Molecular Mechanisms in Understanding Anoxia Tolerance in Rice Seeds under Submergence and Their Implication in Rice Biotechnology
Abstract
1. Introduction
2. Physiological Relevance of Seeds’ Response to Anoxia
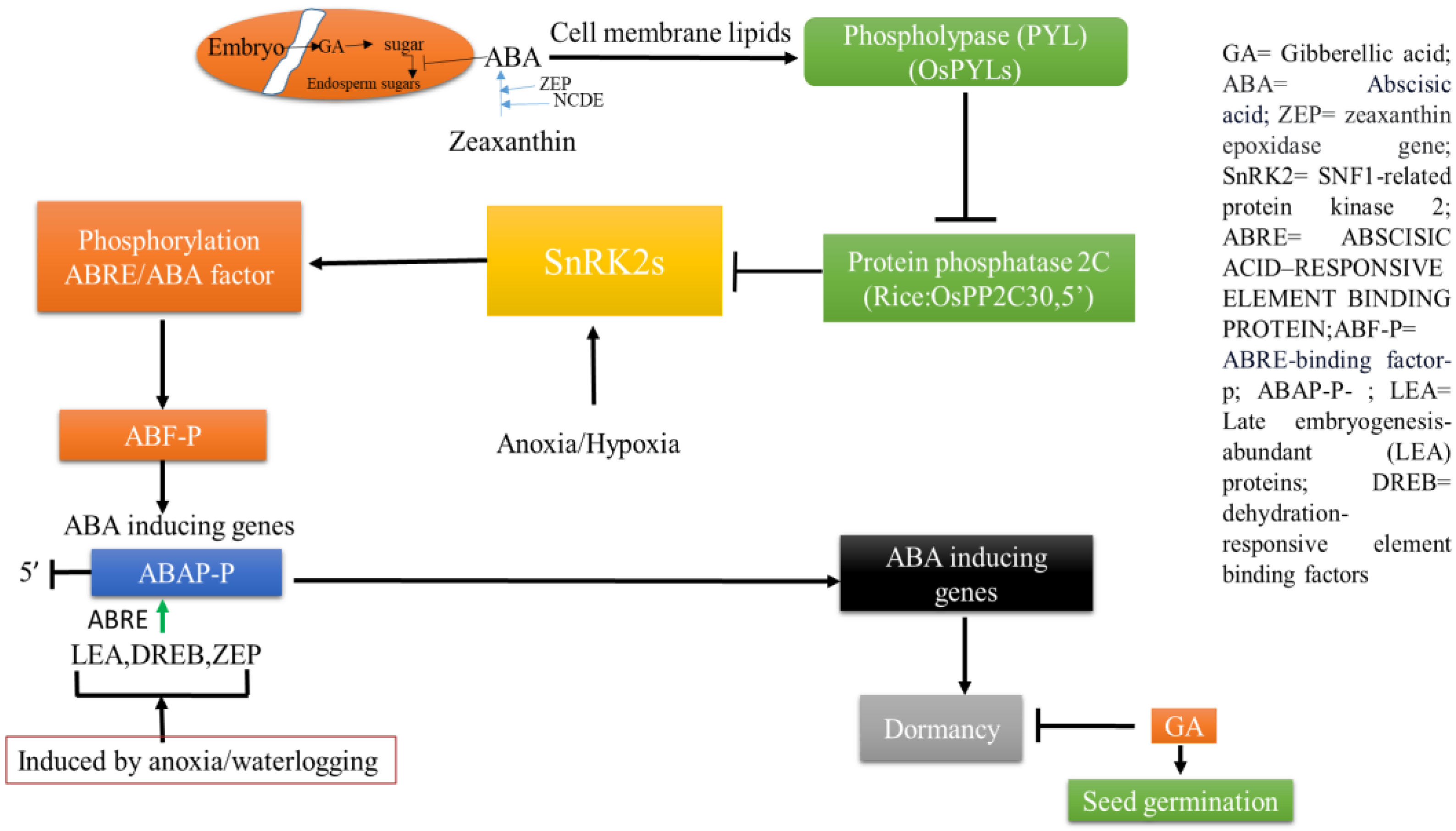
3. Hormonal Interplay between Seed Dormancy and Germination in Rice under Water
4. Development of Rice Seedlings Facing Anoxic Conditions
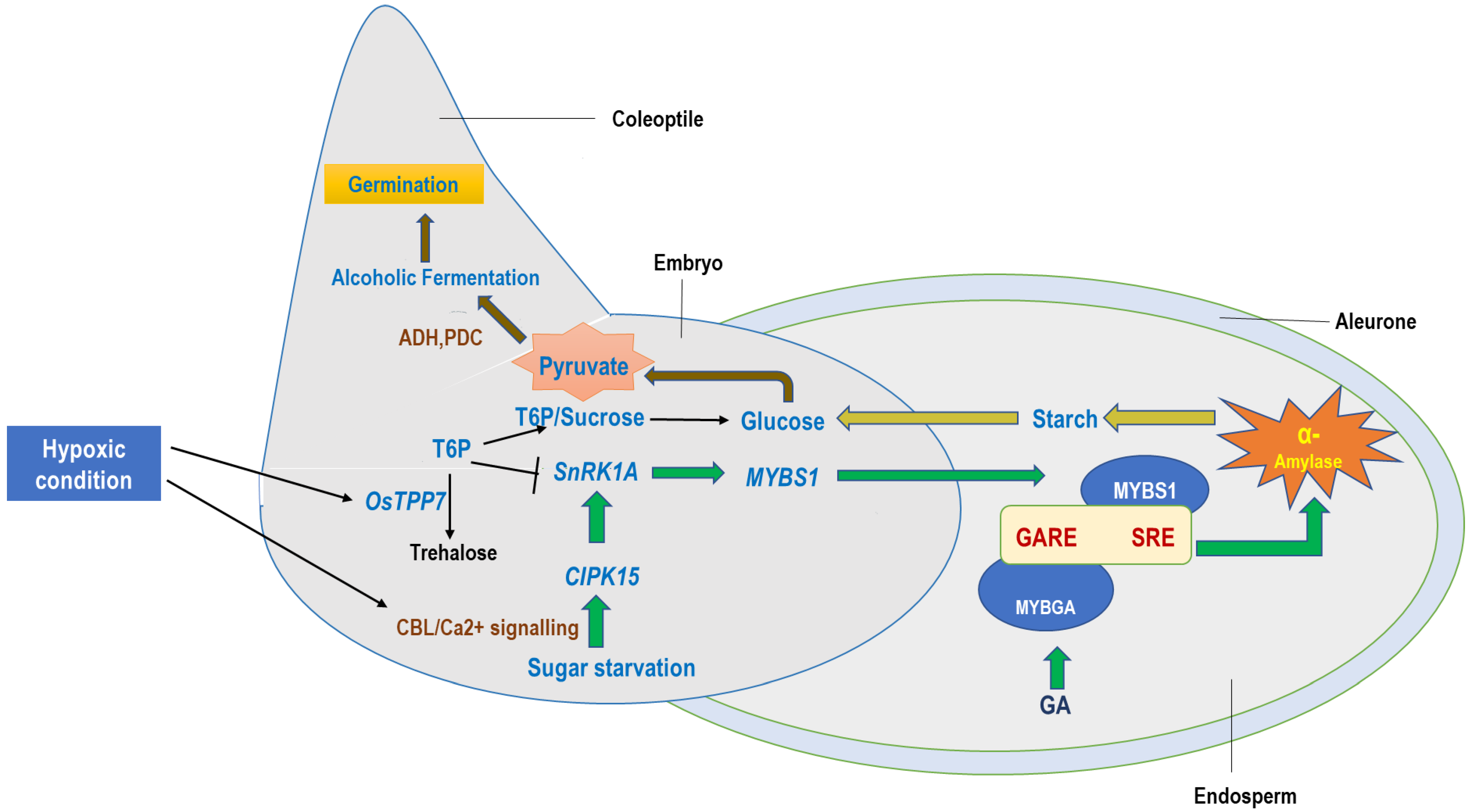
5. Fermentative Mechanism: Pathways for Anaerobic Seeds’ Germination in Rice
6. Molecular Regulation of Major Glycolytic Flux: Amylase Activity with GA Induction
7. Constraints and Remediation of Pre-Harvest Sprouting
8. Metabolomic Approaches for Hypoxia Tolerance in Rice
9. Regulation of Transcripts in Seed Germination under Submergence
10. Biochemical Implications of Anoxic Seed Germination
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talukder, B.; van Loon, G.W.; Hipel, K.W.; Chiotha, S.; Orbinski, J. Health impacts of climate change on smallholder farmers. One Health 2021, 13, 100258. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Datta, K.; Datta, S.K. Rice biofortification: High iron, zinc, and vitamin-A to fight against “hidden hunger”. Agronomy 2019, 9, 803. [Google Scholar] [CrossRef]
- Wiraguna, E. Adaptation of legume seeds to waterlogging at germination. Crops 2022, 2, 111–119. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Gui, R.; Wang, Z.; Li, Z.; Han, Y.; Guo, X.; Sun, J. Comparative multi-omics analysis of hypoxic germination tolerance in weedy rice embryos and coleoptiles. Genomics 2021, 113, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A. Understanding benefits and risks of pesticide use. Sci. Res. Essays 2009, 4, 945–949. [Google Scholar]
- Das, A.; Uchimiya, H. Oxygen stress and adaptation of a semi-aquatic plant: Rice (Oryza sativa). J. Plant Res. 2002, 115, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Septiningsih, E.M.; Ignacio, J.C.I.; Sendon, P.M.; Sanchez, D.L.; Ismail, A.M.; Mackill, D.J. QTL mapping and confirmation for tolerance of anaerobic conditions during germination derived from the rice landrace Ma-Zhan Red. Theor. Appl. Genet. 2013, 126, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Septiningsih, E.M.; Balyan, H.S.; Singh, N.K.; Rai, V. Genetics, physiological mechanisms and breeding of flood-tolerant rice (Oryza sativa L.). Plant Cell Physiol. 2017, 58, 185. [Google Scholar]
- Yu, S.M.; Lo, S.F.; Ho, T.H.D. Source–sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Yu, W.; Peng, F.; Wang, W.; Liang, J.; Xiao, Y.; Yuan, X. SnRK1 phosphorylation of SDH positively regulates sorbitol metabolism and promotes sugar accumulation in peach fruit. Tree Physiol. 2021, 41, 1077–1086. [Google Scholar] [CrossRef]
