Dietary Supplements and the Gut–Brain Axis: A Focus on Lemon, Glycerin, and Their Combinations
Abstract
1. Introduction
2. Dietary Supplements and Their Relationship with the Gut–Brain Axis
3. Lemon Components and Their Relationship with the Gut–Brain Axis
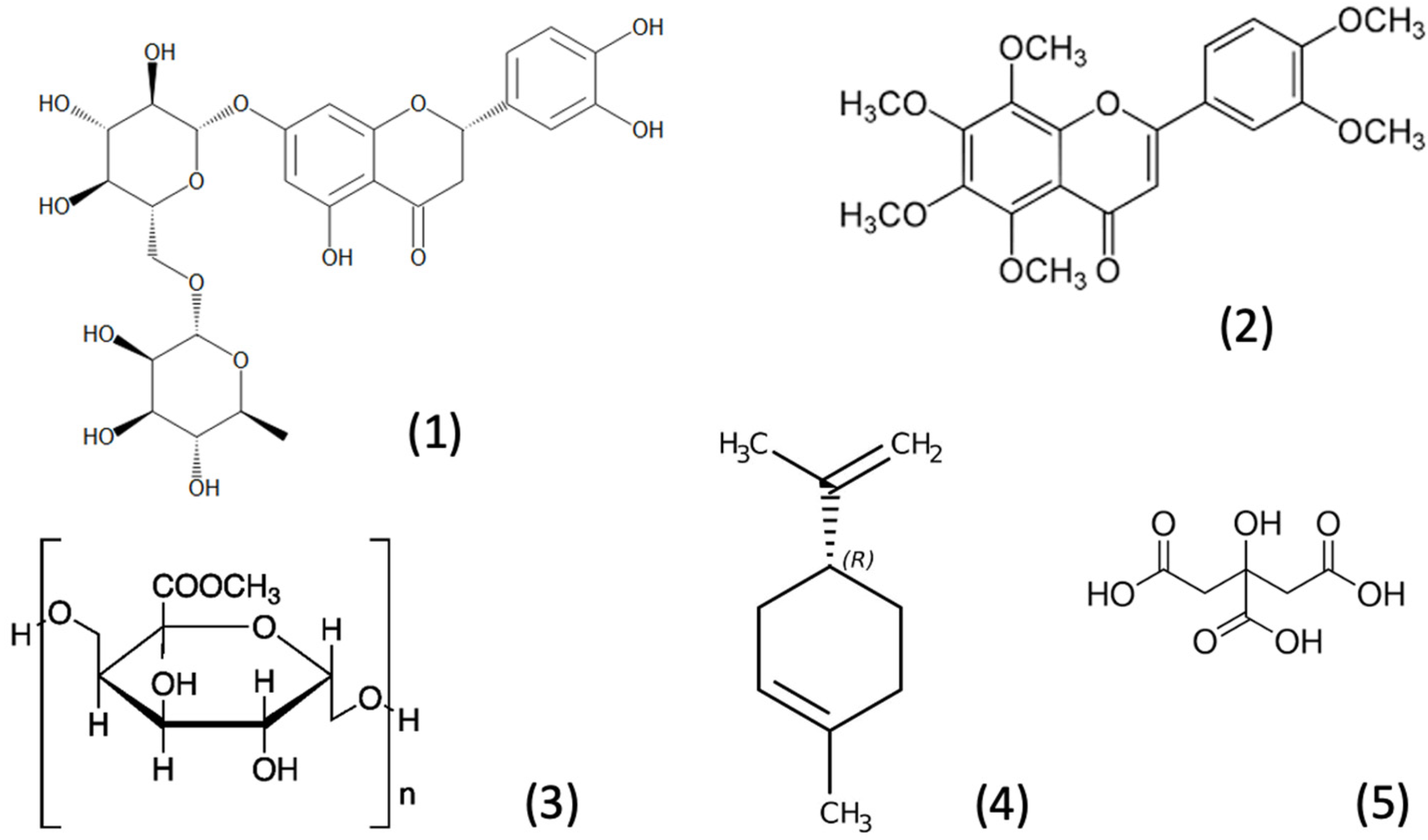
3.1. Nutrients and Bioactive Components of Lemon
3.2. Neurological Effects of Lemon Extracts/Components and Their Relationship with the Gut-Brain Axis
| Lemon Components | Model/Diseases | Model/Population | Exposure Window/Period | Dose/Concentration | Routes of Administration | Diet/Medium | Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Lemon extract | Type 1 diabetes | Adult male NOD mice | 6 months | Not known | Oral gavage | The 5053-PicoLab® Rodent Diet 20 | Decrease in the Verrucomicrobia, Cyanobacteria and TM7 phyla, the RF39 and YS2 orders, F16 and Clostridiaceae families, and the muciniphila species; and an increase in the rc4-4 genus; predicted to decrease both propanoate metabolism and glycolysis/gluconeogenesis | [14] |
| Pectin-derived oligosaccharides | Continuous colon model (TIM-2) | Elderly Donors/GMB | 3 days | 6.5 g per day | In vitro | Standard ileal efflux medium (SIEM) | Increases in beneficial species such as Faecalibacterium prausnitzii and alpha diversity and SCFAs, but not butyrate | [29] |
| Pectin (galacturonide oligosaccharides DP4 and DP5) | In vitro cell-based assays | Eubacterium eligens and Faecalibacterium prausnitzii strains | 24 h | 0.2% | In vitro | M2GSC medium | Promotes the production of the anti-inflammatory cytokine IL-10 | [36] |
| Extracts from citrus fiber | Tolerance to different pH values and bile salts | L. paracasei, L. fermentum, L. rhamnosus and B. animalis subsp. paracasei | 0–24 h | 10 g/L | In vitro | MRS broth | Showed great prebiotic activities | [37] |
| Citrus Pectin Enzyme Hydrolysate | Probiotics | B. bifidum and L. acidophilus cultures | 6, 12, 24, 48 h | 1%, 2%, 4% | Supplemented into growth media | Glucose-free MRS broth | Higher populations of B. bifidum and L. acidophilus | [39] |
| Eriocitrin | Metabolism | Six-week-old male ICR mice | 2 weeks | 100 mg/kg | Oral gavage | Standard chow | Altered the beta diversity; the probiotics such as Lachnospiraceae_UCG_006 were enriched, and the production of butyrate, valerate and hexanoate were increased | [43] |
| Lemon-derived exosome-like nanoparticles | Toleration to bile | C57BL/6J mice | Not given | Not given | Oral gavage | Not given | Lactobacillus rhamnosus GG was increased while the S24-7 was decreased | [44] |
| Citrus extract rich in citrus flavonoids | In vitro model of the colon (TIM-2) | Fecal samples with metabolic syndrome | 3 days | 500 mg/day | In vitro | Standard ileal efflux medium (SIEM) | Increased production of butyrate, acetate, and valerate | [49] |
| Citrus extract rich in citrus flavonoids | Volunteers with metabolic syndrome | Fecal samples | 12 weeks | 500 mg/day | In vivo/Oral | Habitual diet without foods high in citrus flavonoids | Increased SCFAs with significantly more butyrate. A trend towards a reduction in intestinal inflammation (calprotectin) | [49] |
| Citrus Fruit Extract (88.2% hesperidin and 6.5% naringin) | In vitro model of the colon (TIM-2) | Fecal samples of healthy volunteers | 3 days | 250 or 350 mg/day | In vitro | Standard ileal efflux medium (SIEM) | Increased Roseburia, Eubacterium ramulus and Bacteroides eggerthii. Increased acetate while reduced butyrate | [50] |
| Citrus limon peel (LP) powder rich in dietary fibers | Dextran sulfate sodium (DSS)-induced colitis | 7 weeks old male BALB/c mice | 16 days | 5% by diet weight | Oral/diet | AIN-93G-based diet | LP powder increased levels of acetate and n-butyrate, reduced colitis, restored normal colon length and reduced intestinal damage | [51] |
| Lemon fermented with Lactobacillus OPC1 | Obesity | Male Wistar rats | 9 weeks | 2.89 g/kg | Oral gavage | High calorie diet | Reduced the ratio of Firmicutes/Bacteroidetes and increased the abundance of Firmicutes Clostridia, decreased content of acetic acid and propionic acid. | [52] |
