A Comprehensive Review of the Rheological Properties of Powders in Pharmaceuticals
Abstract
:1. Introduction
2. Importance of Powder Flowability in Pharmaceutical Manufacturing
3. Powder Flow Properties and Characterization Techniques
3.1. Particle Size
3.2. Particle Morphology
Particle Shape
3.3. Friability
Dynamic Testing
3.4. Electrostatic Charging
3.5. Permeability
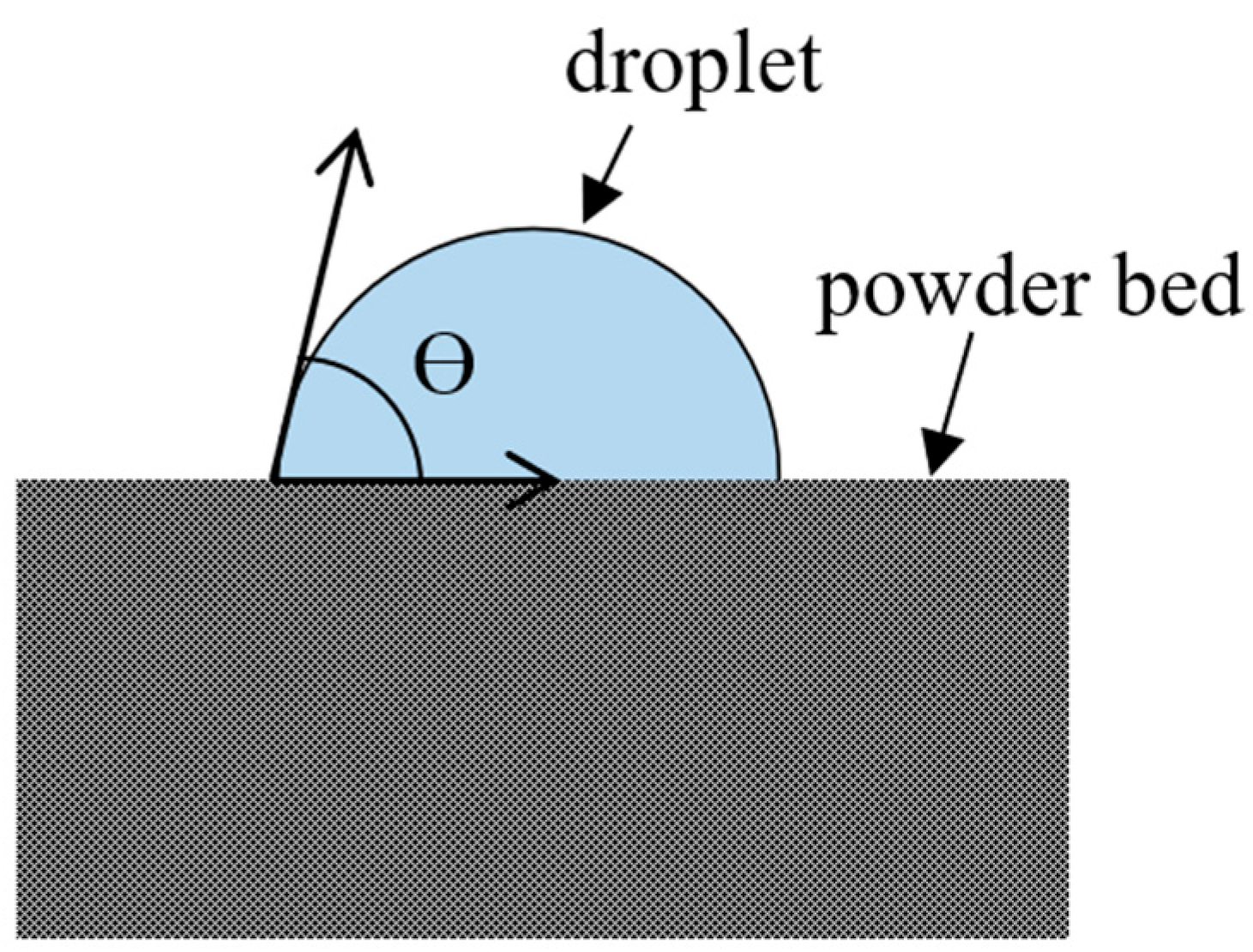
3.6. Wettability
3.6.1. Methods of Characterization
Experimental Methods
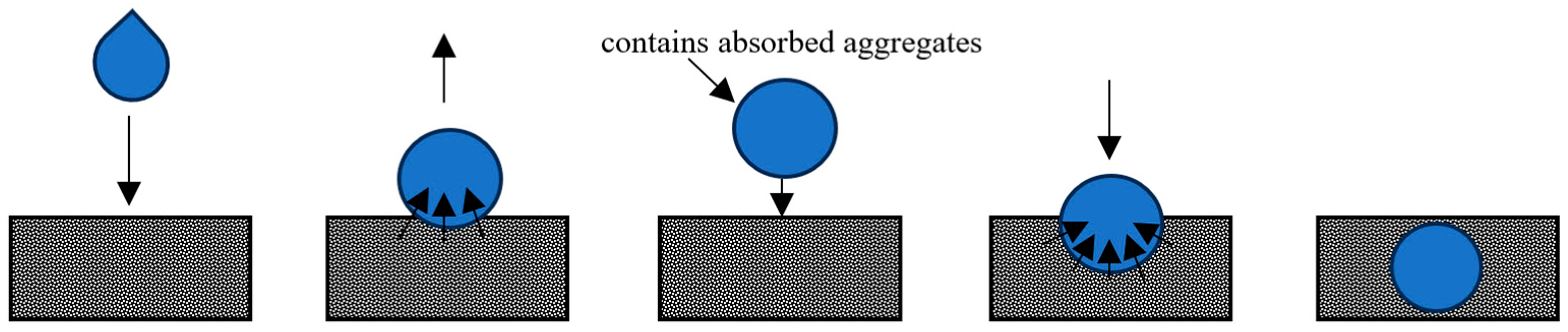
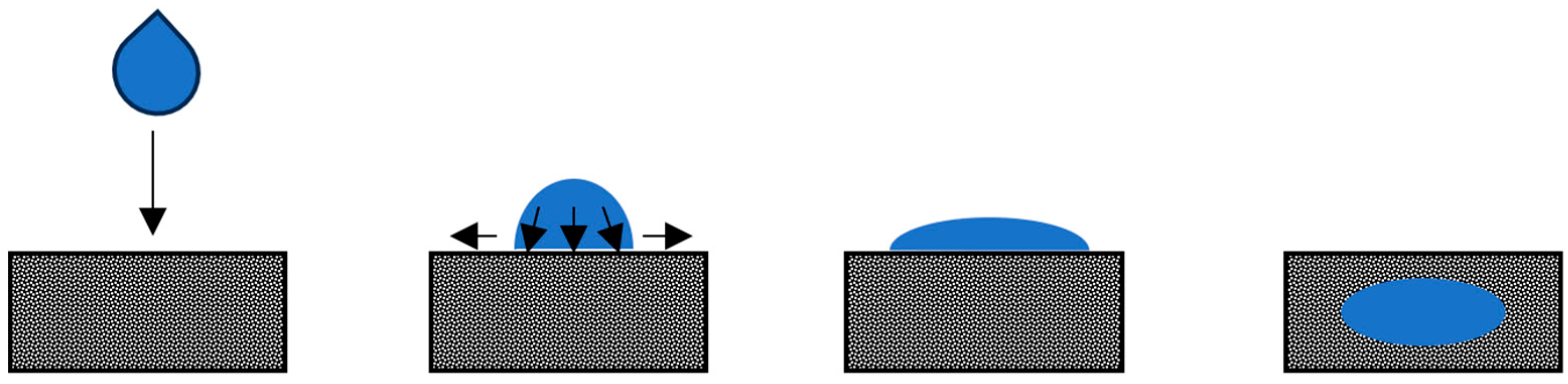
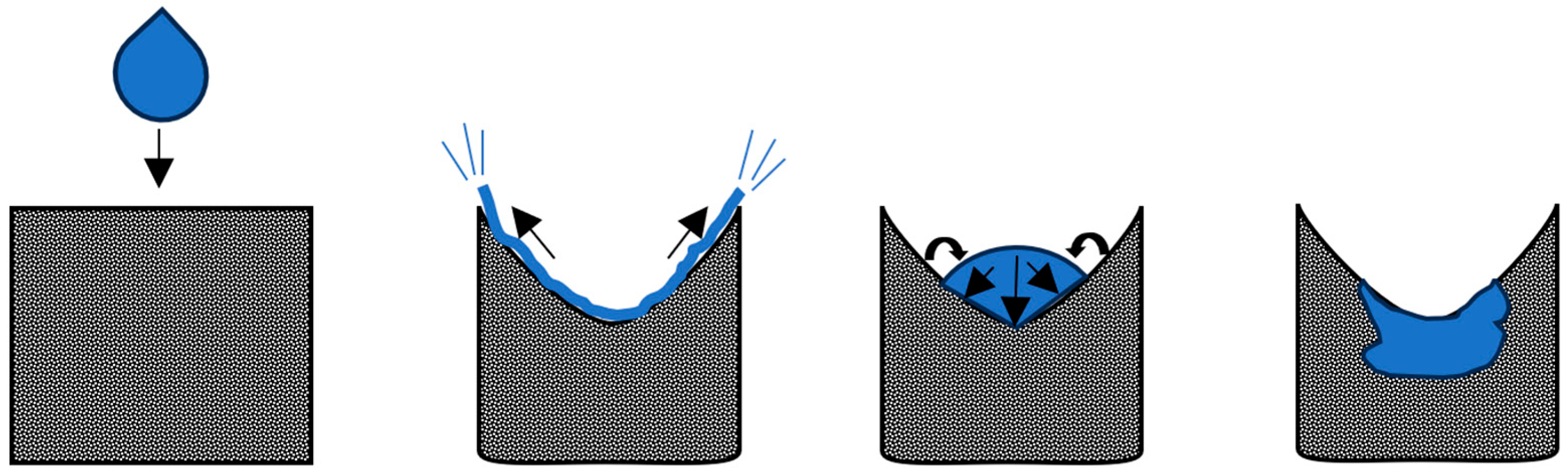
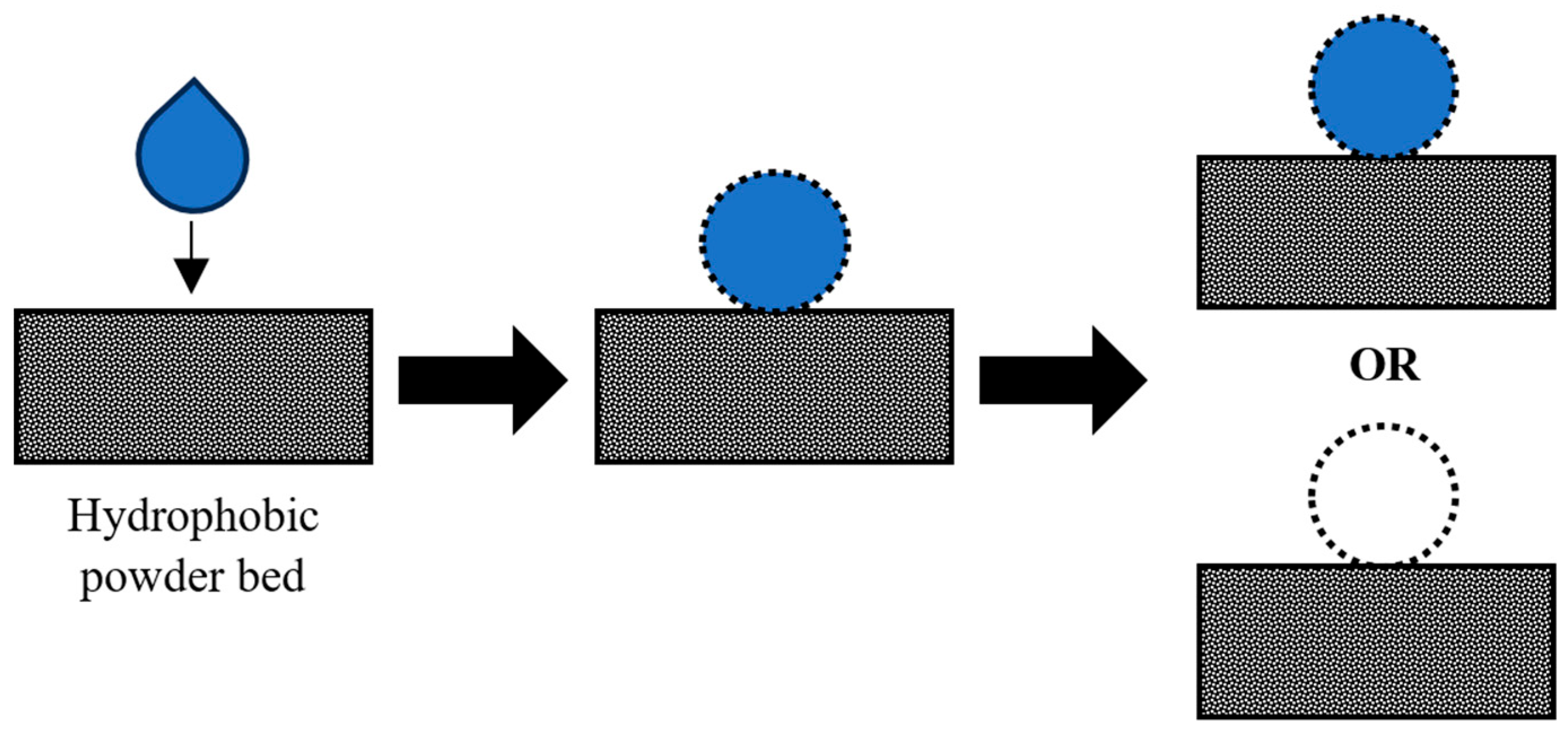
Droplet Mechanisms
Factors Affecting Droplet Impact and Penetration
Sessile Drop Method
Washburn Method
Drop Penetration Method
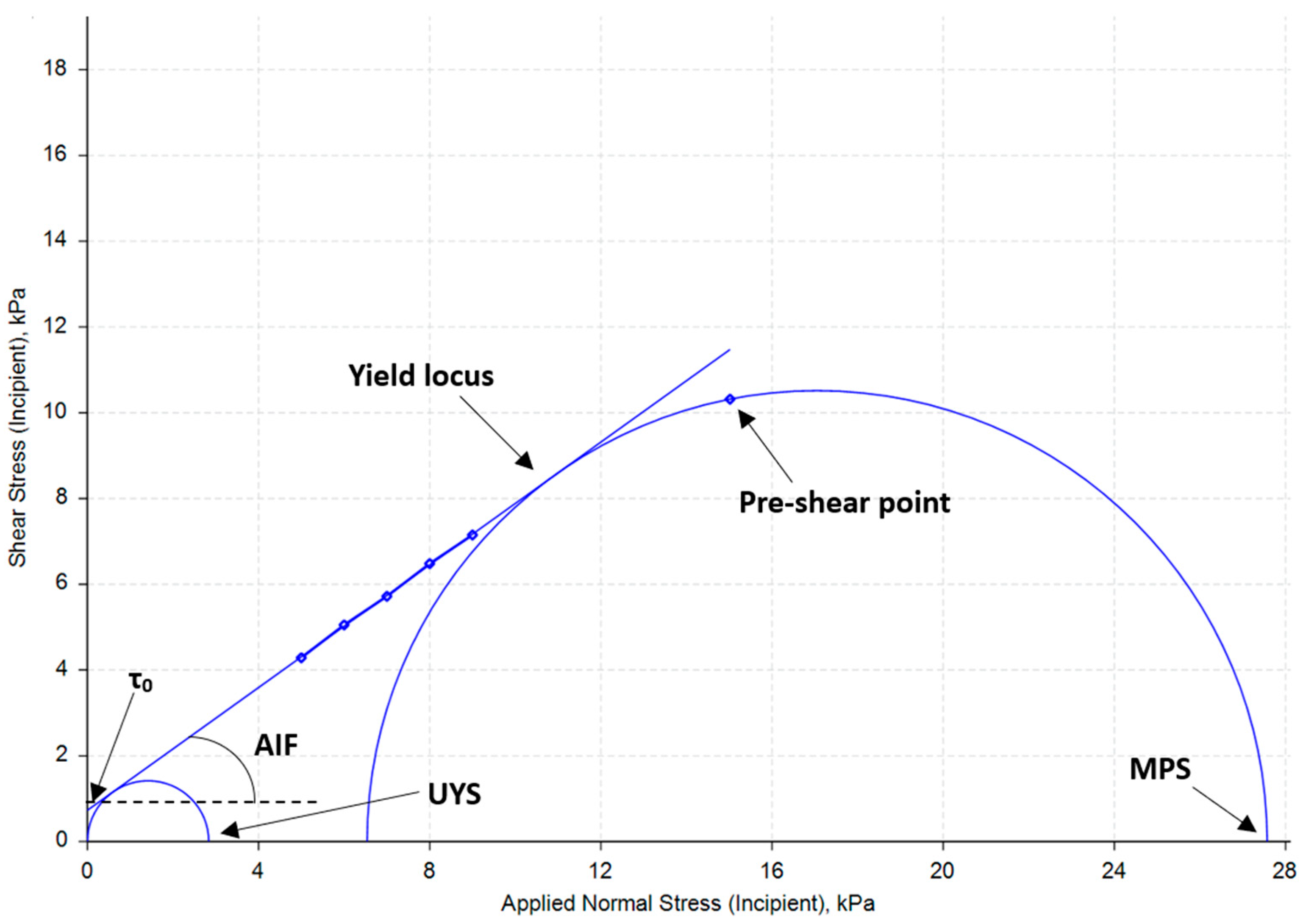
3.7. Cohesion
3.7.1. Shear Cell
3.7.2. Avalanche Testers
3.7.3. Revolution Powder Analyzer
3.7.4. Gravitational Displacement Rheometer
3.7.5. API AeroFlow Avalanche Tester
3.7.6. GranuDrum Instrument
3.7.7. Bond Number
3.8. Bulk Density
3.8.1. Compressibility
3.8.2. Carr Index
3.8.3. Hausner Ratio
3.9. Agglomeration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z. Understanding Powder Behavior in Pharmaceutical Manufacturing to Enhance Drug Productivity and Therapeutic Performance. Ph.D. Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Moghtadernejad, S.; Escotet-Espinoza, M.S.; Oka, S.; Singh, R.; Liu, Z.; Román-Ospino, A.D.; Li, T.; Razavi, S.; Panikar, S.; Scicolone, J.; et al. A training on: Continuous Manufacturing (Direct Compaction) of Solid Dose Pharmaceutical Products. J. Pharm. Innov. 2018, 13, 155–187. [Google Scholar] [CrossRef]
- Ervasti, T.; Niinikoski, H.; Mäki-Lohiluoma, E.; Leppinen, H.; Ketolainen, J.; Korhonen, O.; Lakio, S. The comparison of two challenging low dose APIs in a continuous direct compression process. Pharmaceutics 2020, 12, 279. [Google Scholar] [CrossRef]
- Plumb, K. Continuous Processing in the Pharmaceutical Industry: Changing the Mind Set. Chem. Eng. Res. Des. 2005, 83, 730–738. [Google Scholar] [CrossRef]
- Oka, S.S.; Escotet-Espinoza, M.S.; Singh, R.; Scicolone, J.V.; Hausner, D.B.; Ierapetritou, M.; Muzzio, F.J. Design of an Integrated Continuous Manufacturing System; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Patel, D.B.; Dave, R.H. Predicting lubricants effect on tablet sticking using ketoprofen as model drug and evaluating sticking propensity using different metals and powder rheology. Int. J. Pharm. 2021, 606, 913. [Google Scholar] [CrossRef]
- Garg, V.; Mallick, S.S.; Garcia-Trinanes, P.; Berry, R.J. An investigation into the flowability of fine powders used in pharmaceutical industries. Powder Technol. 2018, 336, 375–382. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Muzzio, F.J.; Glasser, B.J. Predicting feeder performance based on material flow properties. Powder Technol. 2017, 308, 135–148. [Google Scholar] [CrossRef]
- Portillo, P.M.; Ierapetritou, M.G.; Muzzio, F.J. Characterization of continuous convective powder mixing processes. Powder Technol. 2008, 182, 368–378. [Google Scholar] [CrossRef]
- Saddik, J.S.; Dave, R.H. Evaluation of powder rheology as a potential tool to predict tablet sticking. Powder Technol. 2021, 386, 298–306. [Google Scholar] [CrossRef]
- Parekh, B.V.; Saddik, J.S.; Patel, D.B.; Dave, R.H. Evaluating the effect of glidants on tablet sticking propensity of ketoprofen using powder rheology. Int. J. Pharm. 2023, 635, 2710. [Google Scholar] [CrossRef]
