Can Methylococcus capsulatus Revolutionize Methane Capture and Utilization for Sustainable Energy Production?
Abstract
:1. Introduction
2. Methodology
2.1. Literature Search and Selection Criteria
2.2. Data Extraction and Synthesis
3. Characteristics and Metabolic Capabilities
3.1. A Comparison of Methylococcus capsulatus with Other Organisms
3.2. Mechanism of Methane Capture and Utilization by Methylococcus capsulatus
3.2.1. Methane Capture
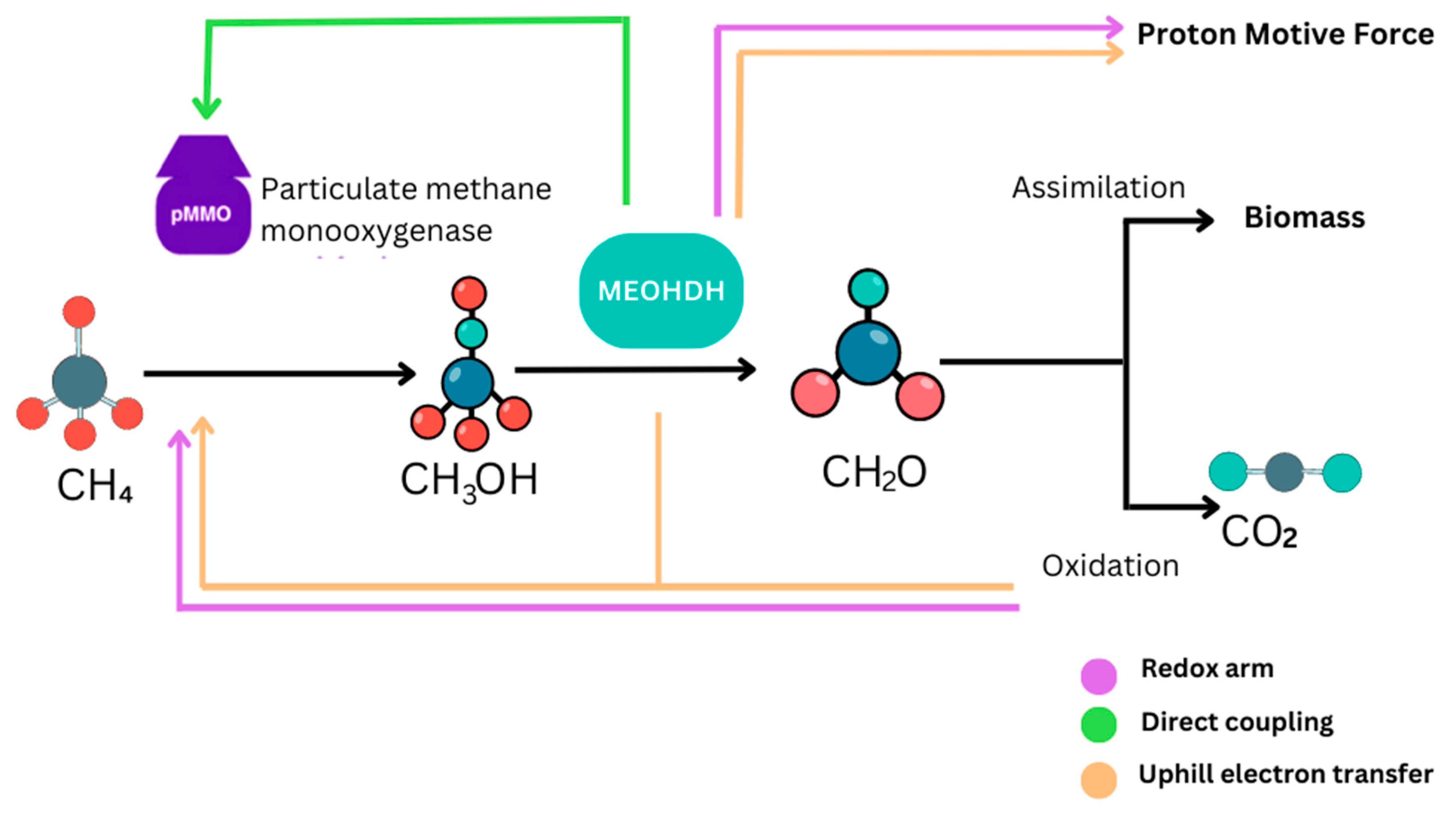
3.2.2. Methane Oxidation
- a.