| Four types of pectins | Fermentation patterns | Male Wistar rats | 7 weeks | 3% by diet weight | Oral/diet | The control diet RMH-B ± pectins | Low methyl esterified citrus pectin increased the production of total SCFAs, propionate and butyrate | [53] |
| Nine structurally diverse pectins | TIM-2 colon model | Fecal samples of healthy volunteers | 24, 48, 56, and 72 h | 7.5 g pectin per day | In vitro fermentation | Simulated ileal efflux medium (SIEM) | Cumulative production of the total short chain fatty acids and propionate was largest in fermentations of the high methoxyl pectins | [54] |
| Rhamnogalacturonan-I (RG-I)-enriched pectin | Gut microbiota | C57BL/6J male mice | 9 weeks | 100 mg/kg | Oral gavage | Standard chow diet | Increased the abundance of prebiotics such as Bifidobacterium spp., Lactobacillus spp., and increased SCFA producers including species in Ruminococcaceae family | [55] |
3.3. Other Effects of Lemon Extracts/Components and Their Side Effects
4. Glycerin and Its Relationship with the Gut–Brain Axis
4.1. Glycerol Metabolism
4.2. Neurological Effects of Glycerin and Its Relationship with the Gut-Brain Axis
| Products | Model/Diseases | Model/Population | Exposure Window/Time | Dose/Concentration | Routes of Administration | Diet/Medium | Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Vegetable glycerin | Type 1 diabetes | Adult male NOD mice | 6 months | Not known | Oral gavage | The 5053-PicoLab® Rodent Diet 20 | Acetate was increased in the fecal Samples; decreased Peptostreptococcaceae, Turicibacter and Coprobacillus genera, and the prausnitzii species | [14] |
| Glycerol | In vitro batch incubations | Fecal samples from 10 individuals | 3 weeks | 140 mM | Supplemented into growth media | Standard culture medium | Altered GMB metabolism and composition. Higher levels of acetate and 1,3-propanediol, and more Lactobacillus-Enterococcus in fast conversion samples | [80] |
| Glycerol | In vitro colonic fermentation model | Adult human immobilized fecal microbiota | 12–24 h | 100 mM | Supplemented into growth media | Standard culture medium | Glycerol increased numbers of Lactobacillus-Enterococcus group and decreased Escherichia coli. In combination with L. Reuteri, glycerol decreased E. coli populations | [82] |
| Glycerol/ Human-derived L. reuteri | Clostridium difficile infection | Human fecal microbial communities | 8–72 h | 2%–10% glycerol | Supplemented into growth media | MRS medium; brain heart infusion (BHI) medium | Codelivery of L. reuteri with glycerol was effective against C. difficile colonization | [83] |
| Glycerol | PolyFermS chicken cecal microbiota models | Immobilized cecal microbiota from broiler chickens | 6–8 days | 50 and 100 mM | Supplemented into growth media | Custom nutritive medium | Increases in butyrate production, reduction in Enterobacteriaceae, and 1,3-propanediol accumulation | [86] |
| Glycerin-containing vitamin D liquid formulations | Fecal microbiota and their metabolites | Infants at 3 months of age | 3 months | Not known | Oral | Infant supplementations were administered to mothers | Fecal 1,2-propanediol and glycerol concentrations were negatively correlated. Positive correlations between fecal 1,2-PD, Bifidobacteriaceae, Lactobacillaceae, Enterobacteriaceae and acetate levels were observed. | [87] |
| Glycerol | Metabolic potential | B. schinkii, the genus Blautia | 15–30 h | 20 mM | Supplemented into growth media | CO2/KHCO3-buffered complex medium | Producing acetate and ethanol | [88] |
| Glycerol | Bovine rumen fluid | 24 h | 25 mM | Supplemented into growth media | Fermentation medium | Acetate, propionate, butyrate, valerate, and caproate concentrations, in decreasing order, all increased with incubation time | [89] | |
| Glycerol | In vitro fermentation | Pig fecal inoculum | 24 and 36 h | 10% | Supplemented into growth media | Normal or modified medium | Increased the abundances of Firmicutes, Anaerovibrio, unclassified_f_Selenomonadaceae, and decreased that of Proteobacteria; decreased the acetate production and increased butyrate | [98] |
4.3. Other Effects of Glycerin and Side Effects
