Physicochemical Properties of Moringa oleifera Leaves Grown in Valencian Community (Spain) †
Abstract
:1. Introduction
2. Materials and Methods
Water Content, Antioxidant Capacity, Optical Properties and Amino Acid Content
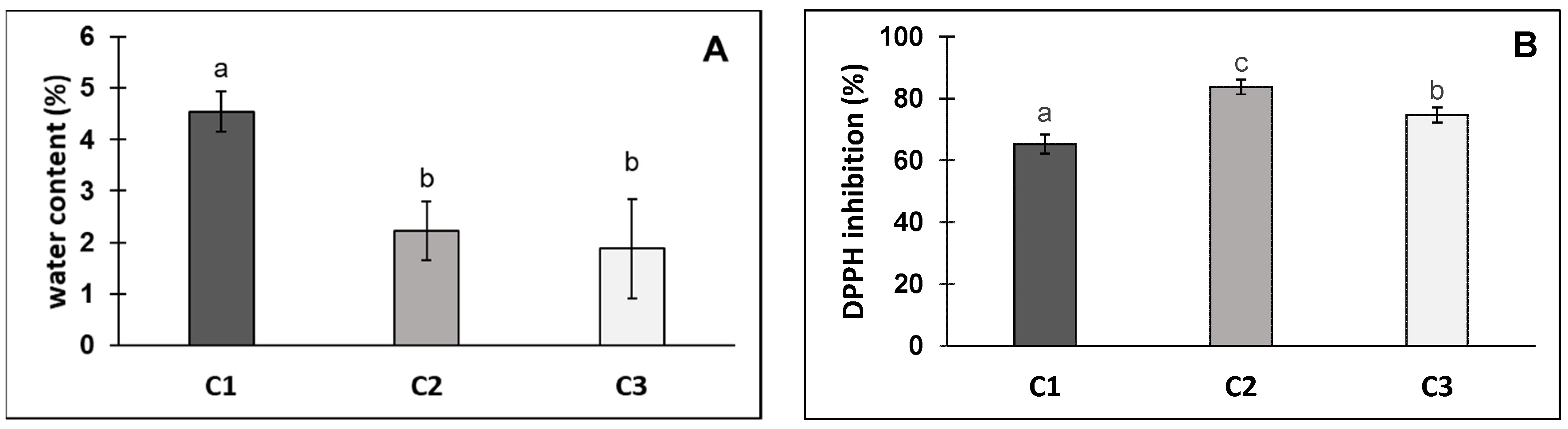
3. Results and Discussion
3.1. Optical Properties
3.2. Amino Acid Content
4. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
References
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Med. Hypotheses 2009, 72, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 2001, 35, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Aderinola, T.A.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Enujiugha, V.N.; Aluko, R.E. In vitro digestibility, structural and functional properties of Moringa oleifera seed proteins. Food Hydrocoll. 2020, 101, 105574. [Google Scholar] [CrossRef]
- Nouman, W.; Anwar, F.; Gull, T.; Newton, A.; Rosa, E.; Domínguez-Perles, R. Profiling of polyphenolics, nutrients and antioxidant potential of germplasm’s leaves from seven cultivars of Moringa oleifera Lam. Ind. Crops Prod. 2016, 83, 166–176. [Google Scholar] [CrossRef]
- Amaglo, N.K.; Bennett, R.N.; Lo Curto, R.B.; Rosa, E.A.S.; Lo Turco, V.; Giuffrida, A.; Lo Curto, A.; Crea, F.; Timpo, G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Periche, A.; Koutsidis, G.; Escriche, I. Composition of antioxidants and amino acids in stevia leaf infusions. Plant Foods Hum. Nutr. 2014, 69, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Gallegos-Corona, M.A.; González de Mejía, E.; Loarca-Piña, G. Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model. Food Res. Int. 2018, 105, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Sledz, M.; Witrowa-Rajchert, D. Influence of microwave-convective drying of chlorophyll content and colour of herbs. Acta Agrophysica 2012, 19, 865–876. [Google Scholar]
- Lalas, S.; Athanasiadis, V.; Karageorgou, I.; Batra, G.; Nanos, G.D.; Makris, D.P. Nutritional characterization of leaves and herbal tea of moringa oleifera cultivated in Greece. J. Herbs Spices Med. Plants 2017, 23, 320–333. [Google Scholar] [CrossRef]
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933. [Google Scholar] [CrossRef] [Green Version]
- Castillo-López, R.I.; León-Félix, J.; Angulo-Escalante, M.Á.; Gutiérrez-Dorado, R.; Muy-Rangel, M.D.; Heredia, J.B. Nutritional and phenolic characterization of moringa Oleifera leaves grown in Sinaloa, México. Pakistan J. Bot. 2017, 49, 161–168. [Google Scholar]
- Natsir, H.; Wahab, A.W.; Budi, P.; Dali, S.; Arif, A.R. Amino acid and mineral composition of moringa oleivera leaves extract and its bioactivity as antioxidant. J. Phys. Conf. Ser. 2019, 1317, 012030. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortolá, M.D.; Castelló, M.L.; Etchevers, M.C.; García-Mares, F.J.; Soriano, M.D. Physicochemical Properties of Moringa oleifera Leaves Grown in Valencian Community (Spain). Biol. Life Sci. Forum 2021, 8, 4. https://doi.org/10.3390/blsf2021008004
Ortolá MD, Castelló ML, Etchevers MC, García-Mares FJ, Soriano MD. Physicochemical Properties of Moringa oleifera Leaves Grown in Valencian Community (Spain). Biology and Life Sciences Forum. 2021; 8(1):4. https://doi.org/10.3390/blsf2021008004
Chicago/Turabian StyleOrtolá, María D., María Luisa Castelló, Maria C. Etchevers, Francisco José García-Mares, and María D. Soriano. 2021. "Physicochemical Properties of Moringa oleifera Leaves Grown in Valencian Community (Spain)" Biology and Life Sciences Forum 8, no. 1: 4. https://doi.org/10.3390/blsf2021008004
APA StyleOrtolá, M. D., Castelló, M. L., Etchevers, M. C., García-Mares, F. J., & Soriano, M. D. (2021). Physicochemical Properties of Moringa oleifera Leaves Grown in Valencian Community (Spain). Biology and Life Sciences Forum, 8(1), 4. https://doi.org/10.3390/blsf2021008004





