An In Silico Approach to Evaluate the Diabetic Wound Healing Potential of Phenylethanoid Glycoside in Inhibiting the Receptor for Advanced Glycation End Products (RAGE) †
Abstract
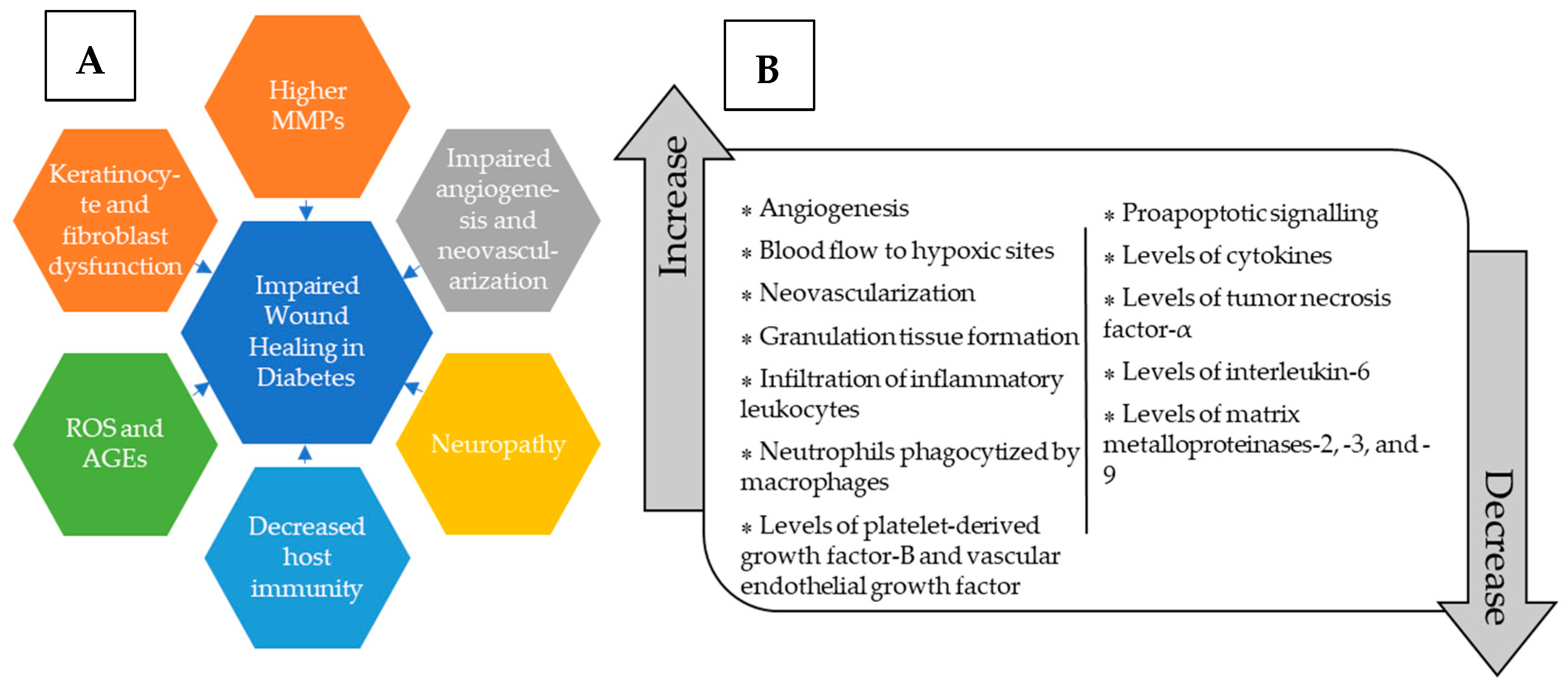
:1. Introduction
2. Materials and Methods
2.1. Protein and Ligand Preparation
2.2. Active Binding Site Selection
2.3. Assessment of Binding Affinities and Interactions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goova, M.T.; Li, J.; Kislinger, T.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Nowygrod, S.; Wolf, B.M.; Caliste, X.; Yan, S.F.; et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001, 159, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, X.; Zhu, G.; Xie, T.; Ge, K.; Niu, Y. Blockade of receptor for advanced glycation end products improved essential response of inflammation in diabetic wound healing. Int. J. Diabetes Dev. Ctries. 2020, 40, 283–289. [Google Scholar] [CrossRef]
- Wang, C.C.; Blomster, J.I.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Fowkes, F.G.; Held, P.; Katona, B.G.; Norgren, L.; Jones, W.S.; et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: The EUCLID trial. J. Am. Coll. Cardiol. 