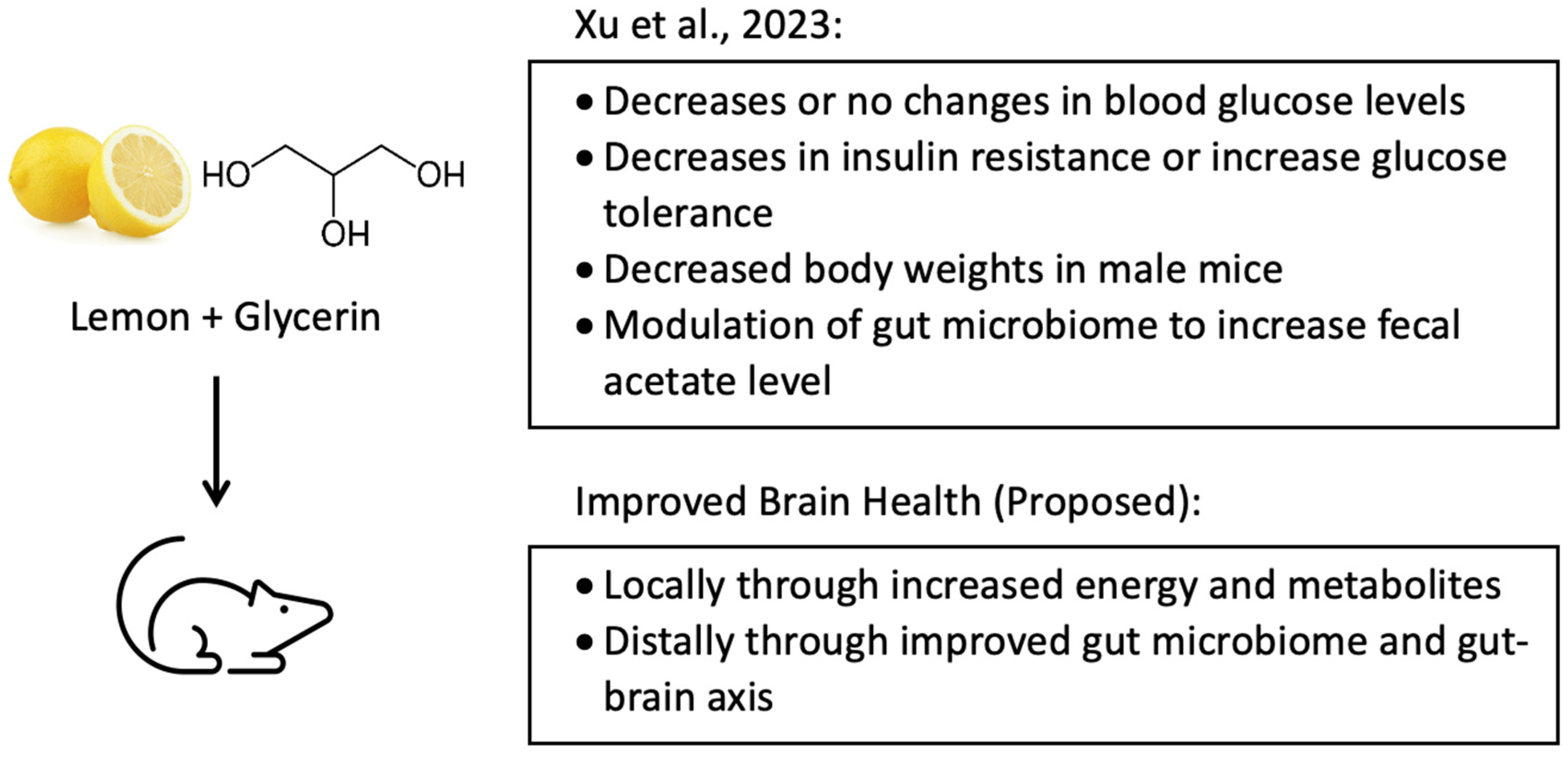
5. The Combination of Lemon and Glycerin and Its Relationship with the Gut–Brain Axis
6. Conclusion and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Schaafsma, A.; Mallee, L.; Van Den Belt, M.; Floris, E.; Kortman, G.; Veldman, J.; Van Den Ende, D.; Kardinaal, A. The Effect of A Whey-Protein and Galacto-Oligosaccharides Based Product on Parameters of Sleep Quality, Stress, and Gut Microbiota in Apparently Healthy Adults with Moderate Sleep Disturbances: A Randomized Controlled Cross-Over Study. Nutrients 2021, 13, 2204. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Meighani, A.; Hart, B.R.; Mittal, C.; Miller, N.; John, A.; Ramesh, M. Predictors of fecal transplant failure. Eur. J. Gastroenterol. Hepatol. 2016, 28, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Bussell, J.K. Medication adherence: WHO cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Teng, Q.; Patel, A.; McDonough, C.; Guo, T.L. Combining Lemon and Glycerin may Beneficially Regulate Blood Glucose Levels by Modulating Gut Microbiota in Non-obese Diabetic Mice. Int. J. Diabetes Manag. 2023, 2, 44–65. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef]
- Brescia, P.; Rescigno, M. The gut vascular barrier: A new player in the gut–liver–brain axis. Trends Mol. Med. 2021, 27, 844–855. [Google Scholar] [CrossRef]
- Hawkins, K.G.; Casolaro, C.; Brown, J.A.; Edwards, D.A.; Wikswo, J.P. The microbiome and the gut-liver-brain axis for central nervous system clinical pharmacology: Challenges in specifying and integrating in vitro and in silico models. Clin. Pharmacol. Ther. 2020, 108, 929–948. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Yu, J.; Shi, M. Gut-Liver-Brain Axis: A Complex Network Influences Human Health and Diseases. Front. Neurosci. 2023, 17, 1241069. [Google Scholar] [CrossRef]
- Guo, T.L.; Chen, Y.; Xu, H.S.; McDonough, C.M.; Huang, G. Gut microbiome in neuroendocrine and neuroimmune interactions: The case of genistein. Toxicol. Appl. Pharmacol. 2020, 402, 115130. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L., IV; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbio. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.; Youness, E.R.; Mohammed, N.A.; Yassen, N.N.; Khadrawy, Y.A.; El-Toukhy, S.E.; Sleem, A.A. Novel neuroprotective and hepatoprotective effects of citric acid in acute malathion intoxication. Asian Pac. J. Trop. Med. 2016, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement Altern. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef]
- He, J.; Zhou, D.; Yan, B. Eriocitrin alleviates oxidative stress and inflammatory response in cerebral ischemia reperfusion rats by regulating phosphorylation levels of Nrf2/NQO-1/HO-1/NF-κB p65 proteins. Ann. Transl. Med. 2020, 8, 757. [Google Scholar] [CrossRef]
- Míguez, B.; Vila, C.; Venema, K.; Parajó, J.C.; Alonso, J.L. Prebiotic effects of pectooligosaccharides obtained from lemon peel on the microbiota from elderly donors using an in vitro continuous colon model (TIM-2). Food Funct. 2020, 11, 9984–9999. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A.; et al. New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model. Antioxidants 2021, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Pu, H.; Zhang, Z.Y.; Song, J. Evaluation of Antioxidant and Antitumour Activities of Lemon Essential Oil. J. Med. Plants Res. 2010, 4, 1910–1915. [Google Scholar]
- Hao, C.W.; Lai, W.S.; Ho, C.T.; Sheen, L.Y. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: Use of the tail suspension test. J. Funct. Foods 2013, 5, 370–379. [Google Scholar] [CrossRef]
- Martial, C.; Poirrier, A.L.; Pottier, L.; Cassol, H.; Mortaheb, S.; Panda, R.; Lopez, M.; Perrin, T.; Boilevin, A.; Gosseries, O.; et al. From nose to brain: The effect of lemon inhalation observed by whole brain voxel to voxel functional connectivity. Cortex 2023, 165, 119–128. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food. 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef]
