Efficient Stereoselective Biotransformation of Prochiral Carbonyls by Endophytic Fungi from Handroanthus impetiginosus
Abstract
:1. Introduction
2. Results
2.1. Screening of Endophytic Fungi
2.2. Optimization of the Bioreduction of Acetophenone (1) by Talaromyces sp. H4
2.3. Bioreduction of Substituted Acetophenones by Talaromyces sp. H4
2.4. Preparative Scale Bioreduction of Acetophenone (1)
3. Discussion
4. Material and Methods
4.1. General Procedures and Chromatographic Analysis
4.2. Screening for Stereoselective Bioreduction of Acetophenone by Endophytic Fungi
4.3. Optimization of the Methodology for Stereoselective Reduction of Acetophenone
4.4. Bioreduction of Substituted Acetophenones by Talaromyces sp. H4
4.5. Scale-Up of Biotransformation of Acetophenone by Talaromyces sp. H4
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hollmann, F.; Opperman, D.J.; Paul, C.E. Biocatalytic Reduction Reactions from a Chemist’s Perspective. Angew. Chem. Int. Ed. 2021, 60, 5644–5665. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing…Only Different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Lin, G.-Q.; Sun, X.-W. Chiral Drugs through Asymmetric Synthesis. In Chiral Drugs; Lin, G., You, Q., Cheng, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 29–76. [Google Scholar]
- Prier, C.K.; Kosjek, B. Recent Preparative Applications of Redox Enzymes. Curr. Opin. Chem. Biol. 2019, 49, 105–112. [Google Scholar] [CrossRef]
- Chadha, A.; Padhi, S.K.; Stella, S.; Venkataraman, S.; Saravanan, T. Microbial Alcohol Dehydrogenases: Recent Developments and Applications in Asymmetric Synthesis. Org. Biomol. Chem. 2024, 22, 228–251. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.-E.-H.H.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.; Shams, K.A.; et al. Microbial Biotransformation as a Tool for Drug Development Based on Natural Products from Mevalonic Acid Pathway: A Review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef]
- Nagaki, M.; Sato, R.; Tanabe, S.; Sato, T.; Hasui, Y.; Chounan, Y.; Tanaka, K.; Harada, Y. Biotransformation of Acetophenone to 1-Phenylethanol by Fungi. Trans. Mater. Res. Soc. Jpn. 2016, 41, 247–250. [Google Scholar] [CrossRef]
- Ogórek, R.; Jarosz, B. Assessment of Headspace Solid-Phase Microextraction (HS-SPME) for Control of Asymmetric Bioreduction of Ketones by Alternaria Alternata. Biocatal. Biotransform. 2020, 38, 75–80. [Google Scholar] [CrossRef]
- Świzdor, A.; Janeczko, T.; Dmochowska-Gładysz, J. Didymosphaeria Igniaria: A New Microorganism Useful for the Enantioselective Reduction of Aryl-Aliphatic Ketones. J. Ind. Microbiol. Biotechnol. 2010, 37, 1121–1130. [Google Scholar] [CrossRef]
- Choi, J.-M.; Han, S.-S.; Kim, H.-S. Industrial Applications of Enzyme Biocatalysis: Current Status and Future Aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Augusto Rodrigues, J.R.; Moran, P.J.S.; Conceição, G.J.A.; Fardelone, L.C. Recent Advances in the Biocatalytic Asymmetric Reduction of Acetophenones and a,b-Unsaturated Carbonyl Compounds. Food Technol. Biotechnol. 2004, 42, 295–303. [Google Scholar]