- Megala, R.; Kalarani, M.K.; Jeyakumar, P.; Senthil, N.; Pushpam, R.; Umapathi, M. Standardization of optimum melatonin concentration for drought tolerance at germination and early development stage in rice (CO-54). J. Appl. Nat. Sci. 2022, 14, 1022–1030. [Google Scholar] [CrossRef]
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 11522. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.; Brunello, L.; Iacopino, S.; Valeri, M.C.; Novi, G.; Dornbusch, T.; Perata, P.; Loreti, E. Arabidopsis phenotyping reveals the importance of alcohol dehydrogenase and pyruvate decarboxylase for aerobic plant growth. Sci. Rep. 2020, 10, 16669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiang, Z.; Liu, M.; Wang, S.; Zhang, L.; Cai, D.; Huang, Y.; Mao, D.; Fu, J.; Chen, L. ABA biosynthesis gene OsNCED3 contributes to preharvest sprouting resistance and grain development in rice. Plant Cell Environ. 2022, 46, 1384–1401. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Pandian, S.; Kumar, T.S.; Zoclanclounon, Y.A.B.; Muthuramalingam, P.; Shilpha, J.; Satish, L.; Ramesh, M. Seed dormancy and pre-harvest sprouting in rice—An updated overview. Int. J. Mol. Sci. 2021, 22, 11804. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A.; Jacobsen, J.V.; Ross, J.J.; Helliwell, C.A.; Poole, A.T.; Scofield, G.; Reid, J.B.; Gubler, F. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8′-hydroxylase. Plant J. 2006, 45, 942–954. [Google Scholar] [CrossRef]
- Calvo, A.P.; Nicolas, C.; Nicolas, G.; Rodríguez, D. Evidence of a cross-talk regulation of a GA 20-oxidase (FsGA20ox1) by gibberellins and ethylene during the breaking of dormancy in Fagus sylvatica seeds. Physiol. Plant. 2004, 120, 623–630. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A. Ectopic expression of C4 photosynthetic pathway genes improves carbon assimilation and alleviate stress tolerance for future climate change. Physiol. Mol. Biol. Plants 2020, 26, 195–209. [Google Scholar] [CrossRef]
- Waseem, M.; Nkurikiyimfura, O.; Niyitanga, S.; Nyimbo, W.J.; Shaheen, I.; Aslam, M.M. Role of Metabolomics and Next-Generation Sequencing for Sustainable Crop Production. In Principles and Practices of OMICS and Genome Editing for Crop Improvement; Prakash, C.S., Fiaz, S., Fahad, S., Eds.; Springer International Publishing: Cham, Switzerland; New York, NY, USA, 2022; pp. 123–147. ISBN 978-3-030-96924-0. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y.; et al. Regain flood adaptation in rice through a 14-3-3 protein OsGF14h. Nat. Commun. 2022, 13, 5664. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, M.; Singh, A.; Das, S.; Panigrahi, K.C. Co-action of ABA, brassinosteriod hormone pathways and differential regulation of different transcript isoforms during cold-and-dark induced senescence in Arabidopsis. J. Plant Biochem. Biotechnol. 2022, 31, 489–510. [Google Scholar] [CrossRef]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.P.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Halford, N.G.; Hey, S.J. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 2009, 419, 247–259. [Google Scholar] [CrossRef]
