Restoration of Arterial Blood Flow Access to Rhomboid Fossa Assists in Left Ventricular Hypertrophy Normalization †
Abstract
:1. Introduction
2. Discussion
3. Conclusions
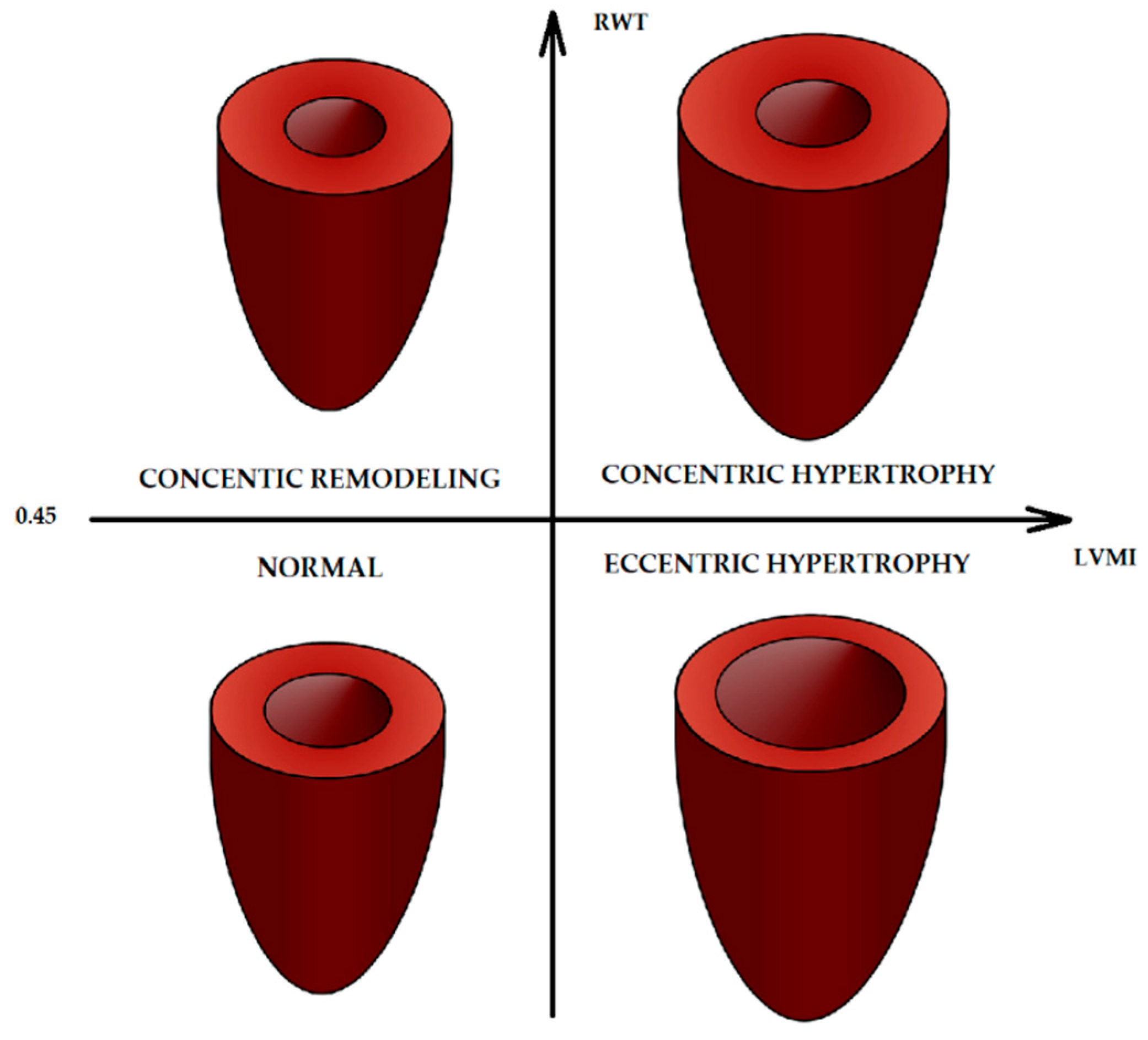
- The development of a mathematical model to determine the correlation between the LVMI and access to information about the flow of blood from the circulatory system to the rhomboid fossa;
- Conducting an experiment on animal model(s) to collect statistical data.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vetcher, A.A.; Zhukov, K.V.; Gasparuan, B.A.; Shishonin, A.Y. The cerebellum role in arterial hypertension. Med. Hypotheses 2022, 162, 110835. [Google Scholar] [CrossRef]
- Zhukov, K.V.; Vetcher, A.A.; Gasparuan, B.A.; Shishonin, A.Y. Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis. Polymers 2021, 13, 4248. [Google Scholar] [CrossRef] [PubMed]
- Vetcher, A.A.; Zhukov, K.V.; Gasparuan, B.A.; Shishonin, A.Y. The Role of Cervical Vertebral Arteries Blood Flow in Centralized Aerobic- Anaerobic Energy Balance Compensation: When Hypothesis Becomes a Theory. Ann. Cardiovasc. Dis. 2021, 5, 1027–1032. [Google Scholar]
- Zhukov, K.V.; Gasparyan, B.A.; Vetcher, A.A.; Shishonin, A.Y. Left ventricular hypertrophy linked with arterial hypertension through centralized aerobicanaerobic energy balance compensation theory. Ann. Clin. Hypertens. 2022, 6, 012–014. [Google Scholar]