- The pMMO Route: The pathway involving particulate methane monooxygenase (pMMO) is a primary process of methane utilization in M. capsulatus. pMMO is an integral membrane enzyme complex that is selectively localized in the bacterial cell membrane. It comprises several copper ions incorporated in the active site of the enzyme, which has central importance for its enzymatic function. In this pathway, methane (CH4) is oxidized in a copper-dependent process [14,38]. During this oxidation process, there is oxidation of ferrous iron (Fe2⁺) which is incorporated within the enzyme into ferric iron (Fe3⁺) which is also involved in the reaction cycle of the enzyme [39]. This process commences when methane molecules are bound to the copper centers within the active site of the pMMO enzyme. The copper centers are necessary for the binding of molecular oxygen (O2) and for the subsequent oxidation of methane. This reaction oxidizes methane to methanol (CH3OH) and water (H2O) [40]. The overall reaction for this pathway is:
- b.
- The sMMO Route: Soluble methane monooxygenase (sMMO) is another form of enzymatic system for the oxidation of methane found in M. capsulatus. sMMO on the other hand is an enzyme complex, soluble and active in the bacterial cytoplasm. It needs other co-factors to function, including iron and alpha-ketoglutarate, which are fundamental to the functioning of the enzyme [38]. The sMMO system functions through a different mechanism that uses a diiron cluster that is positioned in the active site of the enzyme. In this pathway, the diiron cluster is directly involved in the oxidation process and helps to activate molecular oxygen (O2) to react with methane (CH4) and produce methanol (CH3OH) and water (H2O) [41]. The overall reaction catalyzed by sMMO is:
3.3. Conversion to Value-Added Products
3.3.1. Biofuels
- a.
- Dimethyl ether (DME): Methanol is converted to dimethyl ether using dehydration, where water is removed to form DME [44]. The chemical reaction for this transformation is:2CH3OH → CH3OCH3 + H2O
- b.
- Biodiesel: Methanol is also used in large quantities in the manufacture of biodiesel through the transesterification process. This reaction involves the reaction of methanol with vegetable oil or animal fat containing triglycerides to form fatty acid methyl esters (FAMEs) and glycerol [48]. The reaction can be represented as:R-COOH + CH3OH → R-COOCH3 + H2O
3.3.2. Other Chemicals (Formaldehyde)
- (a)
- (b)
- Direct coupling: Electrons generated from the oxidation of methanol are directly transferred to the pMMO [50].
- (c)
3.4. Bioconversion of Methane to Methanol by Methylococcus capsulatus
- a.
- Whole-Cell Methanotroph Cultures
- b.
- MMO Enzyme Isolates
- c.
- Synthetic MMO Analogues
- d.
- Ammonia-Oxidizing Bacteria
3.5. Genetic and Metabolic Engineering Strategies to Boost Methylococcus capsulatus’ Performance to Improve Methane Uptake Rates
- a.
- Genome-Scale Metabolic Model
- b.
- Transcriptional and Metabolomic Responses
3.6. Benefits of Methylococcus capsulatus
3.6.1. Sustainable Energy Production through Methane Utilization
3.6.2. Applications of Methylococcus capsulatus beyond Energy Production
3.6.3. Benefits of Value-Added Products
3.6.4. Synthetic Biology Approaches for Enhanced Methane Oxidation
4. Challenges and Limitations
Critical Analysis and Integration
5. Case Studies and Current Research
6. Conclusions
7. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kemfert, C.; Schill, W.P. Methane: A Neglected Greenhouse Gas. Wkly. Rep. 2009, 5, 218–223. Available online: http://hdl.handle.net/10419/151071 (accessed on 15 May 2024).
- Mohajan, H. Dangerous Effects of Methane Gas in the Atmosphere. 2011. Available online: https://mpra.ub.uni-muenchen.de/50844/ (accessed on 15 May 2024).