- Graeber, K.; Linkies, A.; Müller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-species approaches to seed dormancy and germination: Conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol. Biol. 2010, 73, 67–87. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Paul, S.; Basu, S. Cross-talk between abscisic acid-dependent and abscisic-independent pathways during abiotic stress. Plant Cell Rep. 2013, 32, 985–1006. [Google Scholar] [CrossRef]
- Neuman, H.; Galpaz, N.; Cunningham, F.X., Jr.; Zamir, D.; Hirschberg, J. The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J. 2014, 78, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Miro, B.; Ismail, A.M. Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front. Plant Sci. 2013, 4, 269. [Google Scholar] [CrossRef] [PubMed]
- Mejía, S.M.V.; de Francisco, A.; Bohrer, B. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020, 60, 3693–3704. [Google Scholar] [CrossRef]
- Colmer, T.D.; Armstrong, W.; Greenway, H.; Ismail, A.M.; Kirk, G.J.D.; Atwell, B.J. Physiological mechanisms of flooding tolerance in rice: Transient complete submergence and prolonged standing water. Prog. Bot. 2014, 75, 255–307. [Google Scholar]
- Xu, W.; Dai, M.; Li, F.; Liu, A. Genomic imprinting, methylation and parent-of-origin effects in reciprocal hybrid endosperm of castor bean. Nucleic Acids Res. 2014, 42, 6987–6998. [Google Scholar] [CrossRef] [PubMed]
- Kaspary, T.E.; Roma-Burgos, N.; Merotto, A., Jr. Snorkeling strategy: Tolerance to flooding in rice and potential application for weed management. Genes 2020, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Borisjuk, L.; Junker, B.H.; Mock, H.P.; Rolletschek, H.; Seiffert, U.; Weschke, W.; Wobus, U. Barley grain development: Toward an integrative view. Int. Rev. Cell Mol. Bio. 2010, 281, 49–89. [Google Scholar]
- Yankov, D. Fermentative lactic acid production from lignocellulosic feedstocks: From source to purified product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef]
- Laville, J.; Blumer, C.; Von Schroetter, C.; Gaia, V.; Défago, G.; Keel, C.; Haas, D. Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J. Bacteriol. 1998, 180, 3187–3196. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Panda, D.; Barik, J.; Behera, P.K. Improving Submergence Tolerance in Rice: Recent Progress and Future Perspectives. In Response of Field Crops to Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2022; pp. 111–122. [Google Scholar] [CrossRef]
