Abstract
The following study investigated the effects of blue LED light (410–430 nm) on wound healing in mice with superficial and full-thickness skin wounds. The wounds were treated with blue LED light (20.6 J/cm2, 30 s), and biopsies were collected over 6 days. Results showed modulation of cytokine release, increased number of granulocytes and mast cells, increased density of vessels with mast cells expressing platelet-derived growth factor, and improved collagen deposition compared to untreated wounds. The study concluded that blue light leads to faster and more effective wound healing and improved skin morphology.
1. Introduction
Photobiomodulation, also known as low-level light therapy, is a growing field of research that explores the use of light energy to induce therapeutic effects in living tissue. This non-invasive therapy involves applying low-intensity light in the visible to near-infrared range to target specific cells or tissues [1]. Photobiomodulation treats various conditions, including pain, wound healing, and neurodegenerative diseases. It is also being studied for its potential to improve skin health, increase joint mobility, and enhance athletic performance. Photobiomodulation can be delivered using various devices, including light-emitting diodes (LEDs), laser therapy devices, and infrared lamps [2]. The exact mechanisms of photobiomodulation are still not fully understood, but the involvement of the synthesis of adenosine triphosphate (ATP), mitochondria, and action on specific cellular targets appears evident [3]. Current research suggests that the absorption of light energy by specific cellular components triggers various biological responses [4]. Photobiomodulation has been shown to activate several key cellular processes, including (i) modulation of energy production, where light energy is absorbed by cellular mitochondria, leading to an increase in ATP synthesis; (ii) reduced oxidative stress by increasing the antioxidants and reducing the production of reactive oxygen species (ROS); (iii) stimulated cell proliferation and promotion of tissue repair, making it practical for treating conditions such as wounds and neurodegenerative diseases [5].
In our previous research, we studied the effects of blue light in acute wounds 24 h after the treatment. Our results showed that treated wounds (TW) had an increase in inflammatory cells, starting 1 h after injury until 6 h later. Granulocytes, mast cells (MC), and endothelial cells showed an increase in the timeframe between 6 and 9 h in TW, while in untreated wounds (UTW), the increase occurred later (12 to 24 h). Additionally, the number of M2 macrophages increased earlier in TW. Dendritic cells (DC) played a crucial role in the organization of the inflammatory infiltrate and increased after 6 h, decreasing after 24 h in NTW. The number of M2 macrophages was almost constant in TW. Plasmacytoid cells (pDC) modulate Treg lymphocytes, which are involved in tolerance and suppressing excessive immune responses [6,7]. We found that pDC cells increased at 9 h in TW but remained unchanged in NTW. However, TW and NTW showed a significant decrease in pDC cells 12 h after injury [8]. In another study, 6 days after treatment with blue light, MC led to a well-coordinated cellular response, including an early inflammatory reaction, angiogenesis due to Tumor Necrosis Factor–alpha (TNF-α) secretion [9], and fibroblasts activation [10].
Here, we started studying the effect of blue LED light on full-thickness wounds, which represents a model of chronic wounds. Numerous clinical data support the hypothesis that chronic wounds manifest an excessive inflammatory phase, with high levels of inflammatory infiltrate cells, such as neutrophils and macrophages. The inflammatory environment stimulates cytokines, ROS, and proteases and inhibits factors such as Vascular Endothelial Growth Factor (VEGF) and basic Fibroblasts Growth Factor (bFGF). The consequences lead to a degradation of the extracellular matrix (ECM) and, therefore, the impossibility of progression in the subsequent stages of healing, proliferation, and remodeling [11]. Based on our previous findings regarding the modulation of the inflammatory phase in acute wounds [10,12], here we analyzed cytokine release and growth factors in chronic wounds.
