Thermochemical Pretreatment for Improving the Psychrophilic Anaerobic Digestion of Coffee Husks
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Husk and Inoculum
2.2. Experimental Setup
2.2.1. Adaptation and Degassing Stage
2.2.2. BMP Tests
2.2.3. Monitoring Biogas Production
2.2.4. Theoretical Chemical Oxygen Demand, Theoretical Biomethane Potential, and Biodegradability
2.2.5. First-Order Kinetic Model
3. Results and Discussion
3.1. Changes in Biogas and Methane Productivity
3.2. Stoichiometry, Theoretical COD, Theoretical Biomethane Potential, and Biodegradability
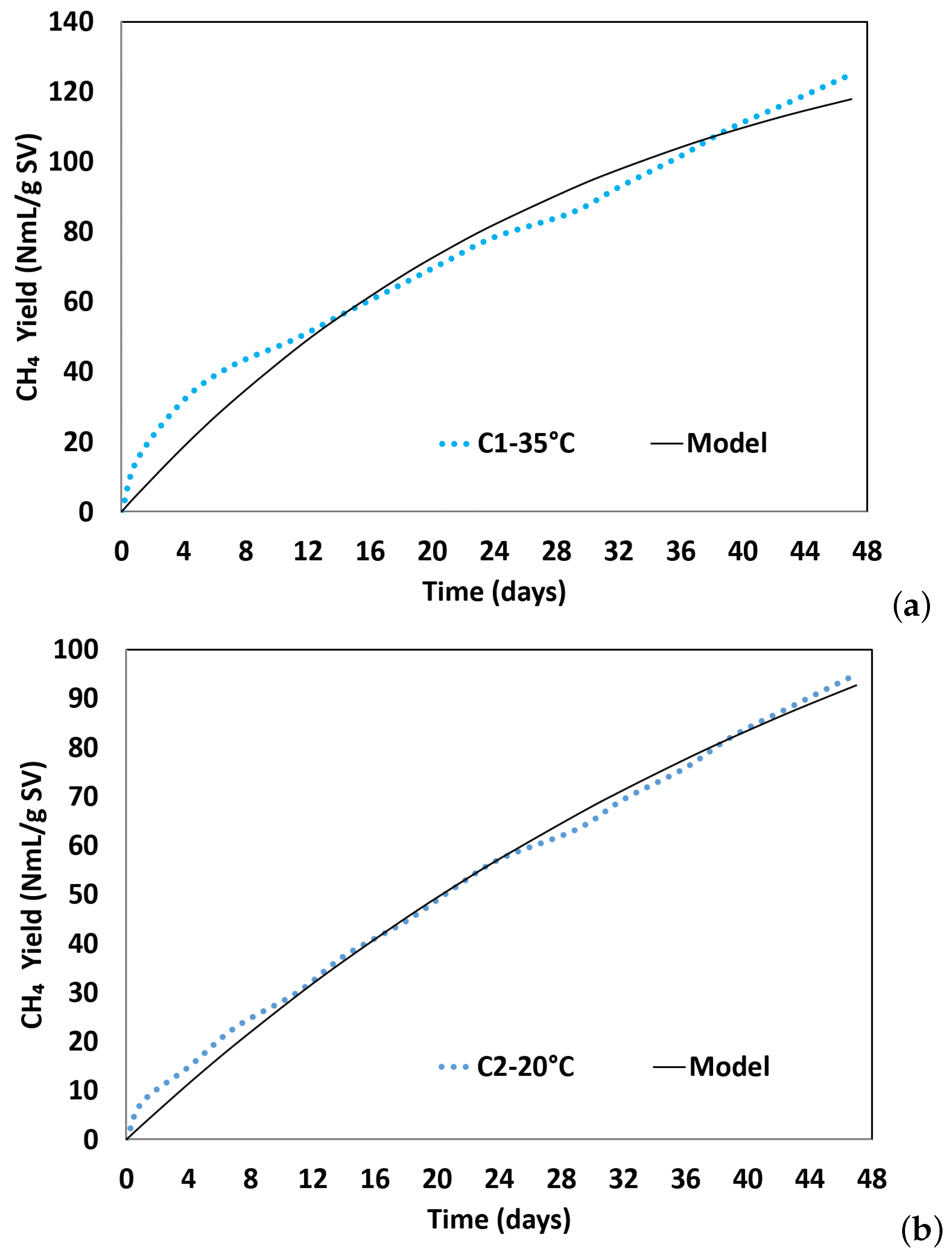
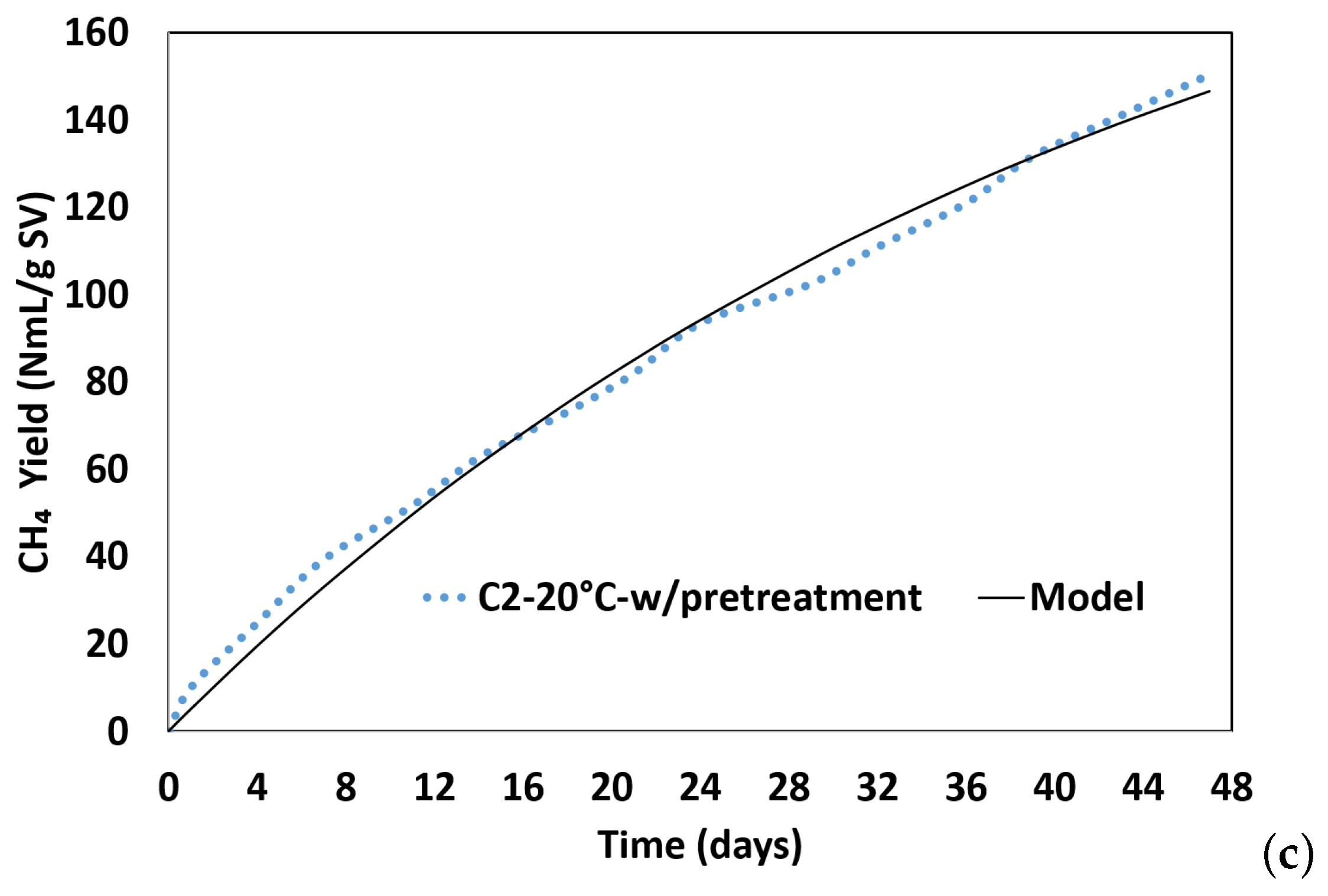
3.3. First-Order Kinetic Model
3.4. Energy Content and Energy Output
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TS | Total Solids |
| VS | Volatile Solids |
| AD | Anaerobic Digestion |
| BD | Biodegradability |
| BMP | Biochemical Methane Potential |
| COD | Chemical Oxygen Demand |
| Theoretical Chemical Oxygen Demand | |
| Energy Content |
References
- Neri, A.; Bernardi, B.; Zimbalatti, G.; Benalia, S. An Overview of Anaerobic Digestion of Agricultural By-Products and Food Waste for Biomethane Production. Energies 2023, 16, 6851. [Google Scholar] [CrossRef]
- Pan, S.Y.; Tsai, C.Y.; Liu, C.W.; Wang, S.W.; Kim, H.; Fan, C. Anaerobic co-digestion of agricultural wastes toward circular bioeconomy. iScience 2021, 24, 102704. [Google Scholar] [CrossRef]
- Lakshana, G.N.; Komal, A.; Pradeep, V. An overview of sustainable approaches for bioenergy production from agro-industrial wastes. Energy Nexus 2022, 6, 100086. [Google Scholar] [CrossRef]
- Gómez-Salcedo, Y.; Baquerizo-Crespo, R.; Da Silva, A.J.; Oliva-Merencio, D.; Pereda-Reyes, I. Anaerobic digestion of solid wastes from coffee wet processing. Rev. Int. Contam. Ambient. 2021, 37, 281–292. [Google Scholar] [CrossRef]
- Peshev, D.; Mitev, D.; Peeva, L.; Peev, G. Valorization of spent coffee grounds—A new approach. Sep. Purif. Technol. 2018, 192, 271–277. [Google Scholar] [CrossRef]
- Atabani, A.E.; Ali, I.; Naqvi, S.R.; Badruddin, I.A.; Aslam, M.; Mahmoud, E.; Almomani, F.; Juchelkova, D.; Atelge, M.R.; Khan, T.Y. A state-of-the-art review on spent coffee ground (SCG) pyrolysis for future biorefinery. Chemosphere 2018, 286, 131730. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Year Production. Coffee Production Report-May 2021. 2021. Available online: https://www.ico.org/prices/po-production.pdf (accessed on 16 September 2023).