- Foti, P.; Ballistreri, G.; Timpanaro, N.; Rapisarda, P.; Romeo, F.V. Prebiotic effects of citrus pectic oligosaccharides. Nat. Prod. Res. 2022, 36, 3173–3176. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods. 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Ho, Y.Y.; Lin, C.M.; Wu, M.C. Evaluation of the prebiotic effects of citrus pectin hydrolysate. J. Food Drug Anal. 2017, 25, 550–558. [Google Scholar] [CrossRef]
- Zafar, J.; Iahtisham-Ul-Haq; Nayik, G.A.; Ramniwas, S.; Mugabi, R.; Ali Alharbi, S.; Ansari, M.J. Studies on the growth of Lactobacillus reuteri, Bifidobacterium and Escherichia coli as affected by prebiotic extracted from citrus peel. Int. J. Food Prop. 2024, 27, 783–798. [Google Scholar] [CrossRef]
- Ojha, S.; Patil, N.; Jain, M.; Kole, C.; Kaushik, P. Probiotics for Neurodegenerative Diseases: A Systemic Review. Microorganisms 2023, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Shin, D. Effect of dietary soluble fiber on neurohormonal profiles in serum and brain of rats. Nutr. Res. Pract. 2007, 1, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wu, H.; Xiong, J.; Li, Y.; Chen, L.; Gu, Q.; Li, P. Metabolism of eriocitrin in the gut and its regulation on gut microbiota in mice. Front. Microbiol. 2023, 13, 1111200. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Teng, Y.; He, L.; Sayed, M.; Mu, J.; Xu, F.; Zhang, X.; Kumar, A.; Sundaram, K.; Sriwastva, M.K.; et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. iScience. 2021, 24, 102511. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.C.; Lunken, G.R.; Kelly, W.J.; Patchett, M.L.; Jordens, Z.; Tannock, G.W.; Sims, I.M.; Bell, T.J.; Hedderley, D.; Henrissat, B.; et al. Genomic insights from Monoglobus pectinilyticus: A pectin-degrading specialist bacterium in the human colon. ISME J. 2019, 13, 1437–1456. [Google Scholar] [CrossRef]
- Belloumi, D.; Calvet, S.; Roca, M.I.; Ferrer, P.; Jiménez-Belenguer, A.; Cambra-López, M.; García-Rebollar, P.; Climent, E.; Martínez-Blanch, J.; Tortajada, M.; et al. Effect of providing citrus pulp-integrated diet on fecal microbiota and serum and fecal metabolome shifts in crossbred pigs. Sci. Rep. 2023, 13, 17596. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Salari, S.; Ghasemi Nejad Almani, P. Antifungal effects of Lactobacillus acidophilus and Lactobacillus plantarum against different oral Candida species isolated from HIV/ AIDS patients: An in vitro study. J. Oral. Microbiol. 2020, 12, 1769386. [Google Scholar] [CrossRef]
- Maurer Sost, M.; Stevens, Y.; Salden, B.; Troost, F.; Masclee, A.; Venema, K. Citrus Extract High in Flavonoids Beneficially Alters Intestinal Metabolic Responses in Subjects with Features of Metabolic Syndrome. Foods 2023, 12, 3413. [Google Scholar] [CrossRef]
- Sost, M.M.; Ahles, S.; Verhoeven, J.; Verbruggen, S.; Stevens, Y.; Venema, K. A Citrus Fruit Extract High in Polyphenols Beneficially Modulates the Gut Microbiota of Healthy Human Volunteers in a Validated In Vitro Model of the Colon. Nutrients 2021, 13, 3915. [Google Scholar] [CrossRef]
- Tinh, N.T.T.; Sitolo, G.C.; Yamamoto, Y.; Suzuki, T. Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods 2021, 10, 240. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, Y.W.; Hou, C.Y.; Chen, Y.T.; Dong, C.D.; Chen, C.W.; Singhania, R.R.; Leang, J.Y.; Hsieh, S.L. Lemon fermented products prevent obesity in high-fat diet-fed rats by modulating lipid metabolism and gut microbiota. J. Food Sci. Technol. 2023, 60, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, B.; Smidt, H.; Scheurink, A.J.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Bussolo de Souza, C.; Krych, L.; Barbosa Cahú, T.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, O.S.; Omale, J.; Omeje, S.C.; Edino, V.O. Antidiarrheal activity of hexane extract of Citrus limon peel in an experimental animal model. J. Integr. Med. 2017, 15, 158–164. [Google Scholar] [CrossRef]
- Murunga, A.N.; Miruka, D.O.; Driver, C.; Nkomo, F.S.; Cobongela, S.Z.; Owira, P.M. Grapefruit Derived Flavonoid Naringin Improves Ketoacidosis and Lipid Peroxidation in Type 1 Diabetes Rat Model. PLoS ONE 2016, 11, e0153241. [Google Scholar] [CrossRef]