- Pettus, A. Methane: Tapping the Untapped Potential; CleanAIR: Boston, MA, USA, 2009. [Google Scholar]
- Sahoo, K.K.; Goswami, G.; Das, D. Biotransformation of Methane and Carbon Dioxide into High-Value Products by Methanotrophs: Current State of Art and Future Prospects. Front. Microbiol. 2021, 12, 636486. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.; Khalil, M. Increase in the concentration of atmospheric methane. Atmos. Environ. 1967 1981, 15, 883–886. [Google Scholar] [CrossRef]
- Khalil, M.; Rasmussen, R. Causes of increasing atmospheric methane: Depletion of hydroxyl radicals and the rise of emissions. Atmos. Environ. 1967 1985, 19, 397–407. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Hayhoe, K. Atmospheric methane and global change. Earth-Sci. Rev. 2002, 57, 177–210. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Zhang, Z.; Poulter, B.; Knox, S.; Stavert, A.; McNicol, G.; Fluet-Chouinard, E.; Feinberg, A.; Zhao, Y.; Bousquet, P.; Canadell, J.G.; et al. Anthropogenic emission is the main contributor to the rise of atmospheric methane during 1993–2017. Natl. Sci. Rev. 2021, 9, nwab200. [Google Scholar] [CrossRef]
- Tauseef, S.; Premalatha, M.; Abbasi, T.; Abbasi, S. Methane capture from livestock manure. J. Environ. Manag. 2013, 117, 187–207. [Google Scholar] [CrossRef]
- Zhou, W. Methane storage in porous metal−organic frameworks: Current records and future perspectives. Chem. Rec. 2010, 10, 200–204. [Google Scholar] [CrossRef]
- Uddin, N.; Blommerde, M.; Taplin, R.; Laurence, D. Sustainable development outcomes of coal mine methane clean development mechanism Projects in China. Renew. Sustain. Energy Rev. 2015, 45, 1–9. [Google Scholar] [CrossRef]
- Verstraete, W.; Yanuka-Golub, K.; Driesen, N.; De Vrieze, J. Engineering microbial technologies for environmental sustainability: Choices to make. Microb. Biotechnol. 2022, 15, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, Y.; Jiang, P.; Zhang, C.; Smith, T.J.; Murrell, J.C.; Xing, X.-H. Methanotrophs: Multifunctional bacteria with promising applications in environmental bioengineering. Biochem. Eng. J. 2010, 49, 277–288. [Google Scholar] [CrossRef]
- Pieja, A.J.; Morse, M.C.; Cal, A.J. Methane to bioproducts: The future of the bioeconomy? Curr. Opin. Chem. Biol. 2017, 41, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J. The methanotrophs—The families Methylococcaceae and Methylocystaceae. Prokaryotes 2006, 5, 266–289. [Google Scholar]
- Islam, T.; Larsen, Ø.; Torsvik, V.; Øvreås, L.; Panosyan, H.; Murrell, J.C.; Birkeland, N.-K.; Bodrossy, L. Novel Methanotrophs of the Family Methylococcaceae from Different Geographical Regions and Habitats. Microorganisms 2015, 3, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Murrell, J.C.; Gilbert, B.; McDonald, I.R. Molecular biology and regulation of methane monooxygenase. Arch. Microbiol. 2000, 173, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Culpepper, M.A.; Rosenzweig, A.C. Architecture and active site of particulate methane monooxygenase. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 483–492. [Google Scholar] [CrossRef]
- Park, D.; Lee, J. Biological conversion of methane to methanol. Korean J. Chem. Eng. 2013, 30, 977–987. [Google Scholar] [CrossRef]
- Fei, Q.; Guarnieri, M.T.; Tao, L.; Laurens, L.M.; Dowe, N.; Pienkos, P.T. Bioconversion of natural gas to liquid fuel: Opportunities and challenges. Biotechnol. Adv. 2014, 32, 596–614. [Google Scholar] [CrossRef]
- Bjorck, C.E.; Dobson, P.D.; Pandhal, J. Biotechnological conversion of methane to methanol: Evaluation of progress and potential. AIMS Bioeng. 2018, 5, 1–38. [Google Scholar] [CrossRef]
- Lee, O.K.; Nguyen, D.T.; Lee, E.Y. Metabolic engineering of methanotrophs for the production of chemicals and fuels. In Methanotrophs: Microbiology Fundamentals and Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–203. [Google Scholar] [CrossRef]