2018, 72, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- Goodson, W.H., III; Hunt, T.K. Studies of wound healing in experimental diabetes mellitus. J. Surg. Res. 1977, 22, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.P. The physiology of wound healing: A summary of normal and abnormal wound healing processes. Adv. Ski. Wound Care 1998, 11, 326–328. [Google Scholar]
- Morain, W.D.; Colen, L.B. Wound healing in diabetes mellitus. Clin. Plast. Surg. 1990, 17, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Koyama, H.; Morioka, T.; Tanaka, S.; Kizu, A.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shioi, A.; Shimogaito, N.; et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes 2006, 55, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.F., Jr.; Hou, F.; Stuart, R.O.; Kay, J.; Boyce, J.; Chertow, G.M.; Schmidt, A.M. β2-Microglobulin modified with advanced glycation end products modulates collagen synthesis by human fibroblasts. Kidney Int. 1998, 53, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, G.; Cao, X.; Dong, J.; Song, F.; Niu, Y. Blocking AGE-RAGE signaling improved functional disorders of macrophages in diabetic wound. J. Diabetes Res. 2017, 2017, 1428537. [Google Scholar] [CrossRef] [PubMed]
- Wicks, K.; Torbica, T.; Mace, K.A. Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2014; Volume 26, pp. 341–353. [Google Scholar]