2. Materials and Methods
2.1. Animal Model
Sixty-three CD1 male mice (15 and 20 g, Envigo, Udine, Italy) were used. The animals were fed with a standard pellet diet and housed in static filter top cages under a 12 h light/dark cycle and controlled temperature (24 °C). The experiments were carried out at the Centre for Laboratory Animal Housing and Experimentation, University of Florence, Italy. In each mouse, one or two full-thickness wounds were performed using a biopsy punch (4 mm in diameter). Irradiation with blue LED light was performed immediately after wound induction: one treatment was performed per animal (410 to 430 nm, 20.6 J/cm2, 30 s treatment time). At 1–3–6–9–24 h and finally 7 days after the treatment, the animals were sacrificed by CO2 inhalation. All experimental procedures were performed in accordance with the European Community guidelines for animal care (86/609/EEC), and written consent was duly obtained from the Italian Ministry of Health (791/2016-PR).
2.2. Samples Preparation for Multiparametric ELISA Tests
After tissue excision, samples were dissolved with a manual homogenizer. The protein content was assessed by comparison with the albumin calibration curve obtained by Bicinchoninic acidic (BCA protein assay, Sigma-Aldrich, Milan, Italy). The samples were then diluted with radioimmunoprecipitation assay buffer (RIPA buffer) as prepared: 10 mM Tris-HCl (tris(hydroxymethyl)aminomethane), 