- Casals, J.B.; Pieri, N.C.; Feitosa, M.L.; Ercolin, A.; Roballo, K.; Barreto, R.S.; Bressan, F.F.; Martins, D.S.; Miglino, M.A.; Ambrósio, C.E. The use of animal models for stroke research: A review. Comp. Med. 2011, 61, 305–313. [Google Scholar]
- Jia, T.; Wang, C.; Han, Z.; Wang, X.; Ding, M.; Wang, Q. Experimental Rodent Models of Cardiovascular Diseases. Front. Cardiovasc. Med. 2020, 7, 588075. [Google Scholar] [CrossRef]
- Zaragoza, C.; Gomez-Guerrero, C.; Martin-Ventura, J.L.; Blanco-Colio, L.; Lavin, B.; Mallavia, B.; Tarin, C.; Mas, S.; Ortiz, A.; Egido, J. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011, 2011, 497841. [Google Scholar] [CrossRef]
- Leong, X.F.; Ng, C.Y.; Jaarin, K. Animal Models in Cardiovascular Research: Hypertension and Atherosclerosis. BioMed Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef]
- de Simone, G.; Mancusi, C.; Esposito, R.; De Luca, N.; Galderisi, M. Echocardiography in Arterial Hypertension. High Blood Press. Cardiovasc. Prev. 2018, 25, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Finocchi, C.; Sassos, D. Headache and arterial hypertension. Neurol. Sci. 2017, 38 (Suppl. 1), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wermelt, J.A.; Schunkert, H. Management of arterial hypertension. Herz 2017, 42, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Ermoshkin, V.I. Hypothesis of Causeless Hypertension. 2011-07-18. Available online: http://www.medlinks.ru/ (accessed on 1 May 2023). (In Russian).