- Janssen, P.H.M.; Depaifve, S.; Neveu, A.; Francqui, F.; Dickhoff, B.H.J. Impact of powder properties on the rheological behavior of excipients. Pharmaceutics 2021, 13, 1198. [Google Scholar] [CrossRef]
- Majerová, D.; Kulaviak, L.; Růžička, M.; Štěpánek, F.; Zámostný, P. Effect of colloidal silica on rheological properties of common pharmaceutical excipients. Eur. J. Pharm. Biopharm. 2016, 106, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Macho, O.; Gabrišová, Ľ.; Brokešová, J.; Svačinová, P.; Mužíková, J.; Galbavá, P.; Blaško, J.; Šklubalová, Z. Systematic study of paracetamol powder mixtures and granules tabletability: Key role of rheological properties and dynamic image analysis. Int. J. Pharm. 2021, 608, 1110. [Google Scholar] [CrossRef] [PubMed]
- Worku, Z.A.; Kumar, D.; Gomes, J.V.; He, Y.; Glennon, B.; Ramisetty, K.A.; Rasmuson, Å.C.; O’Connell, P.; Gallagher, K.H.; Woods, T.; et al. Modelling and understanding powder flow properties and compactability of selected active pharmaceutical ingredients, excipients and physical mixtures from critical material properties. Int. J. Pharm. 2017, 531, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Chendo, C.; Pinto, J.F.; Paisana, M.C. Comprehensive powder flow characterization with reduced testing. Int. J. Pharm. 2023, 642, 3107. [Google Scholar] [CrossRef] [PubMed]
- Koynov, S. Using Statistical Methods to Optimize Powder Flow Measurements and to Predict Powder Processing Performance. Ph.D. Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Navaneethan, C.V.; Missaghi, S.; Fassihi, R. Application of powder rheometer to determine powder flow properties and lubrication efficiency of pharmaceutical particulate systems. AAPS PharmSciTech 2005, 6, 349. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.; Brockbank, K.; Sabathier, J. Characterising powder flow properties—the need for a multivariate approach. EPJ Web Conf. 2017, 140, 3008. [Google Scholar] [CrossRef]
- Ghoroi, C.; Gurumurthy, L.; McDaniel, D.J.; Jallo, L.J.; Davé, R.N. Multi-faceted characterization of pharmaceutical powders to discern the influence of surface modification. Powder Technol. 2013, 236, 63–74. [Google Scholar] [CrossRef]
- Narang, A.S.; Badawy, S.I.F. An Introduction to Powder Characterization. In Handbook of Pharmaceutical Wet Granulation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 569–611. [Google Scholar] [CrossRef]
- Divya, S.; Ganesh, G. Characterization of Powder Flowability Using FT4—Powder Rheometer. J. Pharm. Sci. Res. 2019, 11, 25–29. [Google Scholar]
- Jallo, L.J.; Ghoroi, C.; Gurumurthy, L.; Patel, U.; Davé, R.N. Improvement of flow and bulk density of pharmaceutical powders using surface modification. Int. J. Pharm. 2012, 423, 213–225. [Google Scholar] [CrossRef]
- Lumay, G.; Boschini, F.; Traina, K.; Bontempi, S.; Remy, J.C.; Cloots, R.; Vandewalle, N. Measuring the flowing properties of powders and grains. Powder Technol. 2012, 224, 19–27. [Google Scholar] [CrossRef]
- Krantz, M.; Zhang, H.; Zhu, J. Characterization of powder flow: Static and dynamic testing. Powder Technol. 2009, 194, 239–245. [Google Scholar] [CrossRef]
- Colbert, M.J.; Grandbois, M.; Abatzoglou, N. Identification of inter-particular forces by atomic force microscopy and how they relate to powder rheological properties measured in shearing tests. Powder Technol. 2015, 284, 396–402. [Google Scholar] [CrossRef]