- Wojnar, W.; Zych, M.; Kaczmarczyk-Sedlak, I. Antioxidative effect of flavonoid naringenin in the lenses of type 1 diabetic rats. Biomed. Pharmacother. 2018, 108, 974–984. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Liu, F.; Cui, Y.; Li, X. Dietary citrus and/or its extracts intake contributed to weight control: Evidence from a systematic review and meta-analysis of 13 randomized clinical trials. Phytother. Res. 2020, 34, 2006–2022. [Google Scholar] [CrossRef]
- Tejpal, S.; Wemyss, A.M.; Bastie, C.C.; Klein-Seetharaman, J. Lemon Extract Reduces Angiotensin Converting Enzyme (ACE) Expression and Activity and Increases Insulin Sensitivity and Lipolysis in Mouse Adipocytes. Nutrients 2020, 12, 2348. [Google Scholar] [CrossRef]
- Meydani, M.; Hasan, S.T. Dietary polyphenols and obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaldate, R.; Bisht, A. Chapter4.5—Citric acid, antioxidant effects in health. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–322. [Google Scholar]
- Penniston, K.L.; Steele, T.H.; Nakada, S.Y. Lemonade therapy increases urinary citrate and urine volumes in patients with recurrent calcium oxalate stone formation. Urology 2007, 70, 856–860. [Google Scholar] [CrossRef]
- Cassidy, A.; Rimm, E.B.; O’Reilly, E.J.; Logroscino, G.; Kay, C.; Chiuve, S.E.; Rexrode, K.M. Dietary flavonoids and risk of stroke in women. Stroke 2012, 43, 946–951. [Google Scholar] [CrossRef]
- Shah, A.; Wang, Y.; Wondisford, F.E. Differential Metabolism of Glycerol Based on Oral versus Intravenous Administration in Humans. Metabolites 2022, 12, 890. [Google Scholar] [CrossRef]
- Brisson, D.; Vohl, M.C.; St-Pierre, J.; Hudson, T.J.; Gaudet, D. Glycerol: A neglected variable in metabolic processes? Bioessays 2001, 23, 534–542. [Google Scholar] [CrossRef]
- Frank, M.S.; Nahata, M.C.; Hilty, M.D. Glycerol: A review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy 1981, 1, 147–160. [Google Scholar] [CrossRef]
- Nelson, J.L.; Robergs, R.A. Exploring the potential ergogenic effects of glycerol hyperhydration. Sports Med. 2007, 37, 981–1000. [Google Scholar] [CrossRef]
- Snell, T.W.; Johnston, R.K. Glycerol extends lifespan of Brachionus manjavacas (Rotifera) and protects against stressors. Exp. Gerontol. 2014, 57, 47–56. [Google Scholar] [CrossRef]
- Guisado, R.; Arieff, A.I.; Massry, S.G. Effects of glycerol infusions on brain water and electrolytes. Am. J. Physiol. 1974, 227, 865–872. [Google Scholar] [CrossRef]
- Waterhouse, J.M.; Coxon, R.V. The entry of glycerol into brain tissue. J. Neurol. Sci. 1970, 10, 305–311. [Google Scholar] [CrossRef]
- Meyer, J.S.; Itoh, Y.; Okamoto, S.; Welch, K.M.; Mathew, N.T.; Ott, E.O.; Sakaki, S.; Miyakawa, Y.; Chabi, E.; Ericsson, A.D. Circulatory and metabolic effects of glycerol infusion in patients with recent cerebral infarction. Circulation 1975, 51, 701–712. [Google Scholar] [CrossRef]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in Brain: Distribution, Physiology, and Pathophysiology. J. Cereb. Blood Flow. Metabolism. 2002, 22, 367–378. [Google Scholar] [CrossRef]
- Hibuse, T.; Maeda, N.; Nagasawa, A.; Funahashi, T. Aquaporins and glycerol metabolism. Biochim. Biophys. Acta 2006, 1758, 1004–1011. [Google Scholar] [CrossRef]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef]
- Zahl, S.; Skauli, N.; Stahl, K.; Prydz, A.; Frey, M.M.; Dissen, E.; Ottersen, O.P.; Amiry-Moghaddam, M. Aquaporin-9 in the Brain Inflammatory Response: Evidence from Mice Injected with the Parkinsonogenic Toxin MPP+. Biomolecules 2023, 13, 588. [Google Scholar] [CrossRef]
- Yu, Y.L.; Kumana, C.R.; Lauder, I.J.; Cheung, Y.K.; Chan, F.L.; Kou, M.; Fong, K.Y.; Cheung, R.T.; Chang, C.M. Treatment of acute cortical infarct with intravenous glycerol. A double-blind, placebo-controlled randomized trial. Stroke 1993, 24, 1119–1124. [Google Scholar] [CrossRef]
- Bohn, D.; Daneman, D. Diabetic ketoacidosis and cerebral edema. Curr. Opin. Pediatr. 2002, 14, 287–291. [Google Scholar] [CrossRef]
- Wirtshafter, D.; Davis, J.D. Body weight: Reduction by long-term glycerol treatment. Science 1977, 198, 1271–1274. [Google Scholar] [CrossRef]