- Mazensky, D.; Flesarova, S.; Sulla, I. Arterial Blood Supply to the SpinalCord in Animal Models of Spinal CordInjury. A Review. Anat. Rec. 2017, 300, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J. Animal models of stroke. Anim. Model Exp. Med. 2021, 4, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S.; Ahn, C.; Kronzon, I.; Koenigsberg, M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am. J. Cardiol. 1991, 67, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H. The progression of hypertensive heart disease. Circulation 2011, 123, 327–334. [Google Scholar] [CrossRef]
- Ramer, L.M.; Ramer, M.S.; Bradbury, E.J. Restoring function afterspinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014, 13, 1241–1256. [Google Scholar] [CrossRef]
- Courtine, G.; Bunge, M.B.; Fawcett, J.W.; Grossman, R.G.; Kaas, J.H.; Lemon, R.; Maier, I.; Martin, J.; Nudo, R.J.; Ramon-Cueto, A.; et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 2007, 13, 561–566. [Google Scholar] [CrossRef]
- Geissler, S.A.; Schmidt, C.E.; Schallert, T. Rodent models and behavioral outcomes of cervical spinal cord injury. J. Spine 2013, S4, 1–11. [Google Scholar] [CrossRef]
- Simon, F.; Erhart, P.; Vcelar, B.; Scheuerle, A.; Schelzig, H.; Oberhuber, A. Erythropoietin preconditioning improves clinical and histo-logic outcome in an acute spinal cord ischemia and reperfusionrabbit model. J. Vasc. Surg. 2015, 64, 1797–1804. [Google Scholar] [CrossRef]
- Uezu, T.; Koja, K.; Kuniyoshi, Y.; Miyagi, K.; Shimoji, M.; Arakaki, K.; Yamashiro, S.; Mabuni, K.; Senaha, S. Blood distribution tothe anterior spinal artery from each segment of intercostal andlumbar arteries. J. Cardiovasc. Surg. 2003, 44, 637–645. [Google Scholar]
- Singh, V.K.; Thrall, K.D.; Hauer-Jensen, M. Minipigs as models in drug discovery. Expert Opin. Drug Discov. 2016, 11, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- He, Z.B.; Lv, Y.K.; Li, H.; Yao, Q.; Wang, K.M.; Song, X.G.; Wu, Z.J.; Qin, X. Atlantoaxial Misalignment Causes High Blood Pressure in Rats: A Novel Hypertension Model. Biomed Res Int. 2017, 2017, 5986957. [Google Scholar] [CrossRef] [PubMed]
- Maršala, M. Spinal cord blood flow and metabolism in transient spinal ischemia. In Spinal Cord Monitoring; Stålberg, E., Sharma, H.S., Olsson, Y., Eds.; Springer: New York, NY, USA, 1998; pp. 5–25. [Google Scholar]

| Model Animal | Easy to Transfer Results to Human Clinical Situation(s) | The Absence of Reserve Arterial Way to Rhomboid Fossa | Easy to Measure Blood Pressure | Easy to Boost Blood for Biochemical Analysis | Easy to Measure Linear Blood Flow Velocity through Brachycephalic Arteries |
|---|---|---|---|---|---|
| Mice | + | − | + | + | + |
| Rats | − | − | + | + | + |
| Rabbits | + | − | + | + | + |
| Minipigs | + | + | + | + | + |
| Goats | − | − | + | + | + |
| Sheep | − | − | + | + | − |
| Guinea pigs | − | − | + | + | + |
| Cats | + | + | + | + | + |
| Dogs | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukov, K.V.; Dudnik, G.V.; Vetcher, A.A.; Gasparyan, B.A.; Shishonin, A.Y. Restoration of Arterial Blood Flow Access to Rhomboid Fossa Assists in Left Ventricular Hypertrophy Normalization. Med. Sci. Forum 2023, 21, 16. https://doi.org/10.3390/ECB2023-14551
Zhukov KV, Dudnik GV, Vetcher AA, Gasparyan BA, Shishonin AY. Restoration of Arterial Blood Flow Access to Rhomboid Fossa Assists in Left Ventricular Hypertrophy Normalization. Medical Sciences Forum. 2023; 21(1):16. https://doi.org/10.3390/ECB2023-14551
Chicago/Turabian StyleZhukov, Kirill V., Grigorii V. Dudnik, Alexandre A. Vetcher, Bagrat A. Gasparyan, and Alexander Y. Shishonin. 2023. "Restoration of Arterial Blood Flow Access to Rhomboid Fossa Assists in Left Ventricular Hypertrophy Normalization" Medical Sciences Forum 21, no. 1: 16. https://doi.org/10.3390/ECB2023-14551
APA StyleZhukov, K. V., Dudnik, G. V., Vetcher, A. A., Gasparyan, B. A., & Shishonin, A. Y. (2023). Restoration of Arterial Blood Flow Access to Rhomboid Fossa Assists in Left Ventricular Hypertrophy Normalization. Medical Sciences Forum, 21(1), 16. https://doi.org/10.3390/ECB2023-14551