- Mullarney, M.P.; Beach, L.E.; Davé, R.N.; Langdon, B.A.; Polizzi, M.; Blackwood, D.O. Applying dry powder coatings to pharmaceutical powders using a comil for improving powder flow and bulk density. Powder Technol. 2011, 212, 397–402. [Google Scholar] [CrossRef]
- Sarraguça, M.C.; Cruz, A.V.; Soares, S.O.; Amaral, H.R.; Costa, P.C.; Lopes, J.A. Determination of flow properties of pharmaceutical powders by near infrared spectroscopy. J. Pharm. Biomed. Anal. 2010, 52, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Cordts, E.; Steckel, H. Capabilities and limitations of using powder rheology and permeability to predict dry powder inhaler performance. Eur. J. Pharm. Biopharm. 2012, 82, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Huck, D.; Makein, L.; Armstrong, B.; Willen, U.; Freeman, T. Effect of particle shape and size on flow properties of lactose powders. Particuology 2012, 10, 203–208. [Google Scholar] [CrossRef]
- Susana, L.; Campaci, F.; Santomaso, A.C. Wettability of mineral and metallic powders: Applicability and limitations of sessile drop method and Washburn’s technique. Powder Technol. 2012, 226, 68–77. [Google Scholar] [CrossRef]
- Jallo, L.J.; Dave, R.N. Explaining Electrostatic Charging and Flow of Surface-Modified Acetaminophen Powders as a Function of Relative Humidity Through Surface Energetics. J. Pharm. Sci. 2015, 104, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Blanco, D.; Antikainen, O.; Räikkönen, H.; Mah, P.T.; Healy, A.M.; Juppo, A.M.; Yliruusi, J. Image-based characterization of powder flow to predict the success of pharmaceutical minitablet manufacturing. Int. J. Pharm. 2020, 581, 9280. [Google Scholar] [CrossRef]
- Capece, M.; Ho, R.; Strong, J.; Gao, P. Prediction of powder flow performance using a multi-component granular Bond number. Powder Technol. 2015, 286, 561–571. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Olivas, G.I.; Zamudio-Flores, P.B.; Ortega-Rivas, E.; Perez-Vega, S.; Sepulveda, D.R. Effect of water content on the flowability of hygroscopic powders. J. Food Eng. 2017, 205, 12–17. [Google Scholar] [CrossRef]
- Huang, Z.; Scicolone, J.V.; Gurumuthy, L.; Davé, R.N. Flow and bulk density enhancements of pharmaceutical powders using a conical screen mill: A continuous dry coating device. Chem. Eng. Sci. 2015, 125, 209–224. [Google Scholar] [CrossRef]
- Huang, Z.; Scicolone, J.V.; Han, X.; Davé, R.N. Improved blend and tablet properties of fine pharmaceutical powders via dry particle coating. Int. J. Pharm. 2015, 478, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Setia, G. Mechanical dry particle coating on cohesive pharmaceutical powders for improving flowability—A review. Powder Technol. 2019, 356, 458–479. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Qu, L.; Larson, I.; Stewart, P.J.; Morton, D.A.V. Effect of mechanical dry particle coating on the improvement of powder flowability for lactose monohydrate: A model cohesive pharmaceutical powder. Powder Technol. 2011, 207, 414–421. [Google Scholar] [CrossRef]
- Goh, H.P.; Heng, P.W.S.; Liew, C.V. Comparative evaluation of powder flow parameters with reference to particle size and shape. Int. J. Pharm. 2018, 547, 133–141. [Google Scholar] [CrossRef]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of moist pharmaceutical powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Hausmann, A.; Buck, B.; Shaw, L.; Simons, T.; Jäger, F.K.; Williams, D. The importance of humidity control in powder rheometer studies. Powder Technol. 2023, 421, 8425. [Google Scholar] [CrossRef]