- Homola, P.; Kurák, T.; Illeová, V.; Polakovič, M. Kinetics of Acetophenone Reduction to ( R )-1-Phenylethanol by a Whole-Cell Pichia Capsulata Biocatalyst. Biocatal. Biotransform. 2015, 33, 323–332. [Google Scholar] [CrossRef]
- Chua, L.S.; Sarmidi, M.R. Immobilized Lipase-Catalysed Resolution of (R,S)-1-Phenylethanol in Recirculated Packed Bed Reactor. J. Mol. Catal. B Enzym. 2004, 28, 111–119. [Google Scholar] [CrossRef]
- Nassiri-Koopaei, N.; Faramarzi, M.A. Recent Developments in the Fungal Transformation of Steroids. Biocatal. Biotransform. 2015, 33, 1–28. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The Magnitude of Fungal Diversity: The 1.5 Million Species Estimate Revisited. Mycol. Res. 2001, 105, 1422–1432. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and Host Range Of Foliar Fungal Endophytes: Are Tropical Leaves Biodiversity Hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Tian, Y.; Amand, S.; Buisson, D.; Kunz, C.; Hachette, F.; Dupont, J.; Nay, B.; Prado, S. The Fungal Leaf Endophyte Paraconiothyrium Variabile Specifically Metabolizes the Host-Plant Metabolome for Its Own Benefit. Phytochemistry 2014, 108, 95–101. [Google Scholar] [CrossRef]
- Rodriguez, P.; Gonzalez, D.; Rodríguez Giordano, S. Endophytic Microorganisms: A Source of Potentially Useful Biocatalysts. J. Mol. Catal. B Enzym. 2016, 133, S569–S581. [Google Scholar] [CrossRef]
- Ma, M.F.P.; Li, K.; Zhou, Z.; Tang, C.; Chan, A.S.C. New Chiral Phosphorus Catalysts Derived from (S)-Binaphthol for Highly Enantioselective Reduction of Acetophenone by Borane. Tetrahedron Asymmetry 1999, 10, 3259–3261. [Google Scholar] [CrossRef]
- Decarlini, M.F.; Aimar, M.L.; Vázquez, A.M.; Vero, S.; Rossi, L.I.; Yang, P. Fungi Isolated from Food Samples for an Efficient Stereoselective Production of Phenylethanols. Biocatal. Agric. Biotechnol. 2017, 12, 275–285. [Google Scholar] [CrossRef]
- Prelog, V. Specification of the Stereospecificity of Some Oxido-Reductases by Diamond Lattice Sections. Pure Appl. Chem. 1964, 9, 119–130. [Google Scholar] [CrossRef]
- Itoh, N. Use of the Anti-Prelog Stereospecific Alcohol Dehydrogenase from Leifsonia and Pseudomonas for Producing Chiral Alcohols. Appl. Microbiol. Biotechnol. 2014, 98, 3889–3904. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Srivastava, G.; Sharma, A.N.; Kaur, S.; Jolly, R.S. Biocatalyzed Asymmetric Reduction of Benzils to Either Benzoins or Hydrobenzoins: PH Dependent Switch. Catal. Sci. Technol. 2015, 5, 4017–4028. [Google Scholar] [CrossRef]