- Embil, J.M.; Papp, K.; Sibbald, G.; Tousignant, J.; Smiell, J.M.; Wong, B.; Lau, C.Y.; Canadian Becaplermin Study Group. Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: An open-label clinical evaluation of efficacy. Wound Repair Regen. 2000, 8, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, C.; Kämpfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: Prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef] [PubMed]
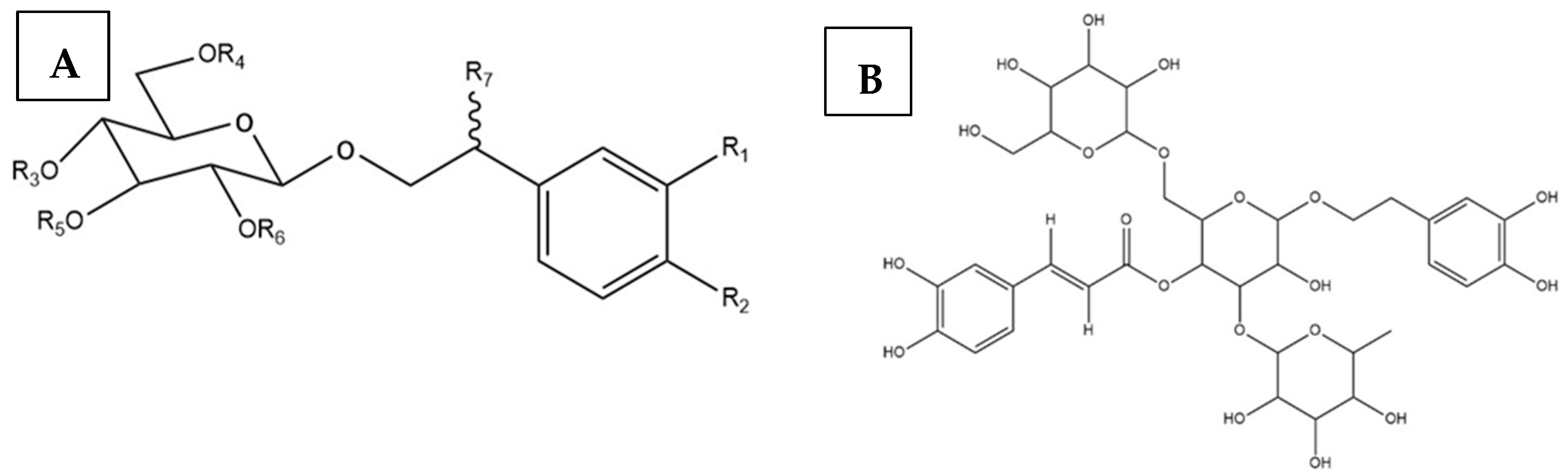
- Tian, X.Y.; Li, M.X.; Lin, T.; Qiu, Y.; Zhu, Y.T.; Li, X.L.; Tao, W.D.; Wang, P.; Ren, X.X.; Chen, L.P. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef] [PubMed]
- Khangholi, S.; Majid, F.A.; Berwary, N.J.; Ahmad, F.; Abd Aziz, R.B. The mechanisms of inhibition of advanced glycation end products formation through polyphenols in hyperglycemic condition. Planta Med. 2016, 82, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, D.; Cui, C.; Hao, J.; Zhang, Z.; Guo, L. Research progress and trends of phenylethanoid glycoside delivery systems. Foods 2022, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gou, C.; Yang, H.; Qiu, J.; Gu, T.; Wen, T. Echinacoside ameliorates D-galactosamine plus lipopolysaccharide-induced acute liver injury in mice via inhibition of apoptosis and inflammation. Scand. J. Gastroenterol. 