- Henard, C.A.; Guarnieri, M.T. Metabolic engineering of methanotrophic bacteria for industrial biomanufacturing. In Methane Biocatalysis: Paving the Way to Sustainability; Springer: Berlin/Heidelberg, Germany, 2018; pp. 117–132. [Google Scholar] [CrossRef]
- Indrelid, S.; Kleiveland, C.; Holst, R.; Jacobsen, M.; Lea, T. The Soil Bacterium Methylococcus capsulatus Bath Interacts with Human Dendritic Cells to Modulate Immune Function. Front. Microbiol. 2017, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T.J.; Rosenzweig, A.C. Methane-Oxidizing Enzymes: An Upstream Problem in Biological Gas-to-Liquids Conversion. J. Am. Chem. Soc. 2016, 138, 9327–9340. [Google Scholar] [CrossRef] [PubMed]
- Chidambarampadmavathy, K.; Obulisamy, P.K.; Heimann, K. Role of copper and iron in methane oxidation and bacterial biopolymer accumulation. Eng. Life Sci. 2015, 15, 387–399. [Google Scholar] [CrossRef]
- Khider, M.L.K.; Brautaset, T.; Irla, M. Methane monooxygenases: Central enzymes in methanotrophy with promising biotechnological applications. World J. Microbiol. Biotechnol. 2021, 37, 72. [Google Scholar] [CrossRef]
- Sirajuddin, S.; Rosenzweig, A.C. Enzymatic Oxidation of Methane. Biochemistry 2015, 54, 2283–2294. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef]
- Khan, S.; Jain, G.; Srivastava, A.; Verma, P.C.; Pande, V.; Dubey, R.S.; Khan, M.; Haque, S.; Ahmad, S. Enzymatic biomethanol production: Future perspective. Sustain. Mater. Technol. 2023, 38, e00729. [Google Scholar] [CrossRef]
- Gan, Y.; Meng, X.; Gao, C.; Song, W.; Liu, L.; Chen, X. Metabolic engineering strategies for microbial utilization of methanol. Eng. Microbiol. 2023, 3, 100081. [Google Scholar] [CrossRef]
- Murrell, J.C.; Smith, T.J. Biochemistry and Molecular Biology of Methane Monooxygenase. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Pham, M.D.; Lin, Y.-P.; Van Vuong, Q.; Nagababu, P.; Chang, B.T.-A.; Ng, K.Y.; Chen, C.-H.; Han, C.-C.; Chen, C.-H.; Li, M.S.; et al. Inactivation of the particulate methane monooxygenase (pMMO) in Methylococcus capsulatus (Bath) by acetylene. Biochim. Biophys. Acta BBA-Proteins Proteom. 2015, 1854, 1842–1852. [Google Scholar] [CrossRef]
- Wang, J.; He, Q.P. Methane Removal from Air: Challenges and Opportunities. Methane 2023, 2, 404–414. [Google Scholar] [CrossRef]
- Feng, J.-C.; Yan, J.; Wang, Y.; Yang, Z.; Zhang, S.; Liang, S.; Li, X.-S. Methane mitigation: Learning from the natural marine environment. Innovation 2022, 3, 100297. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Knief, C. Diversity and phylogeny of described aerobic methanotrophs. In Methane Biocatalysis: Paving the Way to Sustainability; Kalyuzhnaya, M.G., Xing, X.-H., Eds.; Springer: Cham, Switzerland, 2018; pp. 17–42. [Google Scholar]
- Hwang, Y.; Hwang, Y.; Na, J.-G.; Na, J.-G.; Lee, S.J.; Lee, S.J. Transcriptional regulation of soluble methane monooxygenase via enhancer-binding protein derived from Methylosinus sporium 5. Appl. Environ. Microbiol. 2023, 89, e0210422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Koo, C.W.; Cassidy, C.K.; Spink, M.C.; Ni, T.; Zanetti-Domingues, L.C.; Bateman, B.; Martin-Fernandez, M.L.; Shen, J.; Sheng, Y.; et al. Structure and activity of particulate methane monooxygenase arrays in methanotrophs. Nat. Commun. 2022, 13, 5221. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Hung, M.-C.; Chang, B.T.-A.; Lee, T.-L.; Lin, Z.-H.; Tsai, I.-K.; Chen, Y.-S.; Chang, C.-S.; Tsai, Y.-F.; Chen, K.H.-C.; et al. The PmoB subunit of particulate methane monooxygenase (pMMO) in Methylococcus capsulatus (Bath): The CuI sponge and its function. J. Inorg. Biochem. 2019, 196, 110691. [Google Scholar] [CrossRef]
- Ross, M.O.; Rosenzweig, A.C. A tale of two methane monooxygenases. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2017, 22, 307–319. [Google Scholar] [CrossRef]
