with hexane to extract the residual olive oil [1]. In Spain, around 1.2 million tons of this waste are generated every year [2]. According to the chemical composition of EOP, it is considered a promising feedstock for the production of bioenergy, including bioethanol, from its structural carbohydrates (around 35%) and bioactive compounds from the non-structural components (around 50%), which includes phenolic compounds. Therefore, the valorisation of this biomass, considering all these compounds, would allow it to be introduced into the biorefinery concept.
The food industry is currently investigating the possibility of replacing synthetic antioxidants with antioxidants of natural origin, including those of olive origin [3,4]. Due to its bioavailability, chemical properties, bioactivity and low toxicity, these extracts and those rich in hydroxytyrosol could be used as a food additive and functional ingredient for food and nutraceutical applications [5].
Therefore, the extraction of phenolic compounds from olive biomass can be performed as the first step within a biorefinery approach. Conventional techniques such as maceration, Soxhlet extraction and hydrodistillation have been used for years for the recovery of these compounds. These methods have drawbacks such as long extraction time, high solvent consumption, low reproducibility, etc. [6]. These problems have been overcome by employing new extraction techniques such as microwave-assisted extraction (MAE), ultrasound-assisted extraction, accelerated solvent extraction or supercritical fluid extraction [7,8].
Therefore, the purpose of this work was to optimise the extraction of phenolic compounds, including hydroxytyrosol, and the antioxidant activity of the extracts obtained from EOP by MAE using water as solvent. Response surface methodology (RSM) was employed to evaluate the extraction parameters of temperature, extraction time and solid loading using a Box–Behnken experimental design (BBD).
2. Methods
2.1. Raw Material
EOP was obtained from the olive pomace industry “Spuny SA” (Castellar, Jaén, Spain) and milled to a size of 1 mm with a ZM 200 ultracentrifugal mill (Retsch, Haan, Germany).
All the chemicals and reagents were of analytical grade and were supplied by Sigma-Aldrich (St. Louis, MO, USA): Folin–Ciocalteu’s phenol reagent, 2,4,6,-tri (2pyridyl)-1,3,5,-triazine (TPTZ), iron (III) chloride, [2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and standards of gallic acid. Methanol (HPLC grade) was obtained from Honeywell (Morristown, NJ, EEUU) and acetonitrile (HPLC grade) from PanReac AppliChem (Barcelona, Spain). Hydroxytyrosol (98% of purity, w/w) was procured from Extrasynthese (Lyon, France). Ultrapure water was obtained using a Milli-Q system (Millipore, Bedford, MA, USA).
2.2. Microwave-Assisted Extraction of Exhausted Olive Pomace
The extraction was carried out in a microwave reactor Anton Parr Monowave 400 (Graz, Austria). Three independent variables were studied, temperature (40–100 °C), extraction time (4–40 min), and solid loading (3–15%, w/v), applying a BBD. It consisted of 17 experiments, including five central points. The response variables studied were: total phenolic content (TPC), hydroxytyrosol content, and antioxidant activity.
Once the MAE assays were finished, each sample was filtered under a vacuum, and the recovered extract was filtered through syringe filters (nylon, pore size 45 µm) (Grupo SinerLab, Madrid, Spain) for analysis.
2.3. Measurement of the Total Phenolic Content and Antioxidant Activity
TPC was determined using the Folin-Ciocalteu colorimetric assay, according to a procedure described by Singleton and Rossi [9] with a little modification according to Gómez-Cruz et al. [10] and using gallic acid as standard. To determine the antioxidant activity of the extracts, ferric-reducing power assays (FRAP) and ABTS radical scavenging assay were applied according to Martínez-Patiño et al. [3] and using Trolox as a standard. The absorbance was measured using a Bio-Rad iMarkTM microplate reader (Hercules, CA, USA).
2.4. Phenolic Profiling and Quantification of Hydroxytyrosol
The phenolic profile and hydroxytyrosol content of the aqueous extracts from EOP were determined by reversed-phase (RP)-high-performance liquid chromatography (HPLC) in a Shimadzu Prominence UFLC chromatograph (Kyoto, Japan) equipped with a diode-array detector, according to Gómez-Cruz et al. [4]. A calibration curve was built using a commercial standard of hydroxytyrosol.
2.5. Statistical Analysis
The experimental data obtained after applying the designs were analyzed using the Design-Expert® v8.0.7.1 software (Stat-Ease, Inc., Minneapolis, MN, USA). Then, the response variables were fitted to a second order polynomial model equation obtained by response surface methodology (RSM) according to the following equation:
where y is the dependent variable, xi and xj are the independent variables, β0, βi, βij and βii are the regression coefficients.
ANOVA was used to determine the significance of the results.
3. Results and Discussion
Water was the solvent selected for the extraction of phenolic compounds from EOP to develop an eco-friendly extraction methodology since it is abundant, low cost, non-toxic, and is generally recognized as a safe (GRAS) solvent [11]. Then, the conditions were optimised using a BBD design and RSM.