- De Weirdt, R.; Possemiers, S.; Vermeulen, G.; Moerdijk-Poortvliet, T.C.; Boschker, H.T.; Verstraete, W.; Van de Wiele, T. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol. Ecol. 2010, 74, 601–611. [Google Scholar] [CrossRef]
- Yu, L.; Rodriguez, R.A.; Chen, L.L.; Chen, L.Y.; Perry, G.; McHardy, S.F.; Yeh, C.K. 1,3-propanediol binds deep inside the channel to inhibit water permeation through aquaporins. Protein Sci. 2016, 25, 433–441. [Google Scholar] [CrossRef]
- Cleusix, V.; Lacroix, C.; Vollenweider, S.; Le Blay, G. Glycerol induces reuterin production and decreases Escherichia coli population in an in vitro model of colonic fermentation with immobilized human feces. FEMS Microbiol. Ecol. 2008, 63, 56–64. [Google Scholar] [CrossRef]
- Spinler, J.K.; Auchtung, J.; Brown, A.; Boonma, P.; Oezguen, N.; Ross, C.L.; Luna, R.A.; Runge, J.; Versalovic, J.; Peniche, A.; et al. Next-Generation Probiotics Targeting Clostridium difficile through Precursor-Directed Antimicrobial Biosynthesis. Infect. Immun. 2017, 85, e00303-17. [Google Scholar] [CrossRef]
- Gravisse, J.; Barnaud, G.; Hanau-Bercot, B.; Raskine, L.; Riahi, J.; Gaillard, J.L.; Sanson-Le-Pors, M.J. Clostridium difficile brain empyema after prolonged intestinal carriage. J. Clin. Microbiol. 2003, 41, 509–511. [Google Scholar] [CrossRef]
- Rogers, M.A.; Greene, M.T.; Young, V.B.; Saint, S.; Langa, K.M.; Kao, J.Y.; Aronoff, D.M. Depression, antidepressant medications, and risk of Clostridium difficile infection. BMC Med. 2013, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Asare, P.T.; Greppi, A.; Geirnaert, A.; Pennacchia, A.; Babst, A.; Lacroix, C. Glycerol and reuterin-producing Limosilactobacillus reuteri enhance butyrate production and inhibit Enterobacteriaceae in broiler chicken cecal microbiota PolyFermS model. BMC Microbiol. 2023, 23, 384. [Google Scholar] [CrossRef]
- Zhao, X.; Bridgman, S.L.; Drall, K.M.; Tun, H.M.; Mandhane, P.J.; Moraes, T.J.; Simons, E.; Turvey, S.E.; Subbarao, P.; Scott, J.A.; et al. Infant Vitamin D Supplements, Fecal Microbiota and Their Metabolites at 3 Months of Age in the CHILD Study Cohort. Biomolecules 2023, 13, 200. [Google Scholar] [CrossRef]
- Trischler, R.; Poehlein, A.; Daniel, R.; Müller, V. Ethanologenesis from glycerol by the gut acetogen Blautia schinkii. Environ. Microbiol. 2023, 25, 3577–3591. [Google Scholar] [CrossRef]
- Trabue, S.; Scoggin, K.; Tjandrakusuma, S.; Rasmussen, M.A.; Reilly, P.J. Ruminal fermentation of propylene glycol and glycerol. J. Agric. Food Chem. 2007, 55, 7043–7051. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, P.; Jiang, Q.; Xu, Q.; Zheng, Y.; Yan, J.; Ji, H.; Ning, J.; Zhang, X.; Li, C.; et al. Depletion of acetate-producing bacteria from the gut microbiota facilitates cognitive impairment through the gut-brain neural mechanism in diabetic mice. Microbiome 2021, 9, 145. [Google Scholar]
- Waniewski, R.A.; Martin, D.L. Preferential utilization of acetate by astrocytes is attributable to transport. J. Neurosci. 1998, 18, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Reisenauer, C.J.; Bhatt, D.P.; Mitteness, D.J.; Slanczka, E.R.; Gienger, H.M.; Watt, J.A.; Rosenberger, T.A. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J. Neurochem. 2011, 117, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.L.; Smith, M.D.; Houdek, H.M.; Rosenberger, T.A. Acetate supplementation modulates brain histone acetylation and decreases interleukin-1β expression in a rat model of neuroinflammation. J. Neuroinflamm. 2012, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Brissette, C.A.; Houdek, H.M.; Floden, A.M.; Rosenberger, T.A. Acetate supplementation reduces microglia activation and brain interleukin-1beta levels in a rat model of Lyme neuroborreliosis. J. Neuroinflamm. 2012, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Arun, P.; Madhavarao, C.N.; Moffett, J.R.; Namboodiri, M.A. Progress toward acetate supplementation therapy for Canavan disease: Glyceryl triacetate administration increases acetate, but not N-acetylaspartate, levels in brain. J. Pharmacol. Exp. Ther. 2005, 315, 297–303. [Google Scholar] [CrossRef]