- Pal, M.; Srivastava, G.; Moon, L.S.; Jolly, R.S. Bioreduction of Methyl Heteroaryl and Aryl Heteroaryl Ketones in High Enantiomeric Excess with Newly Isolated Fungal Strains. Bioresour. Technol. 2012, 118, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.C.; Ferreira, H.V.; Pimenta, E.F.; Berlinck, R.G.S.; Rezende, M.O.O.; Landgraf, M.D.; Seleghim, M.H.R.; Sette, L.D.; Porto, A.L.M. Biotransformation of α-Bromoacetophenones by the Marine Fungus Aspergillus Sydowii. Mar. Biotechnol. 2010, 12, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Comasseto, J.V.; Assis, L.F.; Andrade, L.H.; Schoenlein-Crusius, I.H.; Porto, A.L.M. Biotransformations of Ortho-, Meta- and Para-Aromatic Nitrocompounds by Strains of Aspergillus Terreus: Reduction of Ketones and Deracemization of Alcohols. J. Mol. Catal. B Enzym. 2006, 39, 24–30. [Google Scholar] [CrossRef]
- Pereira dos Santos, V.H.; Luiz, J.H.H.; dos Anjos, J.P.; de Oliveira Silva, E. Oxidative Potential of Two Brazilian Endophytic Fungi from Handroanthus Impetiginosus towards Progesterone. Steroids 2022, 187, 109101. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, J.S.; Silva, F.M.; Magallanes-Noguera, C.A.; Kurina-Sanz, M.; dos Santos, E.G.; Caldas, I.S.; Luiz, J.H.H.; Silva, E.O. Natural Trypanocidal Product Produced by Endophytic Fungi through Co-Culturing. Folia Microbiol. 2020, 65, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Aimar, M.L.; Bordón, D.L.; Formica, S.M.; Cantero, J.J.; Vazquez, A.M.; Velasco, M.I.; Rossi, L.I. Fruits of the Glossy Privet (Ligustrum Lucidum—Oleaceae) as Biocatalysts for Producing Chiral Aromatic Alcohols. Biocatal. Biotransform. 2014, 32, 348–357. [Google Scholar] [CrossRef]
- Mathre, D.J.; Thompson, A.S.; Douglas, A.W.; Hoogsteen, K.; Carroll, J.D.; Corley, E.G.; Grabowski, E.J.J. A Practical Process for the Preparation of Tetrahydro-1-Methyl-3,3-Diphenyl-1H,3H-Pyrrolo[1,2-c][1,3,2]Oxazaborole-Borane. A Highly Enantioselective Stoichiometric and Catalytic Reducing Agent. J. Org. Chem. 1993, 58, 2880–2888. [Google Scholar] [CrossRef]
- Orden, A.A.; Magallanes-Noguera, C.; Agostini, E.; Kurina-Sanz, M. Anti-Prelog Reduction of Ketones by Hairy Root Cultures. J. Mol. Catal. B Enzym. 2009, 61, 216–220. [Google Scholar] [CrossRef]
- Contente, M.L.; Serra, I.; Palazzolo, L.; Parravicini, C.; Gianazza, E.; Eberini, I.; Pinto, A.; Guidi, B.; Molinari, F.; Romano, D. Enzymatic Reduction of Acetophenone Derivatives with a Benzil Reductase from Pichia Glucozyma (KRED1-Pglu): Electronic and Steric Effects on Activity and Enantioselectivity. Org. Biomol. Chem. 2016, 14, 3404–3408. [Google Scholar] [CrossRef] [PubMed]
- Chanysheva, A.R.; Vorobyova, T.E.; Zorin, V.V. Relative Reactivity of Substituted Acetophenones in Enantioselective Biocatalytic Reduction Catalyzed by Plant Cells of Daucus Carota and Petroselinum Crispum. Tetrahedron 2019, 75, 130494. [Google Scholar] [CrossRef]
- Corey, E.J.; Shibata, S.; Bakshi, R.K. An Efficient and Catalytically Enantioselective Route to (S)-(-)-Phenyloxirane. J. Org. Chem. 1988, 53, 2861–2863. [Google Scholar] [CrossRef]
- Aguirre-Pranzoni, C.; Bisogno, F.R.; Orden, A.A.; Kurina-Sanz, M. Lyophilized Rhodotorula Yeast as All-in-One Redox Biocatalyst: Access to Enantiopure Building Blocks by Simple Chemoenzymatic One-Pot Procedures. J. Mol. Catal. B Enzym. 2015, 114, 19–24. [Google Scholar] [CrossRef]


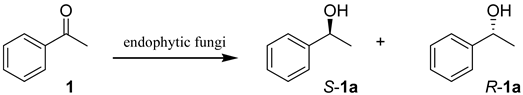 | ||||