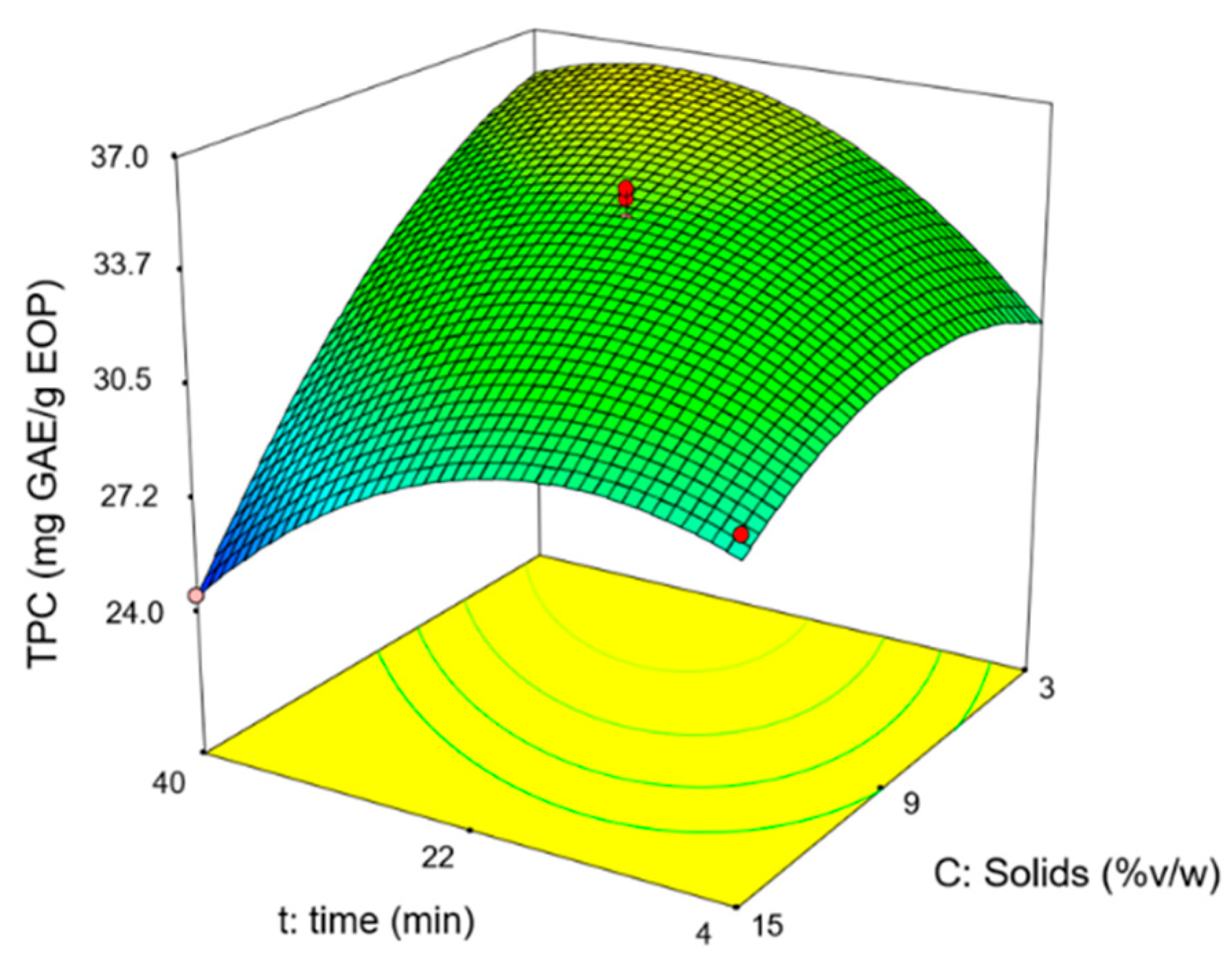
In the experimental design, the values obtained for the total phenol content (TPC) ranged from 25 to 41 mg GAE/g EOP and the hydroxytyrosol content between 4 and 6 mg/g EOP. As an example, Figure 1 shows the response surface for the TPC, where it can be observed that the extraction time and solid loading have a positive influence until a maximum is reached. Alternatively, the temperature was affected positively under the conditions tested. The hydroxytyrosol content showed a similar trend.
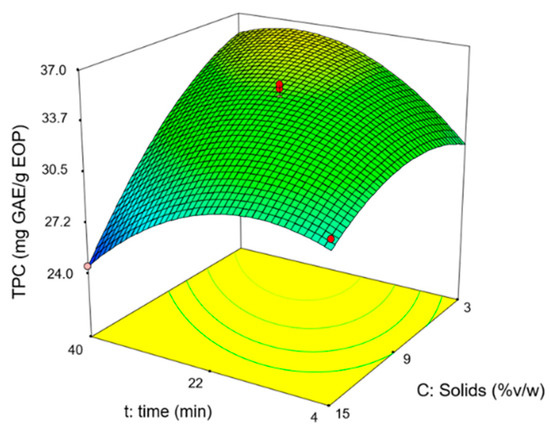
Figure 1.