2014, 49, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Imamura, M.; Akaki, J.; Fujikura, S.; Pan, Y.; Yuan, D.; Yoshikawa, M.; Jia, X.; Li, Z.; et al. Acylated phenylethanoid glycosides, echinacoside and acteoside from Cistanche tubulosa, improve glucose tolerance in mice. J. Nat. Med. 2014, 68, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, H.R.; Wei, C.M.; Luo, X.H.; Sun, M.L.; Yang, Z.Z.; Chen, X.Y.; Wang, H.B. Echinacoside, a phenylethanoid glycoside from Cistanche deserticola, extends lifespan of Caenorhabditis elegans and protects from Aβ-induced toxicity. Biogerontology 2018, 19, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39 (Suppl. 2), W270–W277. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Agrawal, D.K. Therapeutic Potential of Targeting the HMGB1/RAGE Axis in Inflammatory Diseases. Molecules 2022, 27, 7311. [Google Scholar] [CrossRef] [PubMed]
- RCSB PDB. Available online: https://www.rcsb.org/structure/6VXG (accessed on 4 January 2023).
- National Institutes of Health (NIH). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Echinacoside (accessed on 4 January 2023).
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Chem. Biol. Methods Protoc. 2015, 1263, 243–250. [Google Scholar]
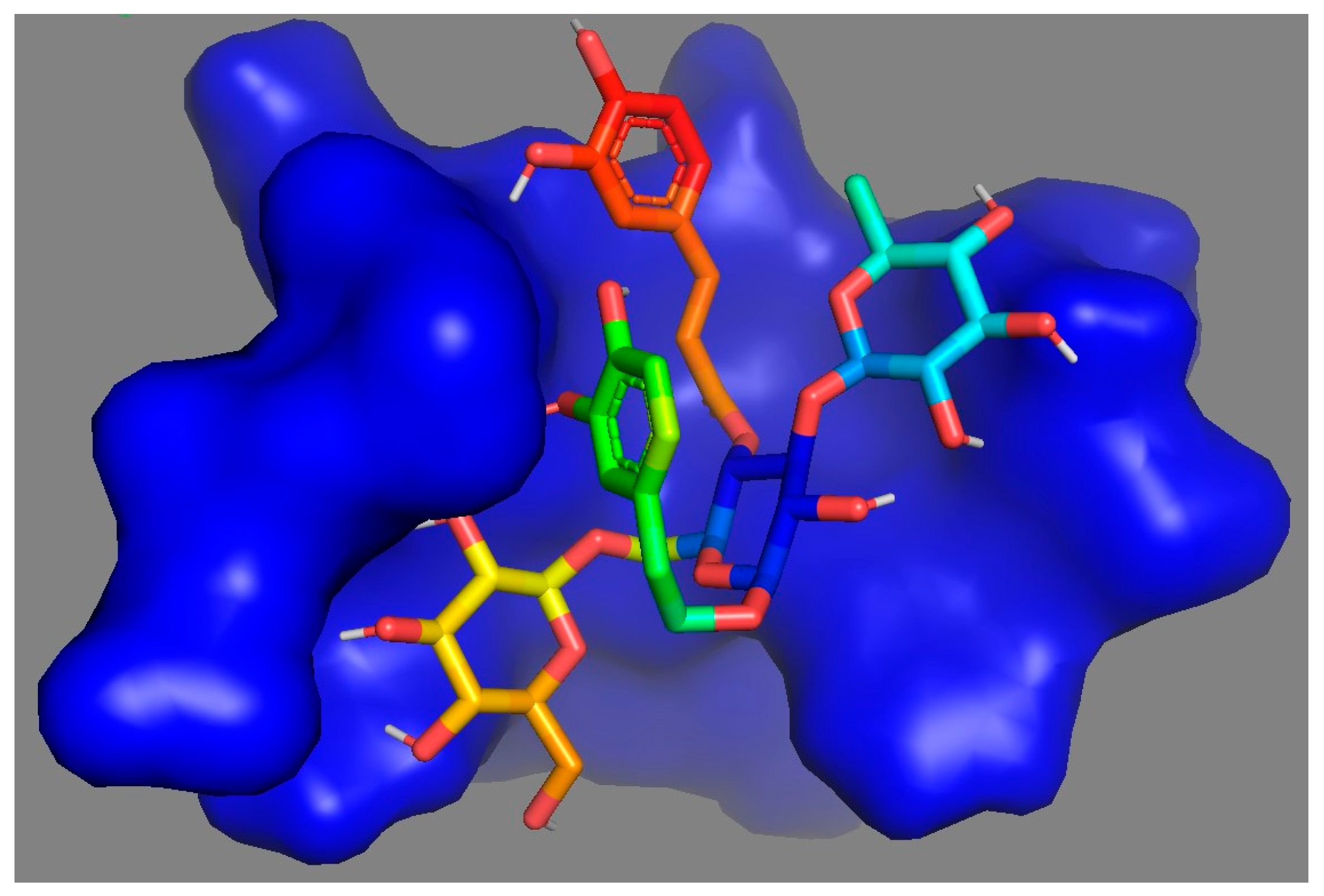
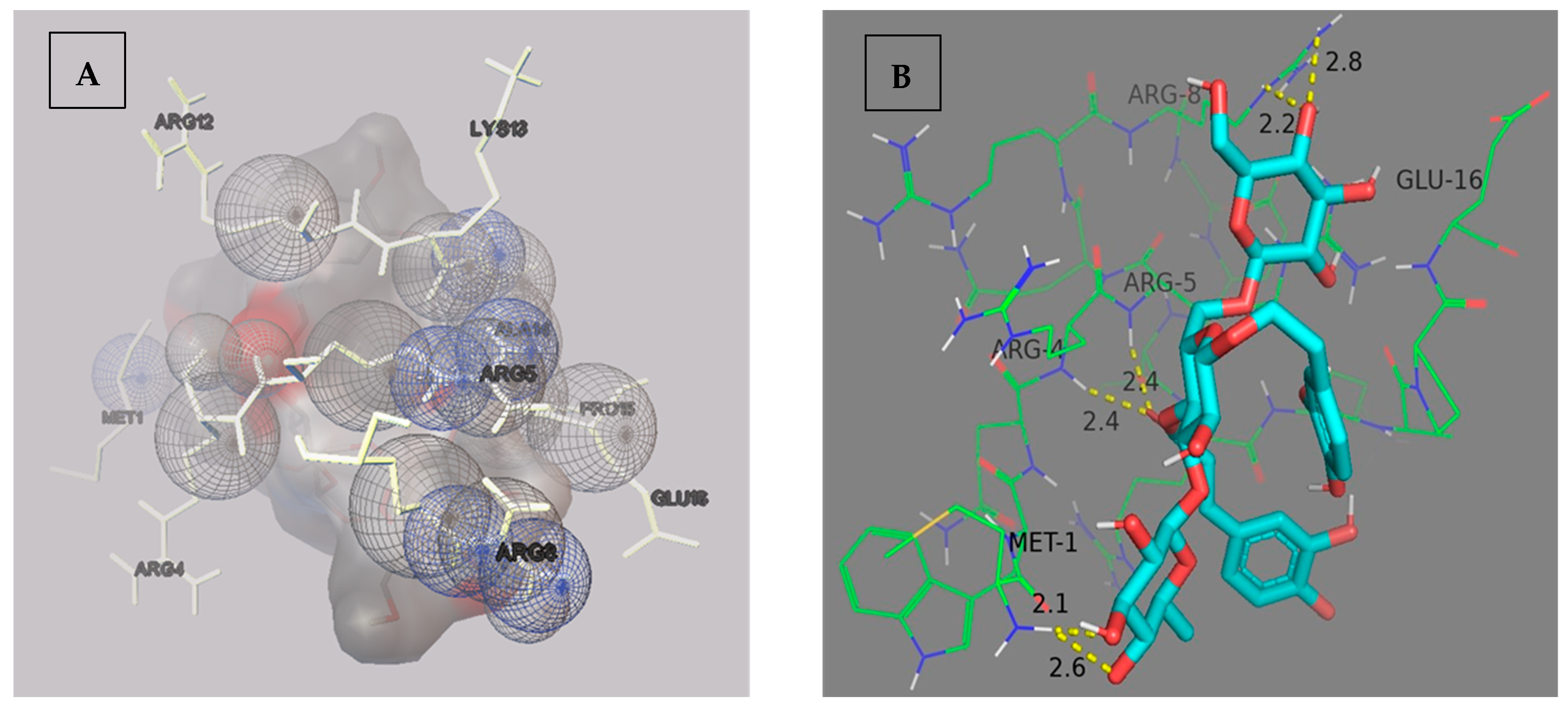
| Docking Poses | Complex | Binding Energy (kcal/mol) | RMSD L.B. | RMSD U.B. |