- Bertau, M.; Offermanns, H.; Plass, L.; Schmidt, F.; Wernicke, H.J. (Eds.) Methanol: The Basic Chemical and Energy Feedstock of the Future; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar] [CrossRef]
- Deka, T.J.; Osman, A.I.; Baruah, D.C.; Rooney, D.W. Methanol fuel production, utilization, and techno-economy: A review. Environ. Chem. Lett. 2022, 20, 3525–3554. [Google Scholar] [CrossRef]
- Chmielarz, L. Dehydration of Methanol to Dimethyl Ether—Current State and Perspectives. Catalysts 2024, 14, 308. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Arundhathi, R.; Kasture, M.W.; Samanta, C.; Vankayala, R.; Thota, C. Temperature-dependent synthesis of dimethyl ether (DME) from methanol over beta zeolite: A novel approach to a sustainable fuel. R. Soc. Open Sci. 2023, 10, 230524. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Chen, Z.; Nourozieh, H.; Yang, M.; Chai, B. Introduce dimethyl ether (DME) as a solvent for steam-assisted gravity drainage (SAGD) co-injection: An effective and environmental application. Fuel 2023, 341, 127639. [Google Scholar] [CrossRef]
- Święs, A.; Kowalczyk, A.; Gil, B.; Chmielarz, L. Dehydration of methanol and ethanol over ferrierite originated layered zeolites—The role of acidity and porous structure. RSC Adv. 2022, 12, 9395–9403. [Google Scholar] [CrossRef]
- Han, B.; Su, T.; Wu, H.; Gou, Z.; Xing, X.-H.; Jiang, H.; Chen, Y.; Li, X.; Murrell, J.C. Paraffin oil as a “methane vector” for rapid and high cell density cultivation of Methylosinus trichosporium OB3b. Appl. Microbiol. Biotechnol. 2009, 83, 669–677. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Huong, D.T.M.; Juan, H.-Y.; Su, C.-H.; Chien, C.-C. Liquid Lipase-Catalyzed Esterification of Oleic Acid with Methanol for Biodiesel Production in the Presence of Superabsorbent Polymer: Optimization by Using Response Surface Methodology. Energies 2018, 11, 1085. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T. A review of the synthesis of activated carbon for biodiesel production: Precursor, preparation, and modification. Energy Convers. Manag. X 2022, 13, 100152. [Google Scholar] [CrossRef]
- Sana, N.; Arnepalli, D.N.; Krishnan, C. Enhanced Bioconversion of Methane to Biodiesel by Methylosarcina sp. LC-4. Sustainability 2022, 15, 505. [Google Scholar] [CrossRef]
- Lieven, C.; Herrgård, M.J.; Sonnenschein, N. Microbial Methylotrophic Metabolism: Recent Metabolic Modeling Efforts and Their Applications in Industrial Biotechnology. Biotechnol. J. 2018, 13, e1800011. [Google Scholar] [CrossRef]
- Lieven, C.; Petersen, L.A.H.; Jørgensen, S.B.; Gernaey, K.V.; Herrgard, M.J.; Sonnenschein, N. A Genome-Scale Metabolic Model for Methylococcus capsulatus (Bath) Suggests Reduced Efficiency Electron Transfer to the Particulate Methane Monooxygenase. Front. Microbiol. 2018, 9, 2947. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Conrad, R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol. Lett. 1992, 101, 261–270. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Biogas Production and Applications in the Sustainable Energy Transition. J. Energy 2022, 2022, 8750221. [Google Scholar] [CrossRef]
- Hakemian, A.S.; Rosenzweig, A.C. The Biochemistry of Methane Oxidation. Annu. Rev. Biochem. 2007, 76, 223–241. [Google Scholar] [CrossRef]
- Bedekar, A.A.; Deewan, A.; Jagtap, S.S.; Parker, D.A.; Liu, P.; Mackie, R.I.; Rao, C.V. Transcriptional and metabolomic responses of Methylococcus capsulatus Bath to nitrogen source and temperature downshift. Front. Microbiol. 2023, 14, 1259015. [Google Scholar] [CrossRef]
- Patel, S.K.; Gupta, R.K.; Kondaveeti, S.; Otari, S.V.; Kumar, A.; Kalia, V.C.; Lee, J.-K. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour. Technol. 2020, 315, 123791. [Google Scholar] [CrossRef] [PubMed]