Response surface for the total phenol content (TPC) at 70 °C.
The antioxidant activity of the aqueous extracts obtained by MAE was determined by the FRAP and ABTS methods. In this case, the three parameters studied also showed a significant influence on these response variables. In general, the solid loading and temperature had the greatest influence on both of them, whose values ranged between 32 and 55 mg TE/g EOP and 61 and 97 mg TE/g EOP.
The quality of fit of the response surface models was assessed by ANOVA. The adjusted coefficient of determination (R2), the coefficient of variation (CV) and the statistical parameters F-value and lack of fit (p-value) were determined for each of the responses. The models developed presented adjusted coefficients of determination (R2 adj) in the range of 0.886–0.964, suggesting that the experimental data matched well with the predicted values. Furthermore, the CV ranged from 1.98 to 3.55%, indicating the reliability and accuracy of the model. ANOVA results showed high F-values for all response variables (21.65–587.22), implying that the model was highly significant and there was no lack of fit.
Once the effect of the three factors (temperature, time, and solid loading) on all responses had been analysed, the software was able to determine the individual optimum conditions for each response. These depended on the response variable and ranged between 99.5 °C (TPC) and 100 °C (FRAP), 22.9 min (FRAP) and 34.3 min (hydroxytyrosol content) and 3% solids (FRAP) and 6.4% solids (ABTS). The predicted optimum values were: 41 mg GAE/g EOP for TPC, 6 mg/g EOP for hydroxytyrosol content, 55 mg TE/g EOP for the antioxidant capacity determined by the FRAP method and 102 mg TE/g EOP by the ABTS method.
The results obtained show that MAE is an efficient technique for the extraction of bioactive compounds from EOP using short extraction times, as other studies suggested for olive pomace [12], and simply using water.
4. Conclusions
MAE, using water as a “green” solvent, was efficient for the extraction of antioxidant compounds from EOP, including hydroxytyrosol. Its content was maximized at 99.7 °C, 34.3 min and a solid loading of 3.9% (w/v): 6 mg/g EOP.
Author Contributions
Conceptualization, I.G.-C., I.R. and M.d.M.C.; methodology, M.d.M.C. and I.G.-C.; software, I.R.; validation, M.d.M.C. and I.G.-C.; investigation, M.d.M.C. and I.G.-C.; writing—original draft preparation, I.G.-C. and M.d.M.C.; writing—review and editing, E.C., I.R. and M.d.M.C.; supervision, E.C. and I.R.; project administration, I.R. and M.d.M.C.; funding acquisition, I.R. and M.d.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Estatal de Investigación (MICINN, Spain) and Fondo Europeo de Desarrollo Regional, reference project ENE2017-85819-C2-1-R. M.D.M.C. would like to express their gratitude to the FEDER UJA project 1260905 funded by “Programa Operativo FEDER 2014-2020” and “Consejería de Economía y Conocimiento de la Junta de Andalucía” and Ramón y Cajal grant (RYC2018-026177-I/AEI/10.13039/501100011033) from the Ministry of Science and Innovation of Spain. I.G.-C. was supported by Universidad de Jaén (research grant R5/04/2017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived biomass as a source of energy and chemicals. Biofuel. Bioprod. Biorefin. 2017, 6, 246–256. [Google Scholar] [CrossRef]
- Del Contreras, M.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Olive pomace-derived biomasses fractionation through a two-step extraction based on the use of ultrasounds: Chemical Characteristics. Foods 2021, 10, 111. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gómez-Cruz, I.; Romero, I.; Gullón, B.; Ruiz, E.; Brnčićc, M.; Castro, E. Ultrasound-assisted extraction as a first step in a biorefinery strategy for valorisation of extracted olive pomace. Energies 2019, 12, 2679. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of exhausted olive pomace by an eco-friendly solvent extraction process of natural antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Domínguez-Rodríguez, G.; Castro-Puyana, M.; Marina, M.L. Polyphenols Analysis and Related Challenges. In Polyphenols: Properties, Recovery and Applications; Galanakis, C.M., Ed.; Elsevier: Helsinki, Finland, 2018; pp. 177–232. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; del Contreras, M.M.; Espínola, F.; Moya, M.; De Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, S. Colorimetric of total phenolics with phosphomolibic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Gómez-Cruz, I.; del Contreras, M.M.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Romero, I.; Castro, E. Recovery of bioactive compounds from industrial exhausted olive pomace through ultrasound-assisted extraction. Biology 2021, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Flórez, N.; Conde, E.; Domínguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive mill and winery wastes as viable sources of bioactive compounds: A study on polyphenols recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).