- Li, J.; Ma, S.; He, X.; Sun, Y.; Zhang, X.; Guan, J.; Mao, S. Exploring the influence of magnesium stearate content and mixing modality on the rheological properties and in vitro aerosolization of dry powder inhaler. Int. J. Pharm. 2023, 642, 3179. [Google Scholar] [CrossRef]
- Almansour, K.; Alfagih, I.M.; Shalash, A.O.; Brockbank, K.; Ali, R.; Freeman, T.; Elsayed, M.M.A. Insights into the potential of rheological measurements in development of dry powder inhalation formulations. Int. J. Pharm. 2022, 614, 121407. [Google Scholar] [CrossRef]
- Tranová, T.; Macho, O.; Loskot, J.; Mužíková, J. Study of rheological and tableting properties of lubricated mixtures of co-processed dry binders for orally disintegrating tablets. Eur. J. Pharm. Sci. 2022, 168, 106035. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, C.; Sun, C.C.; Rantanen, J. Analytical method development for powder characterization: Visualization of the critical drug loading affecting the processability of a formulation for direct compression. J. Pharm. Biomed. Anal. 2016, 128, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Hsiau, S.S.; Huang, T.Y. The effect of vibrating conditions on the electrostatic charge in a vertical vibrating granular bed. Powder Technol. 2011, 208, 1–6. [Google Scholar] [CrossRef]
- Kale, K.; Hapgood, K.; Stewart, P. Drug agglomeration and dissolution—What is the influence of powder mixing? Eur. J. Pharm. Biopharm. 2009, 72, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Schulze, D. Powders and Bulk Solids: Behavior, Characterization, Storage and Flow, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Shi, H.; Mohanty, R.; Chakravarty, S.; Cabiscol, R.; Morgeneyer, M.; Zetzener, H.; Ooi, J.Y.; Kwade, A.; Luding, S.; Magnanimo, V. Effect of particle size and cohesion on powder yielding and flow. KONA Powder Part. J. 2018, 2018, 226–250. [Google Scholar] [CrossRef]
- Tay, J.Y.S.; Liew, C.V.; Heng, P.W.S. Powder Flow Testing: Judicious Choice of Test Methods. AAPS PharmSciTech 2017, 18, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, B.; Dhondt, J.; Pandelaere, K.; Bertels, J.; Mertens, R.; Klingeleers, D.; Di Pretoro, G.; Remon, J.P.; Vervaet, C.; De Beer, T.; et al. A multivariate raw material property database to facilitate drug product development and enable in-silico design of pharmaceutical dry powder processes. Int. J. Pharm. 2018, 549, 415–435. [Google Scholar] [CrossRef]
- Pingali, K.; Mendez, R.; Lewis, D.; Michniak-Kohn, B.; Cuitiño, A.; Muzzio, F. Evaluation of strain-induced hydrophobicity of pharmaceutical blends and its effect on drug release rate under multiple compression conditions. Drug Dev. Ind. Pharm. 2011, 37, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Schoenwald, D.R.D.; Guillory, J.K.; Matheson, L.E.; Parrott, E.L.; Flanagan, D.R.; Wurster, D.E.; Veng-Pedersen, P.; Grant, D.J.W. Particle, powder, and compact characterization. In Developing Solid Oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2009; pp. 163–183. [Google Scholar]
- Kalman, H. Effect of moisture content on flowability: Angle of repose, tilting angle, and Hausner ratio. Powder Technol. 2021, 393, 582–596. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Olivas, G.I.; Ortega-Rivas, E.; Zamudio-Flores, P.B.; Perez-Vega, S.; Sepulveda, D.R. Water activity, not moisture content, explains the influence of water on powder flowability. LWT—Food Sci. Technol. 2019, 100, 35–39. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Moghtadernejad, S.; Scicolone, J.; Wang, Y.; Pereira, G.; Schäfer, E.; Vigh, T.; Klingeleers, D.; Ierapetritou, M.; Muzzio, F.J. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018, 339, 659–676. [Google Scholar] [CrossRef]