- Guerra, A.F.; Santos, J.d.F.; Ferreira, L.T.; Rocha, O.C. Chapter 5. Coffees of Brazil Research, sustainability and innovation. In Land-Saving Technologies; Telhado, S.F.P.e., de Capdeville, G., Eds.; Embrapa: Brasília, Brazil, 2021. [Google Scholar]
- Mazzafera, P. Degradation of caffeine by microorganisms and potential use of decaffeinated coffee husk and pulp in animal feeding. Sci. Agric. 2002, 59, 815–821. [Google Scholar] [CrossRef]
- Karolyne, A.; Lages Leal, A.; Santos Silva, R.; Cristina, E.; Ferreira, S.; Soares, R.; Ferreira, P. The quality of roasted and ground brazilian coffee: Chemical analysis of the effect of fixed mineral residue e different types of packaging on moisture content. Res. Soc. Dev. 2021, 10, 1. [Google Scholar] [CrossRef]
- Oliveira, L.; Franca, A. Chapter 31—An Overview of the Potential Uses for Coffee Husks. In Coffee in Health and Disease Prevention; Academic Press: Massachusetts, MA, USA, 2015; pp. 283–291. ISBN 9780124095175. [Google Scholar] [CrossRef]
- Oliveira, F.; Srinivas, K.; Helms, G.; Isern, N.; Cort, J.; Gonçalves, A.; Ahring, B. Characterization of coffee (Coffea arabica) husk lignin and degradation products obtained after oxygen and alkali addition. Bioresour. Technol. 2018, 257, 172–180. [Google Scholar] [CrossRef]
- De Matos, A.T. Waste treatment in post-harvest coffee. In Pós-Colheita do Café; Borem, F.M., Lavras, M.G., Eds.; UFLA: Lavras, Brazil, 2008; Volume 6, pp. 159–201. [Google Scholar]
- Galanakis, C. Handbook of Coffee Processing By-Product. Sustainable Applications; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-811290-8. [Google Scholar]
- Twinomuhwezi, H.; Wozeyi, P.; Igwe, V.S.; Amagwula, I.O.; Awuchi, C.G. Heat of Combustion of Coffee Pulp and Husks as Alternative Sources of Renewable Energy. Eur. J. Agric. Food Sci. 2021, 3, 1–4. [Google Scholar] [CrossRef]
- Amertet, S.; Mitiku, Y.; Belete, G. Analysis of a Coffee Husk Fired Cogeneration Plant in South Western Ethiopia Coffee Processing Industries. Low Carbon Econ. 2021, 12, 42–62. [Google Scholar]
- Manrique, R.; Vásquez, D.; Ceballos, C.; Chejne, F.; Amell, A. Evaluation of the Energy Density for Burning Disaggregated and Pelletized Coffee Husks. Acs Omega 2019, 4, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Gordillo, G.; Cantor, C. Experimental Gasification of Coffee Husk Using Pure Oxygen-Steam Blends. Front. Energy Res. 2019, 7, 127. [Google Scholar] [CrossRef]