- Arun, P.; Madhavarao, C.N.; Moffett, J.R.; Hamilton, K.; Grunberg, N.E.; Ariyannur, P.S.; Gahl, W.A.; Anikster, Y.; Mog, S.; Hallows, W.C.; et al. Metabolic acetate therapy improves phenotype in the tremor rat model of Canavan disease. J. Inherit. Metab. Dis. 2010, 33, 195–210. [Google Scholar] [CrossRef]
- Gao, Q.; Li, K.; Zhong, R.; Long, C.; Liu, L.; Chen, L.; Zhang, H. Supplementing Glycerol to Inoculum Induces Changes in pH, SCFA Profiles, and Microbiota Composition in In-Vitro Batch Fermentation. Fermentation 2022, 8, 18. [Google Scholar] [CrossRef]
- Lodén, M.; Andersson, A.C.; Anderson, C.; Bergbrant, I.M.; Frödin, T.; Ohman, H.; Sandström, M.H.; Särnhult, T.; Voog, E.; Stenberg, B.; et al. A double-blind study comparing the effect of glycerin and urea on dry, eczematous skin in atopic patients. Acta Derm. Venereol. 2002, 82, 45–47. [Google Scholar] [CrossRef]
- Overgaard Olsen, L.; Jemec, G.B. The influence of water, glycerin, paraffin oil and ethanol on skin mechanics. Acta Derm. Venereol. 1993, 73, 404–406. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Szél, E.; Polyánka, H.; Szabó, K.; Hartmann, P.; Degovics, D.; Balázs, B.; Németh, I.B.; Korponyai, C.; Csányi, E.; Kaszaki, J.; et al. Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Goulet, E.D.; Aubertin-Leheudre, M.; Plante, G.E.; Dionne, I.J. A meta-analysis of the effects of glycerol-induced hyperhydration on fluid retention and endurance performance. Int. J. Sport. Nutr. Exerc. Metab. 2007, 17, 391–410. [Google Scholar] [CrossRef]
- Van Rosendal, S.P.; Strobel, N.A.; Osborne, M.A.; Fassett, R.G.; Coombes, J.S. Performance benefits of rehydration with intravenous fluid and oral glycerol. Med. Sci. Sports Exerc. 2012, 44, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Fellmann, N. Hormonal and plasma volume alterations following endurance exercise: A brief review. Sports Med. 1992, 13, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.J.M. Glycerin. Boston Med. Surg. J. 1865, 72, 240–243. [Google Scholar] [CrossRef]
- Suzuki, R.; Fukuyama, K.; Miyazaki, Y.; Namiki, T. Contact urticaria syndrome and protein contact dermatitis caused by glycerin enema. JAAD Case Rep. 2016, 2, 108–110. [Google Scholar] [CrossRef][Green Version]
- Thornit, D.N.; Sander, B.; la Cour, M.; Lund-Andersen, H. The effects of peroral glycerol on plasma osmolarity in diabetic patients and healthy individuals. Basic. Clin. Pharmacol. Toxicol. 2009, 105, 289–293. [Google Scholar] [CrossRef]
- Ballot, D.; Baynes, R.D.; Bothwell, T.H.; Gillooly, M.; MacFarlane, B.J.; MacPhail, A.P.; Lyons, G.; Derman, D.P.; Bezwoda, W.R.; Torrance, J.D. The effects of fruit juices and fruits on the absorption of iron from a rice meal. Br. J. Nutr. 1987, 57, 331–343. [Google Scholar] [CrossRef]
- Fukuchi, Y.; Hiramitsu, M.; Okada, M.; Hayashi, S.; Nabeno, Y.; Osawa, T.; Naito, M. Lemon Polyphenols Suppress Diet-induced Obesity by Up-Regulation of mRNA Levels of the Enzymes Involved in beta-Oxidation in Mouse White Adipose Tissue. J. Clin. Biochem. Nutr. 2008, 43, 201–209. [Google Scholar] [CrossRef]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef]
- Talib, W.H. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. Nutrition 2017, 43–44, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Neurological disorders associated with impaired glucose tolerance. Nihon Rinsho. 1996, 54, 2704–2708. [Google Scholar] [PubMed]
- Singleton, J.R.; Smith, A.G.; Bromberg, M.B. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001, 24, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.L.; Li, S.Z.; Xiao, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Li, J.; Liu, E.H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Nissim, I.; Horyn, O.; Luhovyy, B.; Luhovyy, B.; Lazarow, A.; Nissim, I. Brain amino acid requirements and toxicity: The example of leucine. J. Nutr. 2005, 135, 1531S–1538S. [Google Scholar] [CrossRef]
- Walker, A.K.; Wing, E.E.; Banks, W.A.; Dantzer, R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol. Psychiatry 2019, 24, 1523–1532. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Xie, F.; Sun, Z.; Guo, B.; Li, F.; Wang, S.; Wang, Y.; Tian, Y.; Zhao, Y.; et al. Leucine mediates cognitive dysfunction in early life stress-induced mental disorders by activating autophagy. Front. Cell Neurosci. 2023, 16, 1060712. [Google Scholar] [CrossRef]