- Capece, M.; Silva, K.R.; Sunkara, D.; Strong, J.; Gao, P. On the relationship of inter-particle cohesiveness and bulk powder behavior: Flowability of pharmaceutical powders. Int. J. Pharm. 2016, 511, 178–189. [Google Scholar] [CrossRef]
- Allenspach, C.; Timmins, P.; Sharif, S.; Minko, T. Characterization of a novel hydroxypropyl methylcellulose (HPMC) direct compression grade excipient for pharmaceutical tablets. Int. J. Pharm. 2020, 583, 119343. [Google Scholar] [CrossRef]
- Lapčík, L.; Otyepka, M.; Otyepková, E.; Lapčíková, B.; Gabriel, R.; Gavenda, A. Prudilová, Surface heterogeneity: Information from inverse gas chromatography and application to model pharmaceutical substances. Curr. Opin. Colloid. Interface Sci. 2016, 24, 64–71. [Google Scholar] [CrossRef]
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar] [CrossRef]
- Jallo, L.J.; Chen, Y.; Bowen, J.; Etzler, F.; Dave, R. Prediction of inter-particle adhesion force from surface energy and surface roughness. J. Adhes. Sci. Technol. 2011, 25, 367–384. [Google Scholar] [CrossRef]
- Zafar, U.; Vivacqua, V.; Calvert, G.; Ghadiri, M.; Cleaver, J.A.S. A review of bulk powder caking. Powder Technol. 2017, 313, 389–401. [Google Scholar] [CrossRef]
- Leturia, M.; Benali, M.; Lagarde, S.; Ronga, I.; Saleh, K. Characterization of flow properties of cohesive powders: A comparative study of traditional and new testing methods. Powder Technol. 2014, 253, 406–423. [Google Scholar] [CrossRef]
- Matsusaka, S.; Maruyama, H.; Matsuyama, T.; Ghadiri, M. Triboelectric charging of powders: A review. Chem. Eng. Sci. 2010, 65, 5781–5807. [Google Scholar] [CrossRef]
- Naik, S.; Mukherjee, R.; Chaudhuri, B. Triboelectrification: A review of experimental and mechanistic modeling approaches with a special focus on pharmaceutical powders. Int. J. Pharm. 2016, 510, 375–385. [Google Scholar] [CrossRef]
- Naik, S.; Sarkar, S.; Gupta, V.; Hancock, B.C.; Abramov, Y.; Yu, W.; Chaudhuri, B. A combined experimental and numerical approach to explore tribocharging of pharmaceutical excipients in a hopper chute assembly. Int. J. Pharm. 2015, 491, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Garg, V.; Bradley, M.S.A. Electrostatic Charging of Fine Powders and Assessment of Charge Polarity Using an Inductive Charge Sensor. Nanomanufacturing 2023, 3, 281–292. [Google Scholar] [CrossRef]
- Kaialy, W. A review of factors affecting electrostatic charging of pharmaceuticals and adhesive mixtures for inhalation. Int. J. Pharm. 2016, 503, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Šupuk, E.; Zarrebini, A.; Reddy, J.P.; Hughes, H.; Leane, M.M.; Tobyn, M.J.; Timmins, P.; Ghadiri, M. Tribo-electrification of active pharmaceutical ingredients and excipients. Powder Technol. 2012, 217, 427–434. [Google Scholar] [CrossRef]
- Pingali, K.C.; Shinbrot, T.; Hammond, S.V.; Muzzio, F.J. An observed correlation between flow and electrical properties of pharmaceutical blends. Powder Technol. 2009, 192, 157–165. [Google Scholar] [CrossRef]
- Hussain, T.; Deng, T.; Bradley, M.S.A.; Armour-Chélu, D.; Gorman, T.; Kaialy, W. Evaluation studies of a sensing technique for electrostatic charge polarity of pharmaceutical particulates. IET Sci. Meas. Technol. 2016, 10, 442–448. [Google Scholar] [CrossRef]
- Ireland, P.M. Triboelectrification of particulate flows on surfaces: Part I—Experiments. Powder Technol. 2010, 198, 189–198. [Google Scholar] [CrossRef]