- Vale, A.; Gentil, L.; Gonçalez, J.C.; Costa, A. Caracterização energética e rendimento da carbonização de resíduos de grãos de café (Coffea arabica, L) e de madeira (Cedrelinga catenaeformis), Duke. Cerne 2007, 13, 416–420. [Google Scholar]
- Serna-Jiménez, J.A.; Siles, J.A.; de los Ángeles Martín, M.; Chica, A.F. A Review on the Applications of Coffee Waste Derived from Primary Processing: Strategies for Revalorization. Processes 2022, 10, 2436. [Google Scholar] [CrossRef]
- Hernández-Sarabia, M.; Sierra-Silva, J.; Delgadillo-Mirquez, L.; Ávila-Navarro, J.; Carranza, L. The Potential of the Biodigester as a Useful Tool in Coffee Farms. Appl. Sci. 2021, 11, 6884. [Google Scholar] [CrossRef]
- Amador-Diaz, I. Anaerobic Digestion of Lignocellulosic Biomass via Cotreatment: A Techno-Economic Analysis. Master’s Thesis, Pennsylvania State University, State College, PA, USA, 19 May 2019. [Google Scholar]
- Akindolire, M.; Rama, H.; Roopnarain, A. Psychrophilic anaerobic digestion: A critical evaluation of microorganisms and enzymes to drive the process. Renew. Sustain. Energy Rev. 2022, 161, 112394. [Google Scholar] [CrossRef]
- Tiwari, B.; Rouissi, T.; Brar, S.; Surampalli, R. Critical insights into psychrophilic anaerobic digestion: Novel strategies for improving biogas production. Waste Manag. 2021, 131, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Lettinga, G.; Rebac, S.; Zeeman, G. Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 2001, 19, 363–370. [Google Scholar] [CrossRef]
- Dhaked, R.K.; Singh, P.; Singh, L. Biomethanation under psychrophilic conditions. Waste Manag. 2010, 30, 2490–2496. [Google Scholar] [CrossRef]
- VDI. Fermentation Tests: Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data; Germany Association of Engineers: Düsseldorf, Germany, 2016. [Google Scholar]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.D.; Fruteau de Laclos, H.; Koch, K.; Holliger, C. Improving inter-laboratory reproducibility in measurement of biochemical methane potential (BMP). Water 2020, 12, 1752. [Google Scholar] [CrossRef]
- Ahmed, A.M.S.; Buezo, K.A.; Saady, N.M.C. Adapting anaerobic consortium to pure and complex lignocellulose substrates at low temperature: Kinetics evaluation. Int. J. Recycl. Org. Waste Agric. 2019, 8, 99–110. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 2009. 59, 927–934. [Google Scholar] [CrossRef]