- Gibbs, J.; Morrell, S.; Valdez, A.; Setter, T.L.; Greenway, H. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. J. Exp. Bot. 2000, 51, 785–796. [Google Scholar] [CrossRef]
- Felle, H.H. pH regulation in anoxic plants. Ann. Bot. 2005, 96, 519–532. [Google Scholar] [CrossRef]
- Perata, P.; Guglielminetti, L.; Alpi, A. Mobilization of endosperm reserves in cereal seeds under anoxia. Ann. Bot. 1997, 79, 49–56. [Google Scholar] [CrossRef]
- Miricescu, A.; Brazel, A.J.; Beegan, J.; Wellmer, F.; Graciet, E. Transcriptional analysis of waterlogging response in barley identifies signatures of waterlogging tolerance/sensitivity. bioRxiv 2022, 26.518028. [Google Scholar] [CrossRef]
- Magneschi, L.; Kudahettige, R.L.; Alpi, A.; Perata, P. Comparative analysis of anoxic coleoptile elongation in rice varieties: Relationship between coleoptile length and carbohydrate levels, fermentative metabolism and anaerobic gene expression. Plant Biol. 2009, 11, 561–573. [Google Scholar] [CrossRef]
- Quimio, C.A.; Torrizo, L.B.; Setter, T.L.; Ellis, M.; Grover, A.; Abrigo, E.M.; Oliva, N.P.; Ella, E.S.; Carpena, A.L.; Ito, O.; et al. Enhancement of submergence tolerance in transgenic rice overproducing pyruvate decarboxylase. J. Plant Physiol. 2000, 156, 516–521. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Hu, G.; Wang, X.; Chen, H.; Shi, Q.; Xiang, J.; Zhang, Y.; Zhu, D.; Zhang, Y. Reduced bioactive gibberellin content in rice seeds under low temperature leads to decreased sugar consumption and low seed germination rates. Plant Physiol. Biochem. 2018, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Álvarez, E.M.; Pucciariello, C. Cereal germination under low oxygen: Molecular processes. Plants 2022, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Lee, Y.H.; Song, S.I. Overexpression of the rice basic leucine zipper TF OsbZIP12 confers drought tolerance to rice and makes seedlings hypersensitive to ABA. Plant Biotech. Rep. 2014, 8, 431–441. [Google Scholar] [CrossRef]
- Gubler, F.; Kalla, R.; Roberts, J.K.; Jacobsen, J.V. Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. Plant Cell 1995, 7, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Herath, V.; Wijaya, E.; Yeo, H.C.; Benildo, G.; Lee, D.Y. Patterns of cis-element enrichment reveal potential regulatory modules involved in the transcriptional regulation of anoxia response of japonica rice. Gene 2012, 511, 235–242. [Google Scholar] [CrossRef]