| Entry | Endophytic fungus | Conversion (%) | ee (%) | Main product |
| 1 | H2 | 6.3 | 89.8 | S-1a |
| 2 | H3 | >99.9 | 76.2 | S-1a |
| 3 | H4 | >99.9 | 82.9 | S-1a |
| 4 | H6 | 47.0 | 87.2 | S-1a |
| 5 | H7 | 71.4 | 90.0 | S-1a |
| 6 | H8 | 2.2 | 21.6 | R-1a |
| Entry | Co-Solvent | Conversion (%) | ee (%) |
|---|---|---|---|
| 1 | None | 99.4 | 89.2 |
| 2 | dimethyl sulfoxide | >99.9 | 82.9 |
| 3 | Ethanol | 98.5 | 95.3 |
| 4 | methanol | 99,1 | 85.2 |
| 5 | isopropanol | 99.4 | 93.9 |
| 6 | Glycerol | 99.7 | 92.5 |
| 7 | Butanol | 29.8 | 67.0 |
| 8 | cyclohexanol | 9.06 | >99.9 |
| 9 | tetrahydrofuran | 99.0 | 91.9 |
| 10 | Acetone | 99.5 | 80.1 |
| Entry | Conversion (%) | ee (%) | |
|---|---|---|---|
| Time (days) | |||
| 1 | 1 | 11.1 | 72.2 |
| 2 | 2 | 18.6 | 66.9 |
| 3 | 3 | >99.9 | 82.9 |
| 4 | 4 | 77.0 | 84.6 |
| 5 | 5 | 90.0 | 86.0 |
| Inoculum charge a | |||
| 6 | 0.619 | 97.9 | 93.6 |
| 7 | 1.186 | 99.3 | 92.5 |
| 8 | 1.816 | >99.9 | 82.9 |
| 9 | 2.732 | 99.6 | 84.9 |
| Shaking speed (rpm) | |||
| 10 | 80 | 98.7 | 93.1 |
| 11 | 120 | >99.9 | 82.9 |
| 12 | 160 | 82.3 | 45.4 |
| pH | |||
| 13 | 5.0 | 99.6 | 20.8 |
| 14 | 6.0 | 98.9 | 59.6 |
| 15 | 7.0 | >99.9 | 82.9 |
| 16 | 8.0 | 99.7 | 87.0 |
| 17 | 9.0 | 99.5 | 86.6 |
| Acetophenone concentration b | |||
| 18 | 0.22 | 78.7 | 72.6 |
| 19 | 0.44 | 99.9 | 82.9 |
| 20 | 0.88 | 58.2 | 48.3 |
| Temperature (°C) | |||
| 21 | 26 | >99.9 | 87.6 |
| 22 | 28 | >99.9 | 82.9 |
| 23 | 30 | 97.1 | 73.9 |
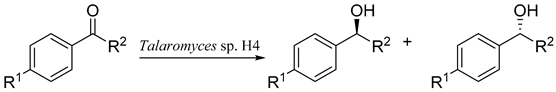 | |||||
| Entry | R1 | R2 | Conversion (%) | ee (%) | Major product |
| 1 | -H | -CH3 | 97.0 | 96.0 | S-1a |
| 2 | -NO2 | -CH3 | 52.2 | 29.3 | S-2a |
| 3 | -OCH3 | -CH3 | 40.0 | 90.9 | S-3a |
| 4 | -Cl | -CH3 | 32.3 | 26.9 | S-4a |
| 5 | -H | -CH2Cl | 11.7 | 61.4 | R-5a a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, V.H.P.; de Souza, M.V.P.; Victor, M.M.; Riatto, V.B.; Silva, E.O. Efficient Stereoselective Biotransformation of Prochiral Carbonyls by Endophytic Fungi from Handroanthus impetiginosus. SynBio 2024, 2, 254-266. https://doi.org/10.3390/synbio2030015
dos Santos VHP, de Souza MVP, Victor MM, Riatto VB, Silva EO. Efficient Stereoselective Biotransformation of Prochiral Carbonyls by Endophytic Fungi from Handroanthus impetiginosus. SynBio. 2024; 2(3):254-266. https://doi.org/10.3390/synbio2030015
Chicago/Turabian Styledos Santos, Valmore Henrique Pereira, Monielly Vasconcellos Pereira de Souza, Maurício Moraes Victor, Valéria Belli Riatto, and Eliane Oliveira Silva. 2024. "Efficient Stereoselective Biotransformation of Prochiral Carbonyls by Endophytic Fungi from Handroanthus impetiginosus" SynBio 2, no. 3: 254-266. https://doi.org/10.3390/synbio2030015
APA Styledos Santos, V. H. P., de Souza, M. V. P., Victor, M. M., Riatto, V. B., & Silva, E. O. (2024). Efficient Stereoselective Biotransformation of Prochiral Carbonyls by Endophytic Fungi from Handroanthus impetiginosus. SynBio, 2(3), 254-266. https://doi.org/10.3390/synbio2030015