- Nurzulaifa, S.; Buyong, F. Comparative of experimental and theoreticaurzulaifal biochemical methane potential generated by municipal solid waste. Environ. Adv. 2023, 11, 100345. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Methods for Determination of Biomethane Potential of Feedstocks: A Review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical methane potential (BMP) of food waste and primary sludge: Influence of inoculum pre-incubation and inoculum source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.; Arantes, Y.; Gomes, A.; Herrera, O.; Alves, L.; Valderrama, J.; Luna, H.; Lobo, B. Coffee Husk Waste Valorization Using Thermal Pretreatment Associated to Bioprocess to Produce Bioproducts: Characterization, Kinetic, Economic Assessment, and Challenges. In Microbial Bioprocessing of Agri-Food Wastes, 1st, ed.; CRC Press: Boca Raton, FL, USA, 2023; p. 29. ISBN 9781003128977. [Google Scholar]
- Reis, J.; Abreu, L.; Rodrigues, L.; Sousa, M. Thermochemical treatment of coffee peels for the production of biogas by anaerobic digestion. In Annals of the Giulio Massarani Journey of Scientific, Technological, Artistic and Cultural Initiation; UFRJ: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Wang, B.; Nges, I.A.; Nistor, M.; Liu, J. Determination of methane yield of cellulose using different experimental setups. Water Sci. Technol. 2014, 70, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef]
- Lübken, M.; Gehring, T.; Wichern, M. Microbiological fermentation of lignocellulosic biomass: Current state and prospects of mathematical modeling. Appl. Microbiol. Biotechnol. 2010, 85, 1643–1652. [Google Scholar] [CrossRef]
- Contreras, L.M.; Schelle, H.; Sebrango, C.R.; Pereda, I. Methane potential and biodegradability of rice straw, rice husk and rice residues from the drying process. Water Sci. Technol. 2012, 65, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Baquerizo-Crespo, R.; Astals, S.; Pérez-Ones, O.; Pereda-Reyes, I. Mathematical Modeling Challenges Associated with Waste Anaerobic Biodegradability. In Advances in the Domain of Environmental Biotechnology; Springer: Singapore, 2021; pp. 357–392. ISBN 978-981-15-8998-0. [Google Scholar]
- Prem, C.; Shanmugam, P. Correlation between empirical formulae based stoichiometric and experimental methane potential and calorific energy values for vegetable solid wastes. Energy Rep. 2021, 7, 19–31. [Google Scholar] [CrossRef]