- Ireland, P.M. Triboelectrification of particulate flows on surfaces: Part II—Mechanisms and models. Powder Technol. 2010, 198, 199–210. [Google Scholar] [CrossRef]
- Zarrebini, A.; Ghadiri, M.; Dyson, M.; Kippax, P.; McNeil-Watson, F. Tribo-electrification of powders due to dispersion. Powder Technol. 2013, 250, 75–83. [Google Scholar] [CrossRef]
- Biegaj, K.W.; Rowland, M.G.; Lukas, T.M.; Heng, J.Y.Y. Surface Chemistry and Humidity in Powder Electrostatics: A Comparative Study between Tribocharging and Corona Discharge. ACS Omega 2017, 2, 1576–1582. [Google Scholar] [CrossRef]
- Intra, P.; Tippayawong, N. Development and evaluation of a faraday cup electrometer for measuring and sampling atmospheric ions and charged aerosols. Part. Sci. Technol. 2015, 33, 257–263. [Google Scholar] [CrossRef]
- Freeman, R. Measuring the flow properties of consolidated, conditioned and aerated powders—A comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007, 174, 25–33. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, D.; Li, J.; Zhao, J.; Feng, Y.; Zhang, X.; Mao, S. Elucidation of lactose fine size and drug shape on rheological properties and aerodynamic behavior of dry powders for inhalation. Eur. J. Pharm. Biopharm. 2022, 179, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, S.; Sun, Y.; Song, R.; Cai, B.; Li, H.; Chen, Y.; Zhang, X.; Guan, J.; Mao, S. Predicting in vitro lung deposition behavior of combined dry powder inhaler via rheological properties. Eur. J. Pharm. Biopharm. 2022, 181, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Llusa, M.; Levin, M.; Snee, R.D.; Muzzio, F.J. Measuring the hydrophobicity of lubricated blends of pharmaceutical excipients. Powder Technol. 2010, 198, 101–107. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Muzzio, F.; Drazer, G. Callegari, A drop penetration method to measure powder blend wettability. Int. J. Pharm. 2018, 538, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tan, H. Three-dimensional simulation of micrometer-sized droplet impact and penetration into the powder bed. Chem. Eng. Sci. 2016, 153, 93–107. [Google Scholar] [CrossRef]
- Alghunaim, A.; Kirdponpattara, S.; Newby, B.M.Z. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Marston, J.O.; Thoroddsen, S.T.; Ng, W.K.; Tan, R.B.H. Experimental study of liquid drop impact onto a powder surface. Powder Technol. 2010, 203, 223–236. [Google Scholar] [CrossRef]
- Mohammadi, M.; Tembely, M.; Dolatabadi, A. Supercooled water droplet impacting superhydrophobic surfaces in the presence of cold air flow. Appl. Sci. 2017, 7, 130. [Google Scholar] [CrossRef]
- Emady, H.N.; Kayrak-Talay, D.; Schwerin, W.C.; Litster, J.D. Granule formation mechanisms and morphology from single drop impact on powder beds. Powder Technol. 2011, 212, 69–79. [Google Scholar] [CrossRef]
- Marston, J.O.; Sprittles, J.E.; Zhu, Y.; Li, E.Q.; Vakarelski, I.U.; Thoroddsen, S.T. Drop spreading and penetration into pre-wetted powders. Powder Technol. 2013, 239, 128–136. [Google Scholar] [CrossRef]
- Charles-Williams, H.R.; Wengeler, R.; Flore, K.; Feise, H.; Hounslow, M.J.; Salman, A.D. Granule nucleation and growth: Competing drop spreading and infiltration processes. Powder Technol. 2011, 206, 63–71. [Google Scholar] [CrossRef]
- Werner, S.R.L.; Jones, J.R.; Paterson, A.H.J.; Archer, R.H.; Pearce, D.L. Droplet impact and spreading: Droplet formulation effects. Chem. Eng. Sci. 2007, 62, 2336–2345. [Google Scholar] [CrossRef]