- Emelianov, G.; Song, D.-U.; Jang, N.; Ko, M.; Kim, S.K.; Rha, E.; Shin, J.; Kwon, K.K.; Kim, H.; Lee, D.-H.; et al. Engineered Methylococcus capsulatus Bath for efficient methane conversion to isoprene. Bioresour. Technol. 2024, 393, 130098. [Google Scholar] [CrossRef] [PubMed]
- Nevzorova, T.; Kutcherov, V. Barriers to the wider implementation of biogas as a source of energy: A state-of-the-art review. Energy Strat. Rev. 2019, 26, 100414. [Google Scholar] [CrossRef]
- Safaeian, P.; Yazdian, F.; Khosravi-Darani, K.; Rashedi, H.; Lackner, M. P3HB from CH4 using methanotrophs: Aspects of bioreactor, fermentation process and modelling for cost-effective biopolymer production. Front. Bioeng. Biotechnol. 2023, 11, 1137749. [Google Scholar] [CrossRef] [PubMed]
- Meraz, J.L.; Abel, A.J.; Clark, D.S.; Criddle, C.S. Biological conversion of methane to bioplastics: Kinetics, stoichiometry, and thermodynamic considerations for process optimization. Chem. Eng. J. 2023, 454, 140166. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of Biopolymers for Drugs and Probiotics Delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Gęsicka, A.; Oleskowicz-Popiel, P.; Łężyk, M. Recent trends in methane to bioproduct conversion by methanotrophs. Biotechnol. Adv. 2021, 53, 107861. [Google Scholar] [CrossRef]
- Hanif, S.; Lateef, M.; Hussain, K.; Hyder, S.; Usman, B.; Zaman, K.; Asif, M. Controlling air pollution by lowering methane emissions, conserving natural resources, and slowing urbanization in a panel of selected Asian economies. PLoS ONE 2022, 17, e0271387. [Google Scholar] [CrossRef]
- Cruz, S.G.; Pijuan, M. Methanotrophic bacterial biorefineries: Resource recovery and GHG mitigation through the production of bacterial biopolymers. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 55–178. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Nguyen, A.D.; Nguyen, T.T.; Nguyen, L.T.; Lee, O.K.; Lee, E.Y. Biological conversion of methane to chemicals and fuels: Technical challenges and issues. Appl. Microbiol. Biotechnol. 2018, 102, 3071–3080. [Google Scholar] [CrossRef]
- Samanta, D.; Sani, R.K. Methane Oxidation via Chemical and Biological Methods: Challenges and Solutions. Methane 2023, 2, 279–303. [Google Scholar] [CrossRef]
- Sanni, G.B. Mobilizing Microbes to Mitigate Climate Change. 2022. Available online: https://theperspectograph.com/mobilizing-microbes-to-mitigate-climate-change/ (accessed on 6 July 2023).
- But, S.Y.; Suleimanov, R.Z.; Oshkin, I.Y.; Rozova, O.N.; Mustakhimov, I.I.; Pimenov, N.V.; Dedysh, S.N.; Khmelenina, V.N. New Solutions in Single-Cell Protein Production from Methane: Construction of Glycogen-Deficient Mutants of M. capsulatus MIR. Fermentation 2024, 10, 265. [Google Scholar] [CrossRef]
| Challenges and Limitations | Solutions |
|---|---|
| Optimization of Methane-to-Methanol Conversion |
|
| |
| |
| Downstream Processes and Purification |
|
| |
| |
| Scaling Up from Laboratory to Industrial Scale |
|
| |
| |
| Long-Term Robustness and Stability |
|
| |
| |
| Regulatory and Economic Considerations |
|
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinsemolu, A.A.; Onyeaka, H.N. Can Methylococcus capsulatus Revolutionize Methane Capture and Utilization for Sustainable Energy Production? SynBio 2024, 2, 311-328. https://doi.org/10.3390/synbio2030019
Akinsemolu AA, Onyeaka HN. Can Methylococcus capsulatus Revolutionize Methane Capture and Utilization for Sustainable Energy Production? SynBio. 2024; 2(3):311-328. https://doi.org/10.3390/synbio2030019
Chicago/Turabian StyleAkinsemolu, Adenike A., and Helen N. Onyeaka. 2024. "Can Methylococcus capsulatus Revolutionize Methane Capture and Utilization for Sustainable Energy Production?" SynBio 2, no. 3: 311-328. https://doi.org/10.3390/synbio2030019
APA StyleAkinsemolu, A. A., & Onyeaka, H. N. (2024). Can Methylococcus capsulatus Revolutionize Methane Capture and Utilization for Sustainable Energy Production? SynBio, 2(3), 311-328. https://doi.org/10.3390/synbio2030019