- Browne, J.D.; Murphy, J.D. Assessment of the resource associated with biomethane from food waste. Appl. Energy 2012, 104, 170–177. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Monlau, M.; Abdellatif, B.; Ferrer, I.; Kaparaju, P.; Trably, E.; Carrère, H. Assessment of hydrothermal pretreatment of various lignocellulosic biomass with CO2 catalyst for enhanced methane and hydrogen production. Water Res. 2017, 120, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Lymperatou, A.; Engelsen, T.; Skiadas, I.; Gavala, H. Prediction of methane yield and pretreatment efficiency of lignocellulosic biomass based on composition. Waste Manag. 2023, 155, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Fernandes, M.; Milagres, A.M.F. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzym. Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef]
- Agregán, R.; Lorenzo, J.M.; Kumar, M.; Shariati, M.A.; Khan, M.U.; Sarwar, A.; Sultan, M.; Rebezov, M.; Usman, M. Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches. Energies 2022, 15, 8413. [Google Scholar] [CrossRef]
- Dou, W.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can Hydrothermal Pretreatment Improve Anaerobic Digestion for Biogas from Lignocellulosic Biomass? Bioresour. Technol. 2018, 249, 117–124. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Dumlu, L.; Ciggin, A.S.; Ručman, S.; Perendeci, N.A. Pretreatment, Anaerobic Codigestion, or Both? Which Is More Suitable for the Enhancement of Methane Production from Agricultural Waste? Molecules 2021, 26, 4175. [Google Scholar] [CrossRef] [PubMed]
- Sagarika, P.; Brajesh, K.D. Electrochemical pretreatment of yard waste to improve biogas production: Understanding the mechanism of delignification, and energy balance. Bioresour. Technol. 2019, 292, 121958. [Google Scholar] [CrossRef]
- Passos, F.; Ortega, V.; Donoso-Bravo, A. Thermochemical pretreatment and AD of dairy cow manure: Experimental and economic evaluation. Bioresour. Technol. 2017, 227, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.; Oliveira, R.; Alves, M.M. Anaerobic co-digestion of coffee waste and sewage sludge. Waste Manag. 2006, 26, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Lobo, B.; Miranda, P.; Passos, F.; Alves, L.; Aquino, S.; Fdz-Polanco, F. Steam explosion pretreatment improved the biomethanization of coffee husks. Bioresour. Technol. 2017, 245, 66–72. [Google Scholar] [CrossRef]
- Passos, F.; Miranda Cordeiro, P.H.; Lobo Baeta, B.; Aquino, S.F.; Perez-Elvira, S.I. Anaerobic co-digestion of coffee husks and microalgal biomass after thermal hydrolysis. Bioresour. Technol. 2018, 253, 49–54. [Google Scholar] [CrossRef]
- CONAB, Companhia Nacional de Abastecimento. 2019. Available online: https://www.conab.gov.br (accessed on 29 March 2019).