1 Mm EDTA (Ethylenediaminetetraacetic acid), 0.5 mM EGTA (egtazic acid), 1% Triton X-100, 0.1% SDS, and 140 mM NaCl (Sodium Chloride). The RIPA buffer was used to obtain a different total protein content. The absorbance at 570 nm was read using a reference wavelength at 630 nm and was evaluated using an automatic microplate absorbance reader (LT-4000 Labtech, Heathfield, East Sussex, UK). Samples were then placed in glass slides and sent to Raybiotech (Peachtree Corners, GA, USA) service for multiparametric ELISA essay. Results were analyzed for means and SD; the Kolmogorov–Smirnov test was then performed.
3. Results
Modulation of Cytokines
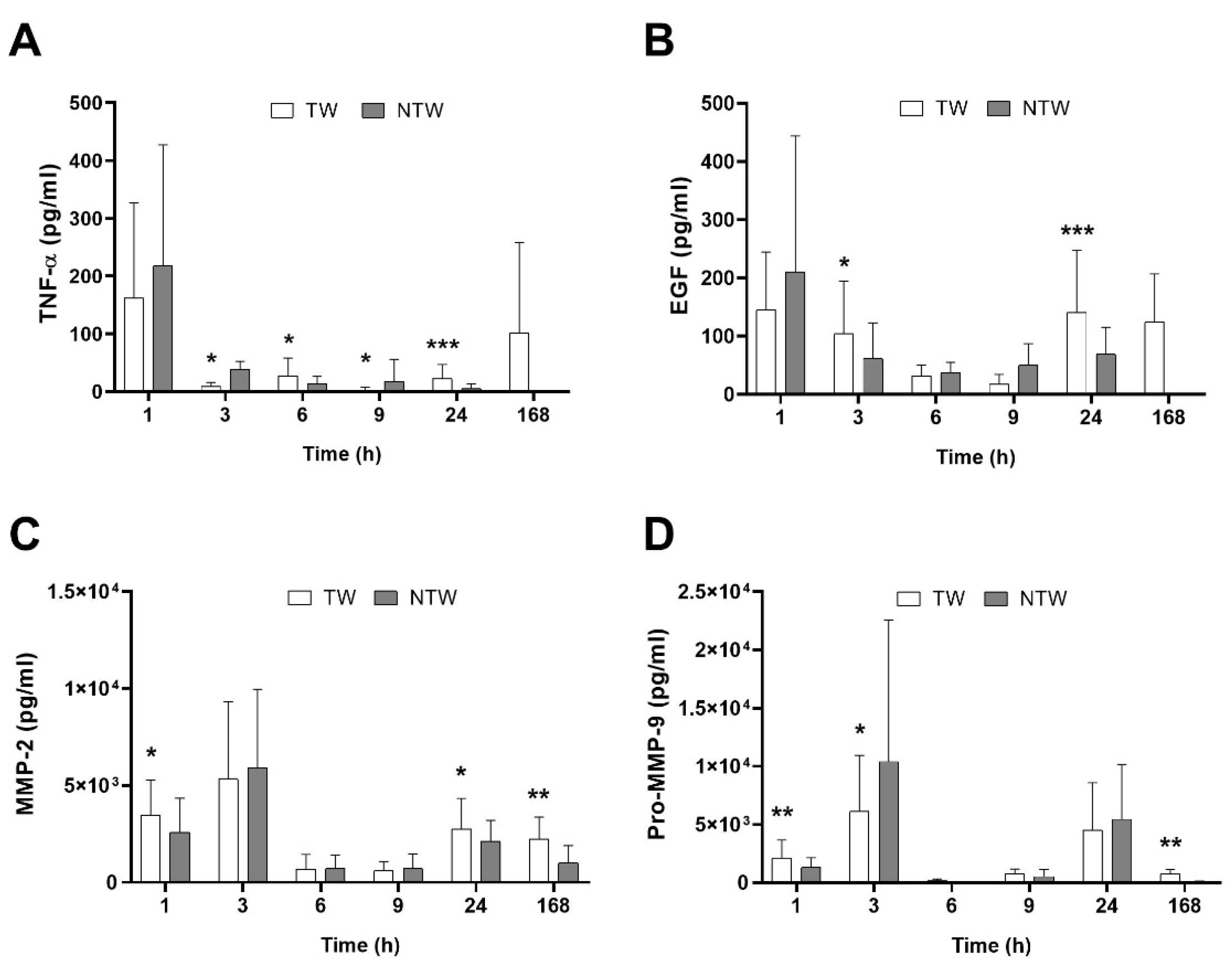
ELISA analysis revealed a differential modulation of signal molecules involved in the inflammatory pathways underlying the healing process. Figure 1A,B shows an overall increase in TNF-α and Epidermal Growth Factor (EGF) in TW, except for the first hour after wound induction. At the same time, Matrix MetalloProteinase-2 (MMP-2) and pro-Matrix MetalloProteinase-9 (pro-MMP-9) (Figure 1C,D) remained constant or slightly augmented.
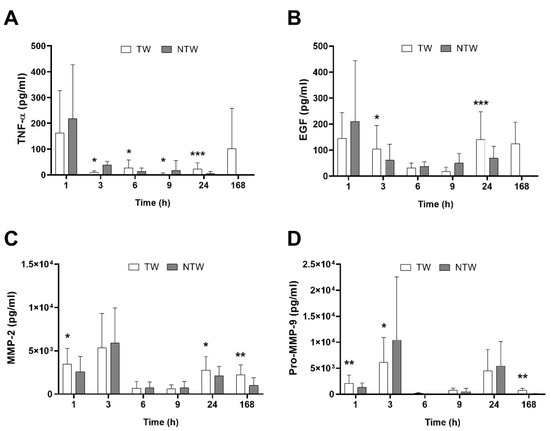
Figure 1.
ELISA test results in samples from TW and NTW in a mice model: TNF-α (A), EGF (B), MMP-2 (C), and pro-MMP-9 (D) trends. Data are expressed as mean ± SD. Significant p-values: * p < 0.1; ** p < 0.05; *** p < 0.01 vs. NTW at the same time point.