- Kalogeropoulou, D.; Lafave, L.; Schweim, K.; Gannon, M.C.; Nuttall, F.Q. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism 2008, 57, 1747–1752. [Google Scholar] [CrossRef]
- Pathak, K.; Zhao, Y.; Calton, E.K.; James, A.P.; Newsholme, P.; Sherriff, J.; Soares, M.J. The impact of leucine supplementation on body composition and glucose tolerance following energy restriction: An 8-week RCT in adults at risk of the metabolic syndrome. Eur. J. Clin. Nutr. 2024, 78, 155–162. [Google Scholar] [CrossRef]
- Baert, F.; Matthys, C.; Maselyne, J.; Van Poucke, C.; Van Coillie, E.; Bergmans, B.; Vlaemynck, G. Parkinson’s disease patients’ short chain fatty acids production capacity after in vitro fecal fiber fermentation. NPJ Park. Dis. 2021, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2276.e7. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Xie, T.; Pan, K.; Deng, Y.; Zhao, Z.; Li, N.; Bian, J.; Deng, X.; Zha, Y. Acetate attenuates perioperative neurocognitive disorders in aged mice. Aging 2020, 12, 3862–3879. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Hou, J.; Wang, X.; Huang, Z.; Cao, H.; Hu, J.; Peng, Q.; Duan, H.; Wang, Q.; Chen, X. Prevotella histicola Transplantation Ameliorates Cognitive Impairment and Decreases Oxidative Stress in Vascular Dementia Rats. Brain Sci. 2023, 13, 1136. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef]
- Gu, N.; Yan, J.; Tang, W.; Zhang, Z.; Wang, L.; Li, Z.; Wang, Y.; Zhu, Y.; Tang, S.; Zhong, J.; et al. Prevotella copri transplantation promotes neurorehabilitation in a mouse model of traumatic brain injury. J. Neuroinflamm. 2024, 21, 147. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Peralta-Marzal, L.N.; Rojas-Velazquez, D.; Rigters, D.; Prince, N.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P.; Lopez-Rincon, A. A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Sci. Rep. 2024, 14, 814. [Google Scholar] [CrossRef]
- Nugumanova, G.; Ponomarev, E.D.; Askarova, S.; Fasler-Kan, E.; Barteneva, N.S. Freshwater Cyanobacterial Toxins, Cyanopeptides and Neurodegenerative Diseases. Toxins 2023, 15, 233. [Google Scholar] [CrossRef]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Lugliè, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef] [PubMed]
- Oluwagbemigun, K.; Schnermann, M.E.; Schmid, M.; Cryan, J.F.; Nöthlings, U. A prospective investigation into the association between the gut microbiome composition and cognitive performance among healthy young adults. Gut Pathog. 2022, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Gorthi, S.P.; Prabhu, A.N.; Das, B.; Mutreja, A.; Vasudevan, K.; Shetty, V.; Ramamurthy, T.; Ballal, M. Dysbiosis of the Beneficial Gut Bacteria in Patients with Parkinson’s Disease from India. Ann. Indian Acad. Neurol. 2023, 26, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.T.; Bråthe, A.; Hassel, B. Neuronal uptake and metabolism of glycerol and the neuronal expression of mitochondrial glycerol-3-phosphate dehydrogenase. J. Neurochem. 2003, 85, 831–842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.L.; Navarro, J.; Luna, M.I.; Xu, H.S. Dietary Supplements and the Gut–Brain Axis: A Focus on Lemon, Glycerin, and Their Combinations. Dietetics 2024, 3, 463-482. https://doi.org/10.3390/dietetics3040034
Guo TL, Navarro J, Luna MI, Xu HS. Dietary Supplements and the Gut–Brain Axis: A Focus on Lemon, Glycerin, and Their Combinations. Dietetics. 2024; 3(4):463-482. https://doi.org/10.3390/dietetics3040034
Chicago/Turabian StyleGuo, Tai L., Jarissa Navarro, Maria Isabel Luna, and Hannah Shibo Xu. 2024. "Dietary Supplements and the Gut–Brain Axis: A Focus on Lemon, Glycerin, and Their Combinations" Dietetics 3, no. 4: 463-482. https://doi.org/10.3390/dietetics3040034
APA StyleGuo, T. L., Navarro, J., Luna, M. I., & Xu, H. S. (2024). Dietary Supplements and the Gut–Brain Axis: A Focus on Lemon, Glycerin, and Their Combinations. Dietetics, 3(4), 463-482. https://doi.org/10.3390/dietetics3040034