- Fernandes, M.; Baeta, B.; Adarme, O.; Fonseca, A. Energy recovery alternatives for coffee husk waste:an LCA-based carbon footprint analysis of anaerobicdigestion technologies. SSRN 2023. [Google Scholar] [CrossRef]
- Soares, N. Evaluation of Hydrothermal Pre-Treatment of Coffee Peels in Enzymatic Hydrolysis and Biogas Production; Federal University of Ouro Preto, Environmental Engineering: Ouro Preto, Brazil, 2019. [Google Scholar]
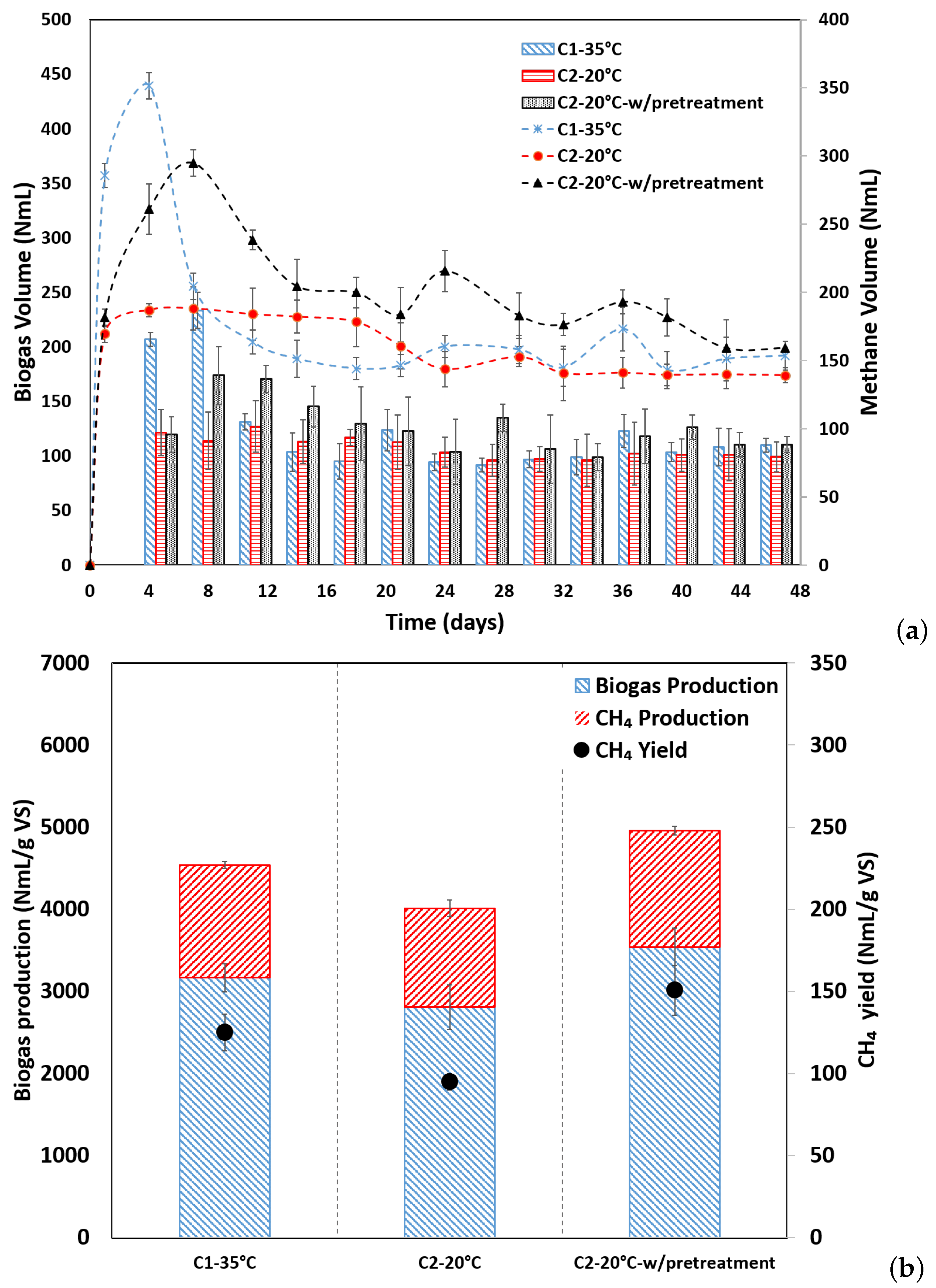
| Parameters | Coffee Husk | Anaerobic Sludge |
|---|---|---|
| Total solids (% TS) | 87.36 ± 0.002 | 6.05 ± 0.003 |
| Total volatile solids (% VS) * | 92.32 ± 0.683 | 85.12 ± 0.149 |
| pH | - | 7.3 ± 0.058 |
| C (%) ** | 44.99 ± 3.423 | – |
| H (%) ** | 5.75 ± 0.738 | – |
| O (%) ** | 47.31 ± 5.950 | – |
| N (%) ** | 1.76 ± 0.971 | – |
| S (%) ** | 0.16 ± 0.100 | – |
| C/N | 25.56 | – |
| Component | Weight Fraction (%) | Contribution Mass (g) | Molecular Weight (g/mol) | Coefficients (mol) |
|---|---|---|---|---|
| Carbon | 44.99 | 6.59 | 12 | 0.55 |
| Hydrogen | 5.79 | 0.848 | 1 | 0.85 |
| Oxygen | 47.31 | 6.938 | 16 | 0.43 |
| Nitrogen | 1.76 | 0.258 | 14 | 0.02 |
| Sulfur | 0.16 | 0.023 | 32 | 0.001 |
| 100% | 14.667 |
| Parameter | Theoretical Energy Content | C1-35 °C | C2-20 °C | C3-20 °C-w/Pretreatment |
|---|---|---|---|---|
| Substrate formula | C0.55H0.85O0.43N0.02 | |||
| BMPEXP (NmL CH4/g VS) | 124.99 ± 11 | 94.96 ± 5 | 150.47 ± 15 | |
| CODTH (g COD/g VS) | 0.93 | |||
| BMPEXP-CODTH (NmL CH4/g VS) | 133.77 | 101.63 | 161.04 | |
| BMPTH (NmL CH4/g VS) | 405.52 | |||