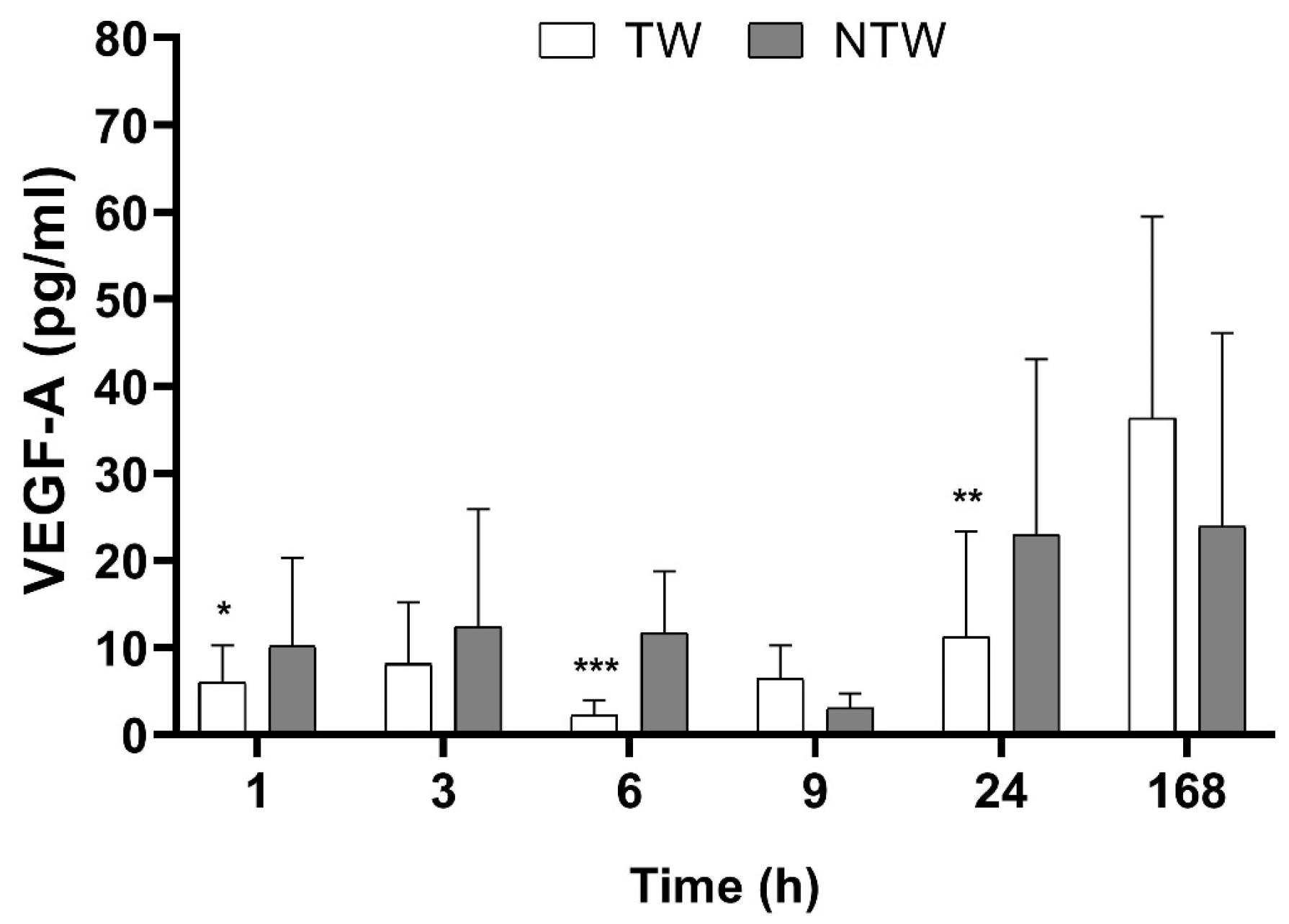
During the first 24 h after the treatment, we observed a modulation of the Vascular Endothelial Growth Factor-A isoform (VEGF-A) level, as shown in Figure 2. During the first hours after the wound was created, the VEGF-A level seemed not to be affected by the treatment, but 9 h later, an increase was observed.
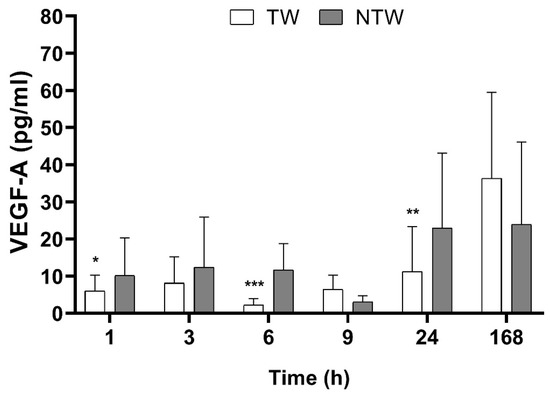
Figure 2.