- Hapgood, K.P.; Farber, L.; Michaels, J.N. Agglomeration of hydrophobic powders via solid spreading nucleation. Powder Technol. 2009, 188, 248–254. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Properties of liquid marbles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2006, 462, 973–999. [Google Scholar] [CrossRef]
- Hapgood, K.P.; Khanmohammadi, B. Granulation of hydrophobic powders. Powder Technol. 2009, 189, 253–262. [Google Scholar] [CrossRef]
- Nguyen, T.; Shen, W.; Hapgood, K. Drop penetration time in heterogeneous powder beds. Chem. Eng. Sci. 2009, 64, 5210–5221. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Shen, W.; Hapgood, K. Effect of formulation hydrophobicity on drug distribution in wet granulation. Chem. Eng. J. 2010, 164, 330–339. [Google Scholar] [CrossRef]
- Mundozah, A.L.; Cartwright, J.J.; Tridon, C.C.; Hounslow, M.J.; Salman, A.D. Hydrophobic/hydrophilic static powder beds: Competing horizontal spreading and vertical imbibition mechanisms of a single droplet. Powder Technol. 2018, 330, 275–283. [Google Scholar] [CrossRef]
- Li, M.; Callegari, G.; Drazer, G. Capillary rise in a closed column: Application to the characterization of powders. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 124822. [Google Scholar] [CrossRef]
- Thakker, M.; Karde, V.; Shah, D.O.; Shukla, P.; Ghoroi, C. Wettability measurement apparatus for porous material using the modified Washburn method. Meas. Sci. Technol. 2013, 24, 125902. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Muzzio, F.J.; Callegari, G.; Drazer, G. Capillary Drop Penetration Method to Characterize the Liquid Wetting of Powders. Langmuir 2017, 33, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Affleck, S.; Thomas, A.L.; Routh, A.F.; Vriend, N.M. Novel protocol for quantifying powder cohesivity through fluidisation tests. Powder Technol. 2023, 415, 118147. [Google Scholar] [CrossRef]
- Crouter, A.; Briens, L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech 2014, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Karner, S.; Urbanetz, N.A. The impact of electrostatic charge in pharmaceutical powders with specific focus on inhalation-powders. J. Aerosol. Sci. 2011, 42, 428–445. [Google Scholar] [CrossRef]
- Trpělková, Ž.; Hurychová, H.; Kuentz, M.; Vraníková, B. Šklubalová, Introduction of the energy to break an avalanche as a promising parameter for powder flowability prediction. Powder Technol. 2020, 375, 33–41. [Google Scholar] [CrossRef]
- Wang, Y.; Koynov, S.; Glasser, B.J.; Muzzio, F.J. A method to analyze shear cell data of powders measured under different initial consolidation stresses. Powder Technol. 2016, 294, 105–112. [Google Scholar] [CrossRef]
- Leung, L.Y.; Mao, C.; Chen, L.P.; Yang, C.Y. Precision of pharmaceutical powder flow measurement using ring shear tester: High variability is inherent to powders with low cohesion. Powder Technol. 2016, 301, 920–926. [Google Scholar] [CrossRef]
- Koynov, S.; Glasser, B.; Muzzio, F. Comparison of three rotational shear cell testers: Powder flowability and bulk density. Powder Technol. 2015, 283, 103–112. [Google Scholar] [CrossRef]
- Salehi, H.; Barletta, D.; Poletto, M. A comparison between powder flow property testers. Particuology 2017, 32, 10–20. [Google Scholar] [CrossRef]
- Spierings, A.B.; Voegtlin, M.; Bauer, T.; Wegener, K. Powder flowability characterisation methodology for powder-bed-based metal additive manufacturing. Progress. Addit. Manuf. 2016, 1, 9–20. [Google Scholar] [CrossRef]