- Guillermo, B.V.; Marisela, R.D.; Rosalba, T.R.; Reginaldo, B.S.; Martin-Ernesto, T.H. Physiological function of rhamnogalacturonan lyase genes based in the analysis of cis-acting elements located in the promoter region. Res. J. Biotechnol. 2017, 12, 6. [Google Scholar]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Hegde, P.S.; White, I.R.; Debouck, C. Interplay of transcriptomics and proteomics. Curr. Opin. Biotech. 2003, 14, 647–651. [Google Scholar] [CrossRef]
- Pawłowski, T.A. Proteome analysis of Norway maple (Acer platanoides L.) seeds dormancy breaking and germination: Influence of abscisic and gibberellic acids. BMC Plant Biol. 2009, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cao, J.; Jiang, H.; Chang, C.; Zhang, H.P.; Sheikh, S.W.; Shah, L.; Ma, C. Unraveling molecular and genetic studies of wheat (Triticum aestivum L.) resistance against factors causing pre-harvest sprouting. Agronomy 2019, 9, 117. [Google Scholar] [CrossRef]
- Ma, M.; Cen, W.; Li, R.; Wang, S.; Luo, J. The molecular regulatory pathways and metabolic adaptation in the seed germination and early seedling growth of rice in response to low O2 stress. Plants 2020, 9, 1363. [Google Scholar] [CrossRef]
- Barnes, W.J.; Anderson, C.T. Release, recycle, rebuild: Cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol. Plant 2018, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.M.; Lunn, J.E. A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Aftab, T.; Roychoudhury, A. (Eds.) Plant Perspectives to Global Climate Changes: Developing Climate-Resilient Plants, 1st ed.; Academic Press: Cambridge, MA, USA, 2021; ISBN 9780323856652. [Google Scholar]
- Hsu, S.K.; Tung, C.W. Genetic mapping of anaerobic germination-associated QTLs controlling coleoptile elongation in rice. Rice 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Wang, Y.; Qin, Y.; Zhou, Y.; Qian, H. Influence and interaction of iron and lead on seed germination in upland rice. Plant Soil 2020, 455, 187–202. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Fan, Y.; Posch, B.C.; Garcia, A.; Coast, O.; Atkin, O.K. Responses of leaf respiration to heatwaves. Plant Cell Environ. 2021, 44, 2090–2101. [Google Scholar] [CrossRef]
- Leyva-González, M.A.; Ibarra-Laclette, E.; Cruz-Ramírez, A.; Herrera-Estrella, L. Functional and transcriptome analysis reveals an acclimatization strategy for abiotic stress tolerance mediated by Arabidopsis NF-YA family members. PLoS ONE 2012, 7, 48138. [Google Scholar] [CrossRef]
- Zhang, P.; Lyu, D.; Jia, L.; He, J.; Qin, S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649. [Google Scholar] [CrossRef] [PubMed]
- Shiao, T.L.; Ellis, M.H.; Dolferus, R.; Dennis, E.S.; Doran, P.M. Overexpression of alcohol dehydrogenase or pyruvate decarboxylase improves growth of hairy roots at reduced oxygen concentrations. Biotech. Bioeng. 2022, 77, 455–461. [Google Scholar] [CrossRef]