|---|---|---|---|---|
| 1 | ECH_RAGE | −6.1 | 0.000 | 0.000 |
| 2 | ECH_RAGE | −6.0 | 2.979 | 6.833 |
| 3 | ECH_RAGE | −5.9 | 2.298 | 4.816 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baidya, R.; Sarkar, B. An In Silico Approach to Evaluate the Diabetic Wound Healing Potential of Phenylethanoid Glycoside in Inhibiting the Receptor for Advanced Glycation End Products (RAGE). Med. Sci. Forum 2023, 21, 24. https://doi.org/10.3390/ECB2023-14137
Baidya R, Sarkar B. An In Silico Approach to Evaluate the Diabetic Wound Healing Potential of Phenylethanoid Glycoside in Inhibiting the Receptor for Advanced Glycation End Products (RAGE). Medical Sciences Forum. 2023; 21(1):24. https://doi.org/10.3390/ECB2023-14137
Chicago/Turabian StyleBaidya, Ritika, and Biswatrish Sarkar. 2023. "An In Silico Approach to Evaluate the Diabetic Wound Healing Potential of Phenylethanoid Glycoside in Inhibiting the Receptor for Advanced Glycation End Products (RAGE)" Medical Sciences Forum 21, no. 1: 24. https://doi.org/10.3390/ECB2023-14137
APA StyleBaidya, R., & Sarkar, B. (2023). An In Silico Approach to Evaluate the Diabetic Wound Healing Potential of Phenylethanoid Glycoside in Inhibiting the Receptor for Advanced Glycation End Products (RAGE). Medical Sciences Forum, 21(1), 24. https://doi.org/10.3390/ECB2023-14137