- Vasilenko, A.; Koynov, S.; Glasser, B.J.; Muzzio, F.J. Role of consolidation state in the measurement of bulk density and cohesion. Powder Technol. 2013, 239, 366–373. [Google Scholar] [CrossRef]
- Vasilenko, A.; Glasser, B.J.; Muzzio, F.J. Shear and flow behavior of pharmaceutical blends—Method comparison study. Powder Technol. 2011, 208, 628–636. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Dave, R.N.; Pfeffer, R. Fluidization of coated group C powders. AIChE J. 2008, 54, 104–121. [Google Scholar] [CrossRef]
- Kalman, H. Quantification of mechanisms governing the angle of repose, angle of tilting, and Hausner ratio to estimate the flowability of particulate materials. Powder Technol. 2021, 382, 573–593. [Google Scholar] [CrossRef]
- Kalman, H.; Portnikov, D. Analyzing bulk density and void fraction: A. the effect of archimedes number. Powder Technol. 2021, 381, 477–487. [Google Scholar] [CrossRef]
- Akseli, I.; Hilden, J.; Katz, J.M.; Kelly, R.C.; Kramer, T.T.; Mao, C.; Osei-Yeboah, F.; Strong, J.C. Reproducibility of the Measurement of Bulk/Tapped Density of Pharmaceutical Powders Between Pharmaceutical Laboratories. J. Pharm. Sci. 2019, 108, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.E.; Cooke, J.R.; Schneider, L.C.R. Measuring shear properties and normal stresses generated within a rotational shear cell for consolidated and non-consolidated powders. Powder Technol. 2009, 190, 65–69. [Google Scholar] [CrossRef]
- Saker, A.; Cares-Pacheco, M.G.; Marchal, P.; Falk, V. Powders flowability assessment in granular compaction: What about the consistency of Hausner ratio? Powder Technol. 2019, 354, 52–63. [Google Scholar] [CrossRef]
- Llusa, M.; Sturm, K.; Sudah, O.; Stamato, H.; Goldfarb, D.J.; Ramachandruni, H.; Hammond, S.; Smith, M.R.; Muzzio, F.J. Effect of high shear blending protocols and blender parameters on the degree of API agglomeration in solid formulations. Ind. Eng. Chem. Res. 2009, 48, 93–101. [Google Scholar] [CrossRef]
- Lim, H.L.; Hapgood, K.P.; Haig, B. Understanding and preventing agglomeration in a filter drying process. Powder Technol. 2016, 300, 146–156. [Google Scholar] [CrossRef]
- Le, V.N.P.; Robins, E.; Flament, M.P. Agglomerate behaviour of fluticasone propionate within dry powder inhaler formulations. Eur. J. Pharm. Biopharm. 2012, 80, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Llusa, M.; Levin, M.; Snee, R.D.; Muzzio, F.J. Shear-induced APAP de-agglomeration. Drug Dev. Ind. Pharm. 2009, 35, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Scherholz, M.L.; Wan, B.; McGeorge, G. A Rational Analysis of Uniformity Risk for Agglomerated Drug Substance Using NIR Chemical Imaging. AAPS PharmSciTech 2017, 18, 432–440. [Google Scholar] [CrossRef]
- FAlfano, O.; Di Maio, F.P.; Di Renzo, A. Deagglomeration of selected high-load API-carrier particles in swirl-based dry powder inhalers. Powder Technol. 2022, 408, 117800. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brubaker, J.; Moghtadernejad, S. A Comprehensive Review of the Rheological Properties of Powders in Pharmaceuticals. Powders 2024, 3, 233-254. https://doi.org/10.3390/powders3020015
Brubaker J, Moghtadernejad S. A Comprehensive Review of the Rheological Properties of Powders in Pharmaceuticals. Powders. 2024; 3(2):233-254. https://doi.org/10.3390/powders3020015
Chicago/Turabian StyleBrubaker, Jack, and Sara Moghtadernejad. 2024. "A Comprehensive Review of the Rheological Properties of Powders in Pharmaceuticals" Powders 3, no. 2: 233-254. https://doi.org/10.3390/powders3020015