ELISA test results in samples from TW and NTW in a mice model: VEGF-A levels over time. Significant p-values for VEGF-A data were: p < 0.1 at 6 h and 9 h, p < 0.05 at 24 h. Data are expressed as mean ± SD. Significant p-values: * p < 0.1; ** p < 0.05; *** p < 0.01 vs. NTW at the same time point.
4. Discussion
Wound healing is a complex and dynamic process. A variety of cell types are involved, orchestrated by several growth factors and cytokines. Blue LED light is successfully used in wound healing, notwithstanding its mechanism of action is still unclear. In our previous work, we demonstrated the beneficial effects of blue LED light irradiation in the healing of superficial abrasions in animal models [10,12,13]. In particular, we studied cell-inflammatory infiltration and new tissue formation. Here, our attention was focused on specific cytokines and growth factors involved in the healing process in chronic wounds.
The ELISA assay showed that blue LED light also affects pro-MMP-9 and MMP-2. We pointed out that in the TW, both markers quickly increased significantly after wound induction (1 h) while reversing at 3 h (significantly only for pro-MMP-9), showing a significant increase in NTW. After 24 h, only MMP-2 appeared to significantly increase in the TW, while after 7 days, both markers were significantly expressed in the TW when compared to the NTW. The involvement of MMP-2 and MMP-9 in wound healing has been demonstrated [14]. MMP-2 was expressed at the edge of the acute wounds. Moreover, it correlated with the appearance of laminin-332 and the increase in keratinocyte migration [15]. Furthermore, MMP-9 knockout mice displayed a delay in wound closure [16], demonstrating that MMP-9 is necessary for the final phase of the wound healing process. According to the literature, our findings indicate that both pro-MMP-9 and MMP-2 levels were increased at the initial and final stage of the wound healing process. We suppose that at early times after wound induction, the blue LED light induced an increase in matrix degradation to prepare the wound bed for new collagen deposition. In contrast, at a later time in the healing process, this increase became an indicator of the angiogenetic process and the closure of the wound. Both MMP-2 and MMP-9 play a role in regulating angiogenesis during wound healing through the activation of proangiogenic cytokines, including TNF-α [17,18,19]. TNF-α is a cytokine involved in acute inflammation, known to be produced by several immune cells, primarily by macrophages, but also by neutrophils and mast cells. As demonstrated in our previous work, an increase in the TNF-α level in treated tissues indicates the activation of inflammatory cells [12]. Surprisingly, we found that blue LED light reduces the levels of VEGF-A in chronic wounds. We suppose these effects may be linked to accelerating inflammation in TW compared to NTW and different time courses of these growth factors during our observations. VEGF affects blood vessel formation and might be enhanced by the treatment, consistently observing a better skin morphology in the later phases [10,13]. Obviously, the considerations made are only preliminary. Experiments relating to the responses of individual cellular components of the cellular infiltrate and angiogenesis are currently being carried out in the laboratory. Globally, no evidence was pointed out in these experiments concerning the cross-talk between TW and NTW in the same animal.
The beneficial effects of blue light, especially in dermatology and wound healing, are indisputable. However, further experiments are necessary to better understand the mechanism behind the light action. The proposed method might provide a helpful approach to skin wound management.