| BMPTH (NmL CH4/g COD) | 434.00 | |||
| BMPE0 (NmL CH4/g VS) | 402.26 | |||
| BMPE0 (NmL CH4/g COD) | 430.50 | |||
| E0 (MJ/kg VS) * | 15.04 | |||
| E0 (MJ/kg COD) * | 16.09 | |||
| BD (%) | 30.82 | 23.42 | 37.11 | |
| BMP Tests | BMPExp (NmL gVS−1) | BMPPred (NmL gVS−1) | k (per Day) | Time (Days) | Adj. R2 | RMSE |
|---|---|---|---|---|---|---|
| C1-35 °C | 124.99 | 149.07 | 0.033 | 30 | 0.970 | 6.19 |
| C2-20 °C | 94.96 | 159.42 | 0.019 | 54 | 0.996 | 2.17 |
| C3-20 °C-w/pretreatment | 150.47 | 221.90 | 0.023 | 43 | 0.994 | 3.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.S.; Flores-Rodriguez, C.; Torres-Albarracin, L.; Silva, A.J.d. Thermochemical Pretreatment for Improving the Psychrophilic Anaerobic Digestion of Coffee Husks. Methane 2024, 3, 214-226. https://doi.org/10.3390/methane3020013
Yang TS, Flores-Rodriguez C, Torres-Albarracin L, Silva AJd. Thermochemical Pretreatment for Improving the Psychrophilic Anaerobic Digestion of Coffee Husks. Methane. 2024; 3(2):214-226. https://doi.org/10.3390/methane3020013
Chicago/Turabian StyleYang, Tzyy Shyuan, Carla Flores-Rodriguez, Lorena Torres-Albarracin, and Ariovaldo José da Silva. 2024. "Thermochemical Pretreatment for Improving the Psychrophilic Anaerobic Digestion of Coffee Husks" Methane 3, no. 2: 214-226. https://doi.org/10.3390/methane3020013
APA StyleYang, T. S., Flores-Rodriguez, C., Torres-Albarracin, L., & Silva, A. J. d. (2024). Thermochemical Pretreatment for Improving the Psychrophilic Anaerobic Digestion of Coffee Husks. Methane, 3(2), 214-226. https://doi.org/10.3390/methane3020013