- Marisco, G.; Saito, S.T.; Ganda, I.S.; Brendel, M.; Pungartnik, C. Low ergosterol content in yeast adh1 mutant enhances chitin maldistribution and sensitivity to paraquat-induced oxidative stress. Yeast 2011, 28, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Sahi, C.; Singh, A.; Kumar, K.; Blumwald, E.; Grover, A. Salt stress response in rice: Genetics, molecular biology, and comparative genomics. Funct. Integr. Genom. 2006, 6, 263–284. [Google Scholar] [CrossRef]
- Llorca, C.M.; Potschin, M.; Zentgraf, U. bZIPs and WRKYs: Two large TF families executing two different functional strategies. Front. Plant Sci. 2014, 5, 169. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Khashayar, P.; Amereh, M.; Tasnim, N.; Hoorfar, M.; Akbari, M. Microfluidic-based oxygen (O2) sensors for on-chip monitoring of cell, tissue and organ metabolism. Biosensors 2022, 12, 6. [Google Scholar] [CrossRef]
- Jurdak, R.; Launay-Avon, A.; Paysant-Le Roux, C.; Bailly, C. Retrograde signalling from the mitochondria to the nucleus translates the positive effect of ethylene on dormancy breaking of Arabidopsis thaliana seeds. New Phytol. 2021, 229, 2192–2205. [Google Scholar] [CrossRef]
- Liu, Z.; Hartman, S.; van Veen, H.; Zhang, H.; Leeggangers, H.A.; Martopawiro, S.; Bosman, F.; de Deugd, F.; Su, P.; Hummel, M.; et al. Ethylene augments root hypoxia tolerance via growth cessation and reactive oxygen species amelioration. Plant Physiol. 2022, 190, 1365–1383. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A. The role of ethylene in metabolic acclimations to low oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adak, M.K.; Das, A.; Kundu, A.; Chatterjee, M.; Hasanuzzaman, M. Molecular Mechanisms in Understanding Anoxia Tolerance in Rice Seeds under Submergence and Their Implication in Rice Biotechnology. Seeds 2023, 2, 246-258. https://doi.org/10.3390/seeds2030019
Adak MK, Das A, Kundu A, Chatterjee M, Hasanuzzaman M. Molecular Mechanisms in Understanding Anoxia Tolerance in Rice Seeds under Submergence and Their Implication in Rice Biotechnology. Seeds. 2023; 2(3):246-258. https://doi.org/10.3390/seeds2030019
Chicago/Turabian StyleAdak, Malay Kumar, Abir Das, Ankita Kundu, Mitali Chatterjee, and Mirza Hasanuzzaman. 2023. "Molecular Mechanisms in Understanding Anoxia Tolerance in Rice Seeds under Submergence and Their Implication in Rice Biotechnology" Seeds 2, no. 3: 246-258. https://doi.org/10.3390/seeds2030019
APA StyleAdak, M. K., Das, A., Kundu, A., Chatterjee, M., & Hasanuzzaman, M. (2023). Molecular Mechanisms in Understanding Anoxia Tolerance in Rice Seeds under Submergence and Their Implication in Rice Biotechnology. Seeds, 2(3), 246-258. https://doi.org/10.3390/seeds2030019