Author Contributions
Conceptualization, F.R., S.B., F.T., and F.S.P.; methodology, S.B., F.T., G.d.S., and R.C.; validation, G.M. and F.T.; formal analysis, M.R.; investigation, S.B., F.T., G.M., and F.R.; resources, F.R. and S.B.; data curation, F.R. and G.M.; writing—original draft preparation, G.M.; writing—review and editing, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EmoLED s.r.l, Fondazione Cassa di Risparmio (grant n. 2017/0771), and the University of Florence.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Italian Ministry of Health (protocol code 791/2016-PR).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available to the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. Light Emitting Diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Bikmulina, P.; Kosheleva, N.; Shpichka, A.; Yusupov, V.; Gogvadze, V.; Rochev, Y.; Timashev, P. Photobiomodulation in 3D tissue engineering. J. Biomed. Opt. 2022, 27, 90901–90902. [Google Scholar] [CrossRef]
- Bathini, M.; Raghushaker, C.R.; Mahato, K.K. The Molecular Mechanisms of Action of Photobiomodulation Against Neurodegenerative Diseases: A Systematic Review. Cell. Mol. Neurobiol. 2022, 42, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Trinchieri, G.; Liu, Y.J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004 512 2004, 5, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Rossi, F.; Tatini, F.; Cicchi, R.; Cavigli, L.; Bacci, S.; Alfieri, D.; Tripodi, C.; Targetti, L.; De Siena, G.; et al. Blue LED treatment of superficial abrasions: In vivo experimental evidence of wound healing improvement. In Biophotonics: Photonic Solutions for Better Health Care VI; SPIE: Bellingham, WA, USA, 2018; Volume 10685, pp. 54–59. [Google Scholar] [CrossRef]
- Wang, R.; Yin, X.; Zhang, H.; Wang, J.; Chen, L.; Chen, J.; Han, X.; Xiang, Z.; Li, D. Effects of a Moderately Lower Temperature on the Proliferation and Degranulation of Rat Mast Cells. J. Immunol. Res. 2016, 2016, 8439594. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Tatini, F.; Siena, G.D.; Pavone, F.S.; Alfieri, D.; Cicchi, R.; Rossi, M.; Murciano, N.; Paroli, G.; Vannucci, C.; et al. Blue-LED-Light Photobiomodulation of Inflammatory Responses and New Tissue Formation in Mouse-Skin Wounds. Life 2022, 12, 1564. [Google Scholar] [CrossRef] [PubMed]
- Remoué, N.; Bonod, C.; Fromy, B.; Sigaudo-Roussel, D. Animal models in chronic wound healing research: For innovations and emerging technologies in wound care. In Innovations and Emerging Technologies in Wound Care; Academic Press: Cambridge, MA, USA, 2020; pp. 197–224. [Google Scholar] [CrossRef]
- Magni, G.; Tatini, F.; Bacci, S.; Paroli, G.; De Siena, G.; Cicchi, R.; Pavone, F.S.; Pini, R.; Rossi, F. Blue LED light modulates inflammatory infiltrate and improves the healing of superficial wounds. Photodermatol. Photoimmunol. Photomed. 2020, 36, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, R.; Rossi, F.; Alfieri, D.; Bacci, S.; Tatini, F.; De Siena, G.; Paroli, G.; Pini, R.; Pavone, F.S. Observation of an improved healing process in superficial skin wounds after irradiation with a blue-LED haemostatic device. J. Biophotonics 2016, 9, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K. The role of nutrition in the management of lower extremity wounds. Int. J. Low. Extrem. Wounds 2005, 4, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, G.P.; Robson, M.C.; Liu, R.; Kuhn, M.A.; Muir, D.F.; Schultz, G.S. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002, 10, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Butte, A.J.; Dzau, V.J.; Glueck, S.B. Further defining housekeeping, or “maintenance,” genes Focus on “A compendium of gene expression in normal human tissues”. Physiol. Genom. 2001, 7, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Lupše, B.; Maedler, K.; Sarma, B.; Radtke, A.; Belge, G.; Dorsch, M.; Wedekind, D.; McCawley, L.J.; Boehm, G.; et al. Matrix metalloproteinase-3 is key effector of TNF-α-induced collagen degradation in skin. Int. J. Mol. Sci. 2019, 20, 5234. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.H.; Pu, C.M.; Liu, C.W.; Chen, Y.C.; Chen, Y.C.; Liang, C.J.; Hsieh, J.H.; Huang, H.F.; Chen, Y.L. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9, α-SMA, and collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Frankova, J.; Diamantova, D.; Vrbkova, J.; Ulrichova, J. Influence of hydrogencalcium salts of oxidized cellulose on MMP-2, MMP-9 and TNF-α production and wound healing in non-healing wounds. Acta Dermatovenerol. Croat. 2013, 4, 219–